Diabetes and Obesity—Cumulative or Complementary Effects On Adipokines, Inflammation, and Insulin Resistance
Abstract
:1. Introduction
2. Experimental Section
3. Results
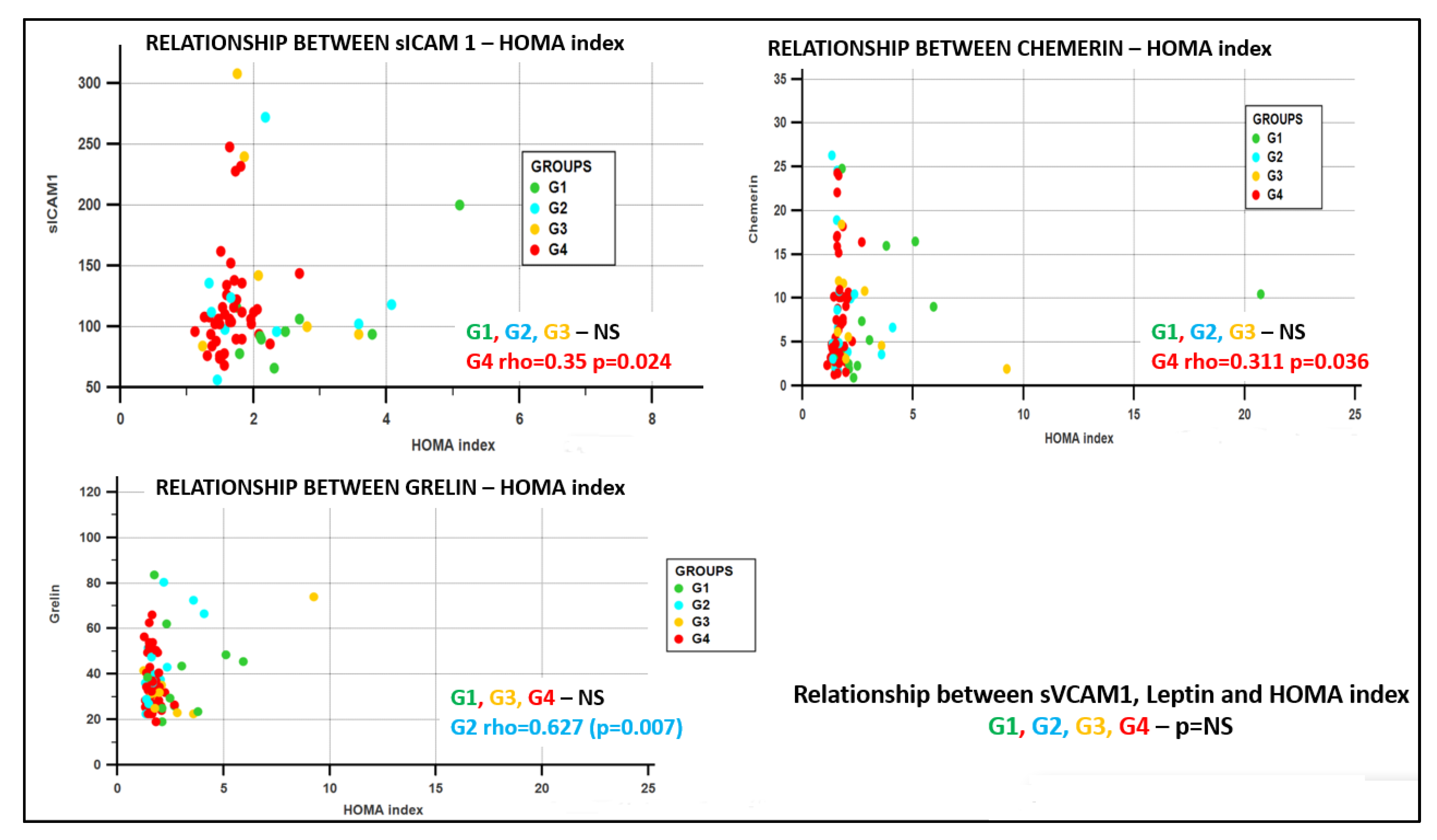
- Group G1 (obesity positive + diabetes positive), in the sICAM1–leptin rho = 0.786 (p = 0.03), sVCAM1–glycemia/insulin rho = −0.85 (p = 0.004);
- Group G2 (obesity positive + diabetes negative), in the sICAM1–sVCAM1 rho = 0.733 (p = 0.025), and in the sVCAM1-chemerin rho = 0.667 (p = 0.05);
- Group G3 (obesity negative + diabetes positive), no significant correlations;
- Group G4 (obesity negative + diabetes negative), in the sICAM1–HOMA rho = 0.35, p = 0.024, sICAM1–QUICKI rho = −0.35, p = 0.023, leptin–glycemia/insulin rho = −0.371 p = 0.024, ghrelin–chemerin rho =−0.350, p = 0.018, chemerin–HOMA rho = 0.311, p = 0.036, and in the chemerin–QUICKI rho = −0.31, p = 0.036.
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Romacho, T.; Elsen, M.; Röhrborn, D.; Eckel, J. Adipose tissue and its role in organ crosstalk. Acta Physiol. 2014, 210, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, K.; Sypniewska, G. Diabetes as a complication of adipose tissue dysfunction. Is there a role for potential new biomarkers? Clin. Chem. Lab. Med. 2013, 51, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poher, A.L.; Tschöp, M.H.; Müller, T.D. Ghrelin regulation of glucose metabolism. Peptides 2018, 100, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Bae, K.-H.; Lee, S.C.; Oh, K.-J. The latest insights into adipokines in diabetes. J. Clin. Med. 2019, 8, 1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelsinger, C.; Tschoner, A.; Kaser, S.; Ebenbichler, C.F. Adipokine update—Neue moleküle, neue funktionen. Wien. Med. Wochenschr. 2010, 160, 377–390. [Google Scholar] [CrossRef]
- Spyrou, N.; Avgerinos, K.I.; Mantzoros, C.S.; Dalamaga, M. Classic and novel adipocytokines at the intersection of obesity and cancer: Diagnostic and therapeutic strategies. Curr. Obes. Rep. 2018, 7, 260–275. [Google Scholar] [CrossRef]
- Roman, A.A.; Parlee, S.D.; Sinal, C.J. Chemerin: A potential endocrine link between obesity and type 2 diabetes. Endocrine 2012, 42, 243–251. [Google Scholar] [CrossRef]
- Dagpo, T.D.; Nolan, C.J.; Delghingaro-Augusto, V. Exploring therapeutic targets to reverse or prevent the transition from metabolically healthy to unhealthy obesity. Cells 2020, 9, 1596. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front. Cardiovasc. Med. 2020, 7, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Internation diabetes federation. IDF Diabetes Atlas Ninth. 2019. ISBN 9782930229874. Available online: https://www.diabetesatlas.org/en/ (accessed on 20 May 2020).
- Weinstein, A.R.; Sesso, H.D.; Lee, I.M.; Cook, N.R.; Manson, J.A.E.; Buring, J.E.; Gaziano, J.M. Relationship of physical activity vs body mass index with type 2 diabetes in women. J. Am. Med. Assoc. 2004, 292, 1188–1194. [Google Scholar] [CrossRef] [Green Version]
- Vinciguerra, F.; Tumminia, A.; Baratta, R.; Ferro, A.; Alaimo, S.; Hagnäs, M.; Graziano, M.; Vigneri, R.; Frittitta, L. Prevalence and clinical characteristics of children and adolescents with metabolically healthy obesity: Role of insulin sensitivity. Life 2020, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A complex interplay of multiple molecular determinants and pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-L.; Chen, H.-H.; Tsai, S.-Y.; Lin, C.-Y.; Liu, S.-J.; Chien, K.-L. The relationship between metabolically healthy obesity and the risk of cardiovascular disease: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 1228. [Google Scholar] [CrossRef] [Green Version]
- Catoi, A.; Parvu, A.; Andreicut, A.; Mironiuc, A.; Craciun, A.; Catoi, C.; Pop, I. Metabolically healthy versus unhealthy morbidly obese: Chronic inflammation, nitro-oxidative stress, and insulin resistance. Nutrients 2018, 10, 1199. [Google Scholar] [CrossRef] [Green Version]
- Eckel, N.; Meidtner, K.; Kalle-Uhlmann, T.; Stefan, N.; Schulze, M.B. Metabolically healthy obesity and cardiovascular events: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2015, 23, 956–966. [Google Scholar] [CrossRef]
- Hamer, M.; Stamatakis, E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J. Clin. Endocrinol. Metab. 2012, 97, 2482–2488. [Google Scholar] [CrossRef]
- Funcke, J.B.; Scherer, P.E. Beyond adiponectin and leptin: Adipose tissue-derived mediators of inter-organ communication. J. Lipid Res. 2019, 60, 1648–1697. [Google Scholar] [CrossRef]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Barchetta, I.; Cimini, F.A.; Ciccarelli, G.; Baroni, M.G.; Cavallo, M.G. Sick fat: The good and the bad of old and new circulating markers of adipose tissue inflammation. J. Endocrinol. Investig. 2019, 42, 1257–1272. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chehimi, M.; Vidal, H.; Eljaafari, A. Pathogenic role of IL-17-producing immune cells in obesity, and related inflammatory diseases. J. Clin. Med. 2017, 6, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.-W.; Lee, M.; Oh, K.-J. Adipose tissue-derived signatures for obesity and type 2 diabetes: Adipokines, batokines and MicroRNAs. J. Clin. Med. 2019, 8, 854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cozma, A.; Sitar-Taut, A.-V.; Fodor, A.; Oltean, M.; Minciuna, I.-A.; Breaban, I.; Matuz, R.; Racataianu, N.; Dogaru, G.; Orăşan, O. Evaluation of endothelial dysfunction and its improvement after cardiac rehabilitation. Balneo Res. J. 2018, 9, 364–375. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; de Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Albu, A.; Para, I. Left ventricular diastolic dysfunction in diabetes mellitus and the therapeutic role of exercise training. Balneo Res. J. 2019, 10, 145–152. [Google Scholar] [CrossRef]
- Gateva, A.; Assyov, Y.; Tsakova, A.; Kamenov, Z. Classical (adiponectin, leptin, resistin) and new (chemerin, vaspin, omentin) adipocytokines in patients with prediabetes. Horm. Mol. Biol. Clin. Investig. 2018, 34, 1–9. [Google Scholar] [CrossRef]
- Wozniak, S.E.; Gee, L.L.; Wachtel, M.S.; Frezza, E.E. Adipose tissue: The new endocrine organ? A review article. Dig. Dis. Sci. 2009, 54, 1847–1856. [Google Scholar] [CrossRef]
- Stejskal, D.; Karpisek, M.; Hanulova, Z.; Svestak, M. Chemerin is an independent marker of the metabolic syndrome in a Caucasian population—A pilot study. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2008, 152, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Stojek, M. The role of chemerin in human disease. Postepy Hig. Med. Dosw. 2017, 71, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.C.; Sinal, C.J. Chemerin: At the crossroads of inflammation and obesity. Trends Endocrinol. Metab. 2010, 21, 660–667. [Google Scholar] [CrossRef]
- Calabrò, P.; Golia, E.; Maddaloni, V.; Malvezzi, M.; Casillo, B.; Marotta, C.; Calabrò, R.; Golino, P. Adipose tissue-mediated inflammation: The missing link between obesity and cardiovascular disease? Intern. Emerg. Med. 2009, 4, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, K.; Shoemaker, J.K.; Overend, T.J.; Petrella, R.J. Metabolic syndrome, endothelial function and lifestyle modification. Diab. Vasc. Dis. Res. 2009, 6, 181–189. [Google Scholar] [CrossRef]
- Schram, M.T.; Stehouwer, C.D.A. Endothelial dysfunction, cellular adhesion molecules and the metabolic syndrome. Horm. Metab. Res. 2005, 37 (Suppl. 1), 49–55. [Google Scholar] [CrossRef]
- Cozma, A.; Sitar-Taut, A.-V.; Orasan, O.; Procopciuc, L.M.; Farcas, A.D.; Stan, A.; Negrean, V.; Sampelean, D.; Pop, D.; Zdrenghea, D.; et al. The relationship between eNOS (G894T) gene polymorphism and arterial stiffness in patients with metabolic syndrome. Rev. Chim. 2018, 69, 2351–2356. [Google Scholar] [CrossRef]
- Sitar-Taut, A.V.; Orasan, O.; Fodor, A.; Farcas, A.D.; Tarmure Sarlea, S.T.; Dogaru, G.; Zdrenghea, D.T.; Pop, D.; Cozma, A. The relationship between inflammation and metabolic syndrome (METS)−A matter of gender? Rev. Chim. 2019, 70, 69–73. [Google Scholar] [CrossRef]
- Song, Y.; Manson, J.E.; Tinker, L.; Rifai, N.; Cook, N.R.; Hu, F.B.; Hotamisligil, G.S.; Ridker, P.M.; Rodriguez, B.L.; Margolis, K.L.; et al. Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes 2007, 56, 1898–1904. [Google Scholar] [CrossRef] [Green Version]
- Sattar, N.; Murray, H.M.; Welsh, P.; Blauw, G.J.; Buckley, B.M.; de Craen, A.J.; Ford, I.; Forouhi, N.G.; Freeman, D.J.; Jukema, J.W.; et al. Are elevated circulating intercellular adhesion molecule 1 levels more strongly predictive of diabetes than vascular risk? Outcome of a prospective study in the elderly. Diabetologia 2009, 52, 235–239. [Google Scholar] [CrossRef] [Green Version]
- Thorand, B.; Baumert, J.; Chambless, L.; Meisinger, C.; Kolb, H.; Döring, A.; Löwel, H.; Koenig, W. Elevated markers of endothelial dysfunction predict type 2 diabetes mellitus in middle-aged men and women from the general population. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Guerra, A.F.; Vargas-Robles, H.; Lozano Nuevo, J.J.; Escalante-Acosta, B.A. Correlation between circulating adhesion molecule levels and albuminuria in type-2 diabetic hypertensive patients. Kidney Blood Press. Res. 2009, 32, 106–109. [Google Scholar] [CrossRef]
- de Marañón, A.M.; Iannantuoni, F.; Abad-Jiménez, Z.; Canet, F.; Díaz-Pozo, P.; López-Domènech, S.; Roldán-Torres, I.; Morillas, C.; Rocha, M.; Víctor, V.M. Association between proinflammatory markers, leukocyte–endothelium interactions, and carotid intima–media thickness in type 2 diabetes: Role of glycemic control. J. Clin. Med. 2020, 9, 2522. [Google Scholar] [CrossRef] [PubMed]
- Ingelsson, E.; Hulthe, J.; Lind, L. Inflammatory markers in relation to insulin resistance and the metabolic syndrome. Eur. J. Clin. Investig. 2008, 38, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Antuna-Puente, B.; Feve, B.; Fellahi, S.; Bastard, J.-P. Adipokines: The missing link between insulin resistance and obesity. Diabetes Metab. 2008, 34, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Lago, F.; Gómez, R.; Gómez-Reino, J.J.; Dieguez, C.; Gualillo, O. Adipokines as novel modulators of lipid metabolism. Trends Biochem. Sci. 2009, 34, 500–510. [Google Scholar] [CrossRef]
- Alamri, B.N.; Shin, K.; Chappe, V.; Anini, Y. The role of ghrelin in the regulation of glucose homeostasis. Horm. Mol. Biol. Clin. Investig. 2016, 26, 3–11. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Lampropoulos, S.; Kapelouzou, A.; Gkontopoulos, A.; Theofilogiannakos, E.K.; Fotiadis, G.; Kottas, G. Serum levels of apelin and ghrelin in patients with acute coronary syndromes and established coronary artery disease-KOZANI STUDY. Transl. Res. 2010, 155, 238–246. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Tsanikidis, H.; Kapelouzou, A.; Vrabas, I.; Vitta, I.; Karayannacos, P.E.; Liapis, C.D.; Sailer, N. Effects of rosiglitazone and metformin treatment on apelin, visfatin, and ghrelin levels in patients with type 2 diabetes mellitus. Metabolism 2010, 59, 373–379. [Google Scholar] [CrossRef]
- Ukkola, O. Ghrelin and metabolic disorders. Curr. Protein Pept. Sci. 2009, 10, 2–7. [Google Scholar] [CrossRef]
- Pop, D.; Peter, P.; Dădârlat, A.; Sitar-Tăut, A.; Zdrenghea, D. Serum ghrelin level is associated with cardiovascular risk score. Rom. J. Intern. Med. 2015, 53, 140–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razzaghy-Azar, M.; Nourbakhsh, M.; Pourmoteabed, A.; Nourbakhsh, M.; Ilbeigi, D.; Khosravi, M. An evaluation of acylated ghrelin and obestatin levels in childhood obesity and their association with insulin resistance, metabolic syndrome, and oxidative stress. J. Clin. Med. 2016, 5, 61. [Google Scholar] [CrossRef]
- Pop, D.; Sitar-Taut, A.; Bodisz, G.; Dadarlat, A.; Stanca, L.; Buzoianu, A.; Zdrenghea, D. Are ghrelin levels directly related with ishemic heart disease. Ind. J. Res. 2013, 2, 222–224. [Google Scholar]
- Helfer, G.; Wu, Q.F. Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Feder, S.; Haberl, E.M.; Aslanidis, C. Chemerin isoforms and activity in obesity. Int. J. Mol. Sci. 2019, 20, 1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, J.; Mattu, H.S.; Chatha, K.; Randeva, H.S. Chemerin in human cardiovascular disease. Vascul. Pharmacol. 2018, 110, 1–6. [Google Scholar] [CrossRef]
- Perumalsamy, S.; Aqilah Mohd Zin, N.A.; Widodo, R.T.; Wan Ahmad, W.A.; Vethakkan, S.R.D.B.; Huri, H.Z. Chemokine like receptor-1 (CMKLR-1) receptor: A Potential therapeutic target in management of chemerin induced type 2 diabetes mellitus and cancer. Curr. Pharm. Des. 2017, 23, 3689–3698. [Google Scholar] [CrossRef] [Green Version]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef]
- Estienne, A.; Reverchon, M.; Partyka, A.; Bourdon, G.; Grandhaye, J.; Barbe, A.; Caldas-Silveira, E.; Rame, C.; Niżański, W.; Froment, P.; et al. Chemerin impairs in vitro testosterone production, sperm motility, and fertility in chicken: Possible involvement of its receptor CMKLR1. Cells 2020, 9, 1599. [Google Scholar] [CrossRef]
- Ernst, M.C.; Haidl, I.D.; Zuńĩga, L.A.; Dranse, H.J.; Rourke, J.L.; Zabel, B.A.; Butcher, E.C.; Sinal, C.J. Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinology 2012, 153, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Karczewska-Kupczewska, M.; Nikolajuk, A.; Stefanowicz, M.; Matulewicz, N.; Kowalska, I.; Straczkowski, M. Serum and adipose tissue chemerin is differentially related to insulin sensitivity. Endocr. Connect. 2020, 9, 360–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, M.; Inomata, S.; Okimura, Y.; Iguchi, G.; Fukuoka, H.; Miyake, K.; Koga, D.; Akamatsu, S.; Kasuga, M.; Takahashi, Y. Decreased serum chemerin levels in male Japanese patients with type 2 diabetes: Sex dimorphism. Endocr. J. 2013, 60, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roguska, J.; Zubkiewicz-Kucharska, A. Chemerin as an early marker of metabolic syndrome. Pediatr. Endocrinol. Diabetes Metab. 2018, 24, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Park, C.Y.; Sweeney, G. Biomarkers of insulin sensitivity and insulin resistance: Past, present and future. Crit. Rev. Clin. Lab. Sci. 2015, 52, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.S.; Butt, Z.; Bader, N.; Pathan, A.Z.; Hussain, S.; Iqbal, N.T. Role of multifunctional chemerin in obesity and preclinical diabetes. Obes. Res. Clin. Pract. 2015, 9, 507–512. [Google Scholar] [CrossRef]
- Han, J.; Kim, S.H.; Suh, Y.J.; Lim, H.A.; Shin, H.; Cho, S.G.; Kim, C.W.; Lee, S.Y.; Lee, D.H.; Hong, S.; et al. Serum chemerin levels are associated with abdominal visceral fat in type 2 diabetes. J. Korean Med. Sci. 2016, 31, 924–931. [Google Scholar] [CrossRef]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef] [Green Version]
- MacDougald, O.A.; Burant, C.F. The rapidly expanding family of adipokines. Cell Metab. 2007, 6, 159–161. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, M.; Ren, L.; Xiang, L.; Chen, J.; Li, M.; Xiao, T.; Ren, P.; Xiong, L.; Zhang, J.V. CMKLR1 deficiency influences glucose tolerance and thermogenesis in mice on high fat diet. Biochem. Biophys. Res. Commun. 2016, 473, 435–441. [Google Scholar] [CrossRef]
- Corona-Meraz, F.-I.; Navarro-Hernández, R.-E.; Ruíz-Quezada, S.-L.; Madrigal-Ruíz, P.-M.; Castro-Albarrán, J.; Chavarría-Ávila, E.; Guzmán-Ornelas, M.-O.; Gómez-Bañuelos, E.; Petri, M.-H.; Ramírez-Cedano, J.-I.; et al. Inverse relationship of the CMKLR1 relative expression and chemerin serum levels in obesity with dysmetabolic phenotype and insulin resistance. Mediators Inflamm. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Niklowitz, P.; Rothermel, J.; Lass, N.; Barth, A.; Reinehr, T. Link between chemerin, central obesity, and parameters of the Metabolic Syndrome: Findings from a longitudinal study in obese children participating in a lifestyle intervention. Int. J. Obes. 2018, 42, 1743–1752. [Google Scholar] [CrossRef]
- Coimbra, S.; Brandão Proença, J.; Santos-Silva, A.; Neuparth, M.J. Adiponectin, leptin, and chemerin in elderly patients with type 2 diabetes mellitus: A close linkage with obesity and length of the disease. Biomed. Res. Int. 2014, 2014, 701915. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.S.; Eshki, A.; AlTassan, B.; Fatani, D.; Helmi, H.; AlSaif, S. Relationship of serum novel adipokine chemerin levels with body composition, insulin resistance, dyslipidemia and diabesity in Saudi women. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1296–1302. [Google Scholar]
- Bozaoglu, K.; Segal, D.; Shields, K.A.; Cummings, N.; Curran, J.E.; Comuzzie, A.G.; Mahaney, M.C.; Rainwater, D.L.; Vandeberg, J.L.; MacCluer, J.W.; et al. Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J. Clin. Endocrinol. Metab. 2009, 94, 3085–3088. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.S.; Rehman, R.; Baig, M.; Khan, T.A. New roles of the multidimensional adipokine: Chemerin. Peptides 2014, 62, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Chakaroun, R.; Raschpichler, M.; Klöting, N.; Oberbach, A.; Flehmig, G.; Kern, M.; Schön, M.R.; Shang, E.; Lohmann, T.; Dreßler, M.; et al. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism 2012, 61, 706–714. [Google Scholar] [CrossRef] [Green Version]
- Jialal, I.; Devaraj, S.; Kaur, H.; Adams-Huet, B.; Bremer, A.A. Increased chemerin and decreased omentin-1 in both adipose tissue and plasma in nascent metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E514–E517. [Google Scholar] [CrossRef]
- Shan, Y.U.; Zhang, Y.; Li, M.Z.; Hua, X.U.; Qian, W.A.N.G.; Jun, S.O.N.G.; Peng, L.I.N.; Zhang, L.; Qian, L.I.U.; Huang, Q.X.; et al. Chemerin and apelin are positively correlated with inflammation in obese type 2 diabetic patients. Chin. Med. J. 2012, 125, 3440–3444. [Google Scholar]
- Adamiak, P.; Łacka, K. Adipose tissue, adipokines and aging. Pol. Merkur. Lekarski 2016, 40, 122. [Google Scholar]
- El-Deeb, T.S.; Bakkar, S.M.; Eltoony, L.; Zakhary, M.M.; Kamel, A.A.; Nafee, A.M.; Hetta, H.F. The adipokine chemerin and fetuin-a serum levels in type 2 diabetes mellitus: Relation to obesity and inflammatory markers. Egypt J. Immunol. 2018, 25, 191–202. [Google Scholar]
- Klöting, N.; Fasshauer, M.; Dietrich, A.; Kovacs, P.; Schön, M.R.; Kern, M.; Stumvoll, M.; Blüher, M. Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Tönjes, A.; Fasshauer, M.; Kratzsch, J.; Stumvoll, M.; Bluher, M. Adipokine pattern in subjects with impaired fasting glucose and impaired glucose tolerance in comparison to normal glucose tolerance and diabetes. PLoS ONE 2010, 5, e13911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouwens, D.M.; Bekaert, M.; Lapauw, B.; Van Nieuwenhove, Y.; Lehr, S.; Hartwig, S.; Calders, P.; Kaufman, J.M.; Sell, H.; Eckel, J.; et al. Chemerin as biomarker for insulin sensitivity in males without typical characteristics of metabolic syndrome. Arch. Physiol. Biochem. 2012, 118, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef] [PubMed]
- Sledzinski, T.; Korczynska, J.; Hallmann, A.; Kaska, L.; Proczko-Markuszewska, M.; Stefaniak, T.; Sledzinski, M.; Swierczynski, J. The increase of serum chemerin concentration is mainly associated with the increase of bodymass index in obese, non-diabetic subjects. J. Endocrinol. Investig. 2013, 36, 428–434. [Google Scholar]
- Bobbert, T.; Schwarz, F.; Fischer-Rosinsky, A.; Maurer, L.; Möhlig, M.; Pfeiffer, A.F.H.; Mai, K.; Spranger, J. Chemerin and prediction of Diabetes mellitus type 2. Clin. Endocrinol. 2015, 82, 838–843. [Google Scholar] [CrossRef]
- Rabe, K.; Lehrke, M.; Parhofer, K.G.; Broedl, U.C. Adipokines and insulin resistance. Mol. Med. 2008, 14, 741–751. [Google Scholar] [CrossRef]
- Fatima, S.; Iqbal, N. Chemerin as a potential screening marker for sub-clinical diabetes? Endocr. Abstr. 2014. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-H.; Chang, Y.; Jung, H.-S.; Shin, H.; Ryu, S. Impact of self-rated health on progression to a metabolically unhealthy phenotype in metabolically healthy obese and non-obese individuals. J. Clin. Med. 2019, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Tsatsoulis, A.; Paschou, S.A. Metabolically healthy obesity: Criteria, epidemiology, controversies, and consequences. Curr. Obes. Rep. 2020, 9, 109–120. [Google Scholar] [CrossRef]
- Esteghamati, A.; Ghasemiesfe, M.; Mousavizadeh, M.; Noshad, S.; Nakhjavani, M. Pioglitazone and metformin are equally effective in reduction of chemerin in patients with type 2 diabetes. J. Diabetes Investig. 2014, 5, 327–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fodor, A.; Cozma, A.; Suharoschi, R.; Sitar-Taut, A.; Roman, G. Clinical and genetic predictors of diabetes drug’s response. Drug Metab. Rev. 2019, 51, 408–427. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, P.; Seres, I.; Lorincz, H.; Harangi, M.; Somodi, S.; Paragh, G. Association of chemerin with oxidative stress, inflammation and classical adipokines in non-diabetic obese patients. J. Cell. Mol. Med. 2014, 18, 1313–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Global | Women | Men | p | ||
|---|---|---|---|---|---|
| Number | 88 | 66 | 22 | ||
| Age (years) | 61.96 ± 10.15 | 60.71 ± 9.94 | 65.72 ± 10.04 | 0.04 | |
| Body mass index (kg/m2) | 28.85 ± 4.22 | 28.88 ± 4.39 | 28.73 ±3.76 | NS | |
| Obesity No (%) | Yes | 31 (35.3) | 24 (36.36) | 7 (31.81) | NS |
| No | 57 (64.7) | 42 (63.63) | 15 (68.18) | ||
| Abdominal circumference (cm) | 98.04 ± 10.41 | 96.27 ± 10.40 | 103.27 ± 8.70 | 0.003 | |
| Systolic blood pressure (mmHg) | 131.59 ± 16.28 (130) | 131.43 ± 16.47 (130) | 132.04 ± 16.08 (130) | NS | |
| Diastolic blood pressure (mmHg) | 82.78 ± 15.38 (80) | 83.56 ± 16.88 (80) | 80.45 ± 9.5 (80) | NS | |
| Hypertension No (%) | Yes | 70 (79.5) | 53 (80.30) | 17 (77.27) | NS |
| No | 18 (20.5) | 13 (19.7) | 5 (22.73) | ||
| Current smokers No(%) | Yes | 15 (17) | 10 (15.15) | 5 (22.72) | NS |
| No | 73 (83) | 56 (84.85) | 17 (77.28) | ||
| Diabetes No (%) | Yes | 24 (27.3) | 16 (24.24) | 8 (36.36) | NS |
| No | 64 (72.7) | 50 (75.75) | 14 (63.63) | ||
| Glycemia (mg/dL) | 100.35 ± 34.77 | 101.34 ± 38.87 | 97.36 ± 17.83 | NS | |
| Dyslipidemia | Yes | 59 (67) | 48 (72.72) | 11 (50) | 0.08 |
| No | 29 (33) | 18 (27.27) | 11 (50) | ||
| Total cholesterol (mg/dL) | 213.07 ± 52.82 | 222.03 ± 48.09 | 186.22 ± 58.24 | 0.014 | |
| LDL cholesterol (mg/dL) | 137.05 ± 41.34 | 142.87 ± 37.60 | 119.59 ± 47.72 | 0.045 | |
| Triglycerides (mg/dL) | 154.50 ± 69.15 | 158.65 ± 73.22 | 142.04 ± 54.81 | NS | |
| HDL cholesterol (mg/dL) | 42.82 ± 9.21 | 44.34 ± 8.80 | 38.27 ± 9.10 | 0.006 | |
| sICAM1 (ng/mL) * | 120.36 ± 51.11 (106.00) | 123.58 ± 46.74 (108.00) | 111.77 ± 61.97 (95.00) | 0.034 | |
| sVCAM1 (ng/mL) * | 1106.48 ± 452.89 (998.00) | 1114.33 ± 460.16 (998.00) | 1085.55 ± 445.17 (988.00) | NS | |
| Leptin (pg/mL) * | 26,201.16 ± 23,946.59 (20,195.00) | 32,758.40 ± 24,533.35 (24,155.00) | 8168.75 ± 7559.82 (5150.00) | <0.0001 | |
| Ghrelin (pg/mL) * | 39.16 ± 17.34 (34.50) | 40.37 ± 19 (35) | 35.70 ± 10.97 (33.75) | NS | |
| Chemerin (pg/mL) * | 8.60 ± 7.22 (6.30) | 8.97 ± 7.72 (6.3) | 7.47 ± 5.44 (5.95) | NS | |
| Insulin (µU/mL) | 8.50 ± 5.19 (7.40) | 8.88 ± 5.96 (7.4) | 7.38 ± 0.5 (7.25) | 0.049 | |
| HOMA index * | 2.23 ± 2.29 (1.67) | 2.38 ± 2.62 (1.66) | 1.78 ± 0.39 (1.78) | NS | |
| QUICKI index * | 0.34 ± 0.01 (0.35) | 0.34 ± 0.02 (0.35) | 0.35 ± 0.01 (0.34) | NS | |
| McAuley Score | 1.93 ± 0.28 | 1.90 ± 0.29 | 2.00 ± 0.24 | NS | |
| Glycemia/insulin * | 12.55 ± 3.62 (12.16) | 12.35 ± 3.99 (11.83) | 13.17 ± 2.13 (12.32) | 0.05 |
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| O + D + | O + D - | O - D + | O -D - | |
| Number of subjects | 13 | 18 | 11 | 46 |
| Women | 10 (76.9) | 14 (77.77) | 6 (54.54) | 36 (78.6) |
| Men | 3 (23.07) | 4 (22.22) | 5 (45.450 | 10 (21.7) |
| GROUPS | Group 1 O + D + 13 Subjects | Group 2 O + D - 18 Subjects | Group 3 O - D + 11 Subjects | Group 4 O - D - 46 Subjects | Global p Trend (Sig Diff between Groups) | p Trend Women (Sig Diff between Groups) | p Trend Men (Sig Diff Groups) |
|---|---|---|---|---|---|---|---|
| Age | 62.23 ± 10.05 | 53.83 ± 7.95 | 69.18 ± 10.6 | 63.34 ± 9.06 | <0.001 G1 vs. G2, G2 vs. G3, G2 vs. G4 | <0.001 G1 vs. G2, G2 vs. G3, G2 vs. G4, G3 vs. G4 | NS |
| sICAM1 (ng/mL) * | 104.22 ± 38.70 (94) | 123.77 ± 59.99 (112) | 167.42 ± 85.54 (142) | 115.12 ± 40.63 (106) | NS | NS | NS |
| sVCAM1 (ng/mL) * | 790.88 ± 164.87 (742) | 995.55 ± 297 (928) | 1562.57 ± 858 (1140) | 1122.24 ± 371 (1054) | 0.0134 G1 vs. G3, G1 vs. G4 | 0.0385 G1 vs. G3, G1 vs. G4 | NS |
| Leptin (pg/mL) * | 38,121.25 ± 24,875 (32,810) | 40,318.88 ± 28,541 (36,650) | 33,893.33 ± 39,946 (14,505) | 18,942.43 ± 16,474 (15,950) | 0.0265 G1 vs. G4, G2 vs. G4 | 0.0048 G1 vs. G4, G2 vs. G4 G3 vs. G4 | 0.0175 G1 vs. G4, G2 vs. G3, G2 vs. G4 |
| Ghrelin (pg/mL) * | 38.30 ± 18.38 (29.5) | 43.61 ± 15.90 (39) | 34.80 ± 14.93 (31.7) | 38.70 ± 18.22 (34.5) | NS | NS | NS |
| Chemerin * (pg/mL) | 7.98 ± 7.22 (5.2) | 8.42 ± 7.55 (5.8) | 7.27 ± 5.24 (5.6) | 9.15 ± 7.64 (7.15) | NS | NS | 0.0259 G1 vs. G3, G1 vs. G4, G2 vs. G4 |
| Insulin | 12.51 ± 12.65 (7.6) | 8.07 ± 2.19 (7.4) | 8.17 ± 1.66 (7.4) | 7.60 ± 0.92 (7.3) | 0.023 G1 vs. G2, G1 vs. G3, G1 vs. G4 | 0.023 G1 vs. G2, G1 vs. G4 | NS |
| HOMA index * | 4.24 ± 5.12 (2.48) | 1.90 ± 0.78 (1.59) | 2.69 ± 2.26 (1.87) | 1.66 ± 0.28 (1.64) | 0.0002 G1 vs. G2, G1 vs. G4, G3 vs. G4 | 0.0002 G1 vs. G2, G1 vs. G4, G2 vs. G3, G3 vs. G4 | NS |
| QUICKI index * | 0.32 ± 0.02 (0.33) | 0.35 ± 0.01 (0.35) | 0.33 ± 0.02 (0.34) | 0.35 ± 0.008 (0.35) | 0.0002 G1 vs. G2, G1 vs. G4, G3 vs. G4 | 0.0001 G1 vs. G2, G1 vs. G4, G2 vs. G3, G3 vs. G4 | NS |
| McAuley Score | 1.73 ± 0.38 | 1.90 ± 0.27 | 1.88 ± 0.27 | 2.01 ± 0.22 | 0.014 G1 vs. G4 | 0.006 G1 vs. G4 | NS |
| Glycemia/insulin * | 13.86 ± 5.34 (14.42) | 12.19 ± 4.10 (11.60) | 14.79 ± 5.5 (13.75) | 11.79 ± 1.54 (11.76) | 0.0076 G1 vs. G2, G1 vs. G4, G2 vs. G3, G3 vs. G4 | 0.06 | NS |
| sICAM1 | sVCAM1 | LEPTIN | GHRELIN | CHEMERIN | HOMA | QUICKI | McAuley Score | GLYC/INS | ||
|---|---|---|---|---|---|---|---|---|---|---|
| BMI | Global | 0.045 | −0.31 * | 0.402 ** | 0.10 | −0.28 | 0.338 ** | −0.339 ** | −0.328 ** | 0.11 |
| G1 | −0.183 | 0.083 | −0.19 | −0.209 | −0.247 | −0.005 | 0.005 | −0.204 | −0.27 | |
| G2 | −0.3 | −0.4 | 0.3 | 0.04 | −0.15 | 0.01 | −0.01 | 0.179 | 0.14 | |
| G3 | 0.75 * | 0.107 | 0.02 | −0.17 | 0.39 | 0.18 | −0.18 | 0.139 | 0.39 | |
| G4 | 0.19 | −0.09 | 0.179 | 0.05 | 0.142 | 0.319 * | −0.33 * | −0.365 * | −0.125 | |
| WC | Global | 0.034 | −0.22 | 0.098 | 0.086 | −0.36 | 0.334 ** | −0.334 ** | −0.225 * | 0.220 * |
| G1 | −0.08 | −0.21 | −0.31 | 0.166 | −0.43 | −0.088 | 0.088 | 0.07 | 0.441 | |
| G2 | 0.025 | 0.084 | 0.377 | 0.006 | −0.09 | 0.005 | −0.005 | −0.05 | 0.18 | |
| G3 | 0.306 | −0.306 | −0.493 | −0.134 | 0.11 | 0.128 | −0.128 | 0.398 | 0.604 | |
| G4 | 0.142 | −0.03 | −0.18 | 0.069 | 0.224 | 0.229 | −0.242 | −0.191 | 0.08 | |
| TC | Global | 0.092 | −0.187 | 0.249 | 0.04 | 0.087 | −0.009 | 0.012 | −0.495 ** | −0.14 |
| G1 | 0.75 * | −0.133 | 0.23 | 0.236 | 0.165 | 0.40 | −0.401 | −0.628 * | 0.148 | |
| G2 | 0.517 | −0.033 | 0.667 * | 0.061 | 0.32 | 0.04 | −0.04 | −0.653 ** | −0.512 * | |
| G3 | −0.28 | −0.286 | 0.60 | −0.05 | −0.345 | −0.05 | 0.05 | −0.37 | 0.009 | |
| G4 | −0.062 | −0.147 | 0.124 | −0.037 | −0.038 | −0.106 | 0.112 | −0.514** | −0.08 | |
| LDL-C | Global | 0.133 | −0.145 | −0.140 | 0.004 | 0.042 | 0.05 | −0.49 | −0.384 ** | −0.103 |
| G1 | 0.603 | −0.293 | 0.036 | 0.124 | 0.047 | 0.316 | −0.316 | −0.536 | 0.206 | |
| G2 | 0.750 * | 0.383 | 0.317 | 0.126 | 0.198 | 0.190 | −0.19 | −0.629 ** | −0.583 * | |
| G3 | −0.179 | −0.429 | 0.771 | −0.03 | −0.255 | −0.023 | 0.023 | −0.17 | 0.182 | |
| G4 | −0.001 | −0.162 | 0.11 | −0.024 | −0.06 | −0.017 | 0.022 | −0.409 ** | −0.05 | |
| HDL-C | Global | 0.176 | 0.054 | 0.169 | 0.178 | 0.196 | −0.254 * | 0.256 * | 0.096 | −0.205 |
| G1 | 0.795 * | 0.059 | 0.503 | 0.515 | 0.454 | 0.263 | −0.263 | −0.338 | 0.069 | |
| G2 | 0.343 | 0.393 | 0.803 ** | −0.224 | 0.562 * | −0.221 | 0.221 | −0.08 | 0.09 | |
| G3 | −0.286 | 0.01 | 0.314 | 0.330 | −0.40 | −0.2 | 0.20 | −0.08 | 0.05 | |
| G4 | 0.075 | 0.009 | 0.081 | 0.118 | 0.086 | −0.166 | 0.167 | 0.325 * | −0.219 | |
| TG | Global | −0.161 | −0.22 | 0.08 | 0.052 | 0.052 | 0.145 | −0.143 | −0.863 ** | −0.06 |
| G1 | 0.45 | 0.1 | 0.286 | 0.264 | 0.407 | 0.385 | −0.385 | −0.791 ** | −0.038 | |
| G2 | 0.183 | −0.283 | 0.233 | 0.432 | 0.22 | 0.125 | −0.125 | −0.91 ** | −0.677 ** | |
| G3 | −0.714 | −0.25 | 0.371 | 0.212 | −0.582 * | 0.291 | −0.291 | −0.957 ** | −0.236 | |
| G4 | −0.295 | −0.129 | −0.08 | −0.175 | 0.021 | −0.017 | 0.026 | −0.901 ** | 0.113 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitar-Taut, A.-V.; Coste, S.C.; Tarmure, S.; Orasan, O.H.; Fodor, A.; Negrean, V.; Pop, D.; Zdrenghea, D.; Login, C.; Tiperciuc, B.; et al. Diabetes and Obesity—Cumulative or Complementary Effects On Adipokines, Inflammation, and Insulin Resistance. J. Clin. Med. 2020, 9, 2767. https://doi.org/10.3390/jcm9092767
Sitar-Taut A-V, Coste SC, Tarmure S, Orasan OH, Fodor A, Negrean V, Pop D, Zdrenghea D, Login C, Tiperciuc B, et al. Diabetes and Obesity—Cumulative or Complementary Effects On Adipokines, Inflammation, and Insulin Resistance. Journal of Clinical Medicine. 2020; 9(9):2767. https://doi.org/10.3390/jcm9092767
Chicago/Turabian StyleSitar-Taut, Adela-Viviana, Sorina Cezara Coste, Simina Tarmure, Olga Hilda Orasan, Adriana Fodor, Vasile Negrean, Dana Pop, Dumitru Zdrenghea, Cezar Login, Brandusa Tiperciuc, and et al. 2020. "Diabetes and Obesity—Cumulative or Complementary Effects On Adipokines, Inflammation, and Insulin Resistance" Journal of Clinical Medicine 9, no. 9: 2767. https://doi.org/10.3390/jcm9092767
APA StyleSitar-Taut, A.-V., Coste, S. C., Tarmure, S., Orasan, O. H., Fodor, A., Negrean, V., Pop, D., Zdrenghea, D., Login, C., Tiperciuc, B., & Cozma, A. (2020). Diabetes and Obesity—Cumulative or Complementary Effects On Adipokines, Inflammation, and Insulin Resistance. Journal of Clinical Medicine, 9(9), 2767. https://doi.org/10.3390/jcm9092767






