Dynamic Risk Stratification for Predicting Treatment Response in Differentiated Thyroid Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kilfoy, B.A.; Zheng, T.; Holford, T.R.; Han, X.; Ward, M.H.; Sjodin, A.; Zhang, Y.; Bai, Y.; Zhu, C.; Guo, G.L.; et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 2009, 20, 525–531. [Google Scholar] [CrossRef] [PubMed]
- von der Recke, P.; Hansen, M.A.; Hassager, C. Increasing incidence of thyroid cancer in the United States, 1973–2002. J. Am. Med. Assoc. 2006, 295, 2164–2167. [Google Scholar] [CrossRef]
- Aschebrook-Kilfoy, B.; Kaplan, E.L.; Chiu, B.C.H.; Angelos, P.; Grogan, R.H. The acceleration in papillary thyroid cancer incidence rates is similar among racial and ethnic groups in the United States. Ann. Surg. Oncol. 2013, 20, 2746–2753. [Google Scholar] [CrossRef]
- Carling, T.; Ocal, I.T.; Udelsman, R. Special variants of differentiated thyroid cancer: Does it alter the extent of surgery versus well-differentiated thyroid cancer? World J. Surg. 2007, 31, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Gillanders, S.L.; O’Neill, J.P. Prognostic markers in well differentiated papillary and follicular thyroid cancer (WDTC). Eur. J. Surg. Oncol. 2018, 44, 286–296. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Alzahrani, A.S. Risk stratification in differentiated thyroid cancer: From detection to final follow-up. J. Clin. Endocrinol. Metab. 2019, 104, 4087–4100. [Google Scholar] [CrossRef]
- Haugen, B.R. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Tuttle, R.M. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: Using response to therapy variables to modify the initial risk estimates predicted by the new American thyroid association staging system. Thyroid 2010, 20, 1341–1349. [Google Scholar] [CrossRef]
- Prognostic Factors in Differentiated Thyroid Cancer Revisited—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/28457063/ (accessed on 30 June 2020).
- Frangos, S.; Iakovou, I.P.; Marlowe, R.J.; Eftychiou, N.; Patsali, L.; Vanezi, A.; Savva, A.; Mpalaris, V.; Giannoula, E.I. Difficulties in deciding whether to ablate patients with putatively ‘low–intermediate-risk’ differentiated thyroid carcinoma: Do guidelines mainly apply in the centres that produce them? Results of a retrospective, two-centre quality assurance study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 2045–2055. [Google Scholar] [CrossRef]
- Frangos, S.; Iakovou, I.P.; Marlowe, R.J.; Eftychiou, N.; Patsali, L.; Vanezi, A.; Savva, A.; Mpalaris, V.; Giannoula, E.I. Acknowledging gray areas: 2015 vs. 2009 American Thyroid Association differentiated thyroid cancer guidelines on ablating putatively low-intermediate-risk patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 185–189. [Google Scholar] [CrossRef]
- Rahbari, R.; Zhang, L.; Kebebew, E. Thyroid cancer gender disparity. Future Oncol. 2010, 6, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Micheli, A.; Ciampichini, R.; Oberaigner, W.; Ciccolallo, L.; de Vries, E.; Izarzugaza, I.; Zambon, P.; Gatta, G.; De Angelis, R.; EUROCARE Working Group. The advantage of women in cancer survival: An analysis of EUROCARE-4 data. Eur. J. Cancer 2009, 45, 1017–1027. [Google Scholar] [CrossRef]
- Sakoda, L.C.; Horn-Ross, P.L. Reproductive and menstrual history and papillary thyroid cancer risk: The San Francisco Bay Area thyroid cancer study. Cancer Epidemiol. Biomark. Prev. 2002, 11, 51–57. [Google Scholar]
- Lang, B.H.; Lo, C.Y.; Chan, W.F.; Lam, K.Y.; Wan, K.Y. Staging systems for papillary thyroid carcinoma: A review and comparison. Ann. Surg. 2007, 245, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Thyroid Carcinoma: Epidemiology, Histology, and Diagnosis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26430868/ (accessed on 30 June 2020).
- Sipos, J.A.; Mazzaferri, E.L. Thyroid cancer epidemiology and prognostic variables. Clin. Oncol. 2010, 22, 395–404. [Google Scholar] [CrossRef]
- Gulcelik, M.A.; Gulcelik, N.E.; Kuru, B.; Camlibel, M.; Alagol, H. Prognostic factors determining survival in differentiated thyroid cancer. J. Surg. Oncol. 2007, 96, 598–604. [Google Scholar] [CrossRef]
- Clinically Significant Prognostic Factors for Differentiated Thyroid Carcinoma: A Population-Based, Nested Case-Control Study—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/16369995/ (accessed on 30 June 2020).
- Krajewska, J.; Chmielik, E.; Jarząb, B. Dynamic risk stratification in the follow-up of thyroid cancer: What is still to be discovered in 2017? Endocr. Relat. Cancer 2017, 24, R387–R402. [Google Scholar] [CrossRef]
- Lo, T.E.N.; Canto, A.U.; Maningat, P.D.D. Risk factors for recurrence in filipinos with well-differentiated thyroid cancer. Endocrinol. Metab. 2015, 30, 543–550. [Google Scholar] [CrossRef]
- Lymph Node and Distant Metastases of Thyroid Gland Cancer. Metastases in the Thyroid Glands—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26357953/ (accessed on 30 June 2020).
- Mao, J.; Zhang, Q.; Zhang, H.; Zheng, K.; Wang, R.; Wang, G. Risk Factors for Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Front. Endocrinol. (Lausanne) 2020, 11. [Google Scholar] [CrossRef]
- Yu, X.M.; Lo, C.Y.; Alfred, K.Y.L.; Leung, P.; Luk, J.M. Serum vascular endothelial growth factor c correlates with lymph node metastases and high-risk tumor profiles in papillary thyroid carcinoma. Ann. Surg. 2008, 247, 483–489. [Google Scholar] [CrossRef]
- Tam, A.A.; Özdemir, D.; Çuhacı, N.; Başer, H.; Aydın, C.; Yazgan, A.K.; Ersoy, R.; Çakır, B. Association of multifocality, tumor number, and total tumor diameter with clinicopathological features in papillary thyroid cancer. Endocrine 2016, 53, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Tomoda, C.; Uruno, T.; Takamura, Y.; Miya, A.; Kobayashi, K.; Matsuzuka, F.; Kuma, K.; Miyauchi, A. Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: Massive but not minimal extension affects the relapse-free survival. World J. Surg. 2006, 30, 780–786. [Google Scholar] [CrossRef]
- Youngwirth, L.M.; Adam, M.A.; Scheri, R.P.; Roman, S.A.; Sosa, J.A. Extrathyroidal Extension Is Associated with Compromised Survival in Patients with Thyroid Cancer. Thyroid 2017, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- So, Y.K.; Son, Y.I.; Hong, S.D.; Seo, M.Y.; Baek, C.H.; Jeong, H.S.; Chung, M.K. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: A study of 551 resections. Surgery 2010, 148, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.; Levy, S.; Tsvetov, G.; Gorshtein, A.; Slutzky-Shraga, I.; Akirov, A.; Robenshtok, E.; Shimon, I.; Benbassat, C.A. long-term outcomes and prognostic factors in patients with differentiated thyroid cancer and distant metastases. Endocr. Pract. 2017, 23, 1193–1200. [Google Scholar] [CrossRef]
- Bhargav, P.R.K.; Mishra, A.; Agarwal, G.; Agarwal, A.; Pradhan, P.K.; Gambhir, S.; Verma, A.K.; Mishra, S.K. Long-term outcome of differentiated thyroid carcinoma: Experience in a developing country. World J. Surg. 2010, 34, 40–47. [Google Scholar] [CrossRef]
- Measuring Quality in Thyroid Cancer Surgery. Available online: https://www.hindawi.com/journals/aen/2014/714291/ (accessed on 30 June 2020).
- Kendler, D.B.; Vaisman, F.; Corbo, R.; Martins, R.; Vaisman, M. Preablation stimulated thyroglobulin is a good predictor of successful ablation in patients with differentiated thyroid cancer. Clin. Nucl. Med. 2012, 37, 545–549. [Google Scholar] [CrossRef]
- Kim, M.H.; Ko, S.H.; Bae, J.S.; Lim, D.J.; Baek, K.H.; Lee, J.M.; Kang, M.I.; Cha, B.Y. Combination of initial stimulation thyroglobulins and staging system by revised ATA guidelines can elaborately discriminate prognosis of patients with differentiated thyroid carcinoma after high-dose remnant ablation. Clin. Nucl. Med. 2012, 37, 1069–1074. [Google Scholar] [CrossRef]
- Melo, M.; Costa, G.; Ribeiro, C.; Carrilho, F.; Martins, M.J.; da Rocha, A.G.; Sobrinho-Simões, M.; Carvalheiro, M.; Soares, P. Stimulated thyroglobulin at recombinant human TSH-aided ablation predicts disease-free status one year later. J. Clin. Endocrinol. Metab. 2013, 98, 4364–4372. [Google Scholar] [CrossRef][Green Version]
- Predictive Value for Disease Progression of Serum Thyroglobulin Levels Measured in the Postoperative Period and After (131)I Ablation Therapy in Patients with Differentiated Thyroid Cancer—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/15181134/ (accessed on 30 June 2020).
- Ha, J.; Kim, M.H.; Jo, K.; Lim, Y.; Bae, J.S.; Lee, S.; Kang, M.I.; Cha, B.Y.; Lim, D.J. Recombinant human TSH stimulated thyroglobulin levels at remnant ablation predict structural incomplete response to treatment in patients with differentiated thyroid cancer. Medicine (U.S.) 2017, 96. [Google Scholar] [CrossRef]
- Spencer, C.A. Clinical review: Clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC). J. Clin. Endocrinol. Metab. 2011, 96, 3615–3627. [Google Scholar] [CrossRef] [PubMed]
- Verburg, F.A.; Luster, M.; Cupini, C.; Chiovato, L.; Duntas, L.; Elisei, R.; Feldt-Rasmussen, U.; Rimmele, H.; Seregni, E.; Smit, J.W.; et al. Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: A clinical position statement. Thyroid 2013, 23, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
| Histological Type/Subtype % n | |
|---|---|
| Papillary, classical variant (P) | 66.3% (364) |
| Papillary, follicular variant or mixed type (FVP) | 29.5% (162) |
| Follicular (F) | 8.9% (49) |
| Hurthle Cell (HC) | 2.0% (11) |
| Insular Cell (IC) | 0.7% (4) |
| Trabecular Variant Papillary (TVP) | 1.8% (10) |
| Anaplastic (A) | 0.9% (5) |
| Medullary Thyroid Cancer (MTC) | 1.1% (6) |
| TNM a Stage % n | |
| Τ1 | 53.9% (295) |
| T2 | 12.8% (70) |
| Τ3 | 31.4% (172) |
| T4 | 2.0% (11) |
| N0 | 33.9% (186) |
| Ν1 | 41.1% (225) |
| Nx | 25.1% (138) |
| M0 | 94.7% (520) |
| M1 | 5.3% (29) |
| Number of Lesions | Mean (SD): 2.48 (2.52) |
| Multifocality: 54.7% (299) | |
| Longest Diameter of Primary Tumor | Mean (SD): 1.66 (1.16) |
| >1 cm: 65.4% (359) | |
| Invasive (I) b | 40.6% (222) |
| Number of excised lymph nodes | Mean (SD): 8.59 (12.01) |
| Number of metastasized lymph nodes | Mean (SD): 2.98 (6.03) |
| Referrals | Median (IQR): 2.0 (1, 4) |
|---|---|
| Surgeons’ score (among 95 surgeons) | |
| 1 | 76.84% (73) |
| 2 | 8.42% (8) |
| 3 | 6.32% (6) |
| 4 | 8.42% (8) |
| Characteristic | Post-Surgery Scintigraphy a (n = 274) | RAI (n = 539) | 1st WBS (n = 501) | 2nd WBS (n = 55) |
|---|---|---|---|---|
| Tg-Ab, Median, Mean (IQR) | 20.0, 65.78 (10.34, 32.30) | 20.0, 58.35 (13.0, 34.12) | 20.0, 52.47 (12.93, 27.0) | 21.30, 55.37 (20.40) |
| TgAb > 30 IU/mL (n) | 26.6(73) | 28.6 (154) | 21.6 (108) | 30.9 (17) |
| Anti-TPO, Median, Mean (IQR) (n = 218) | 20.85, 25.37 (11.43, 59.32) | - | ||
| Anti-TPO > 30, % (n) | 38.5% (84) | - | ||
| TSH, Median, Mean (IQR) | 95.48, 97.59 (60.90, 126.40) | 120.0, 120.75 (85.0, 146.70) | 108.0, 114.67 (83.46, 140.0) | 116.50, 136.78 (89.0, 161.03) |
| Tg, Median, Mean (IQR) | 2.09, 35.25 (0.82, 5.94) | 2.10, 22.47 (0.69, 6.85) | 0.44, 10.27 (0.30, 1.18) | 3.50, 47.03 (0.62, 33.73) |
| Tg < 1, % (n) | 30.5 (84) | 33.2 (179) | 73.5 (369) | 37.3 (22) |
| Tg 1 < 5, % (n) | 42.5 (117) | 36.5 (197) | 14.5 (73) | 15.3 (9) |
| Tg 5–10, % (n) | 9.1 (25) | 10.8 (58) | 4.0 (20) | 10.2 (6) |
| Tg > 10, % (n) | 17.8 (49) | 19.5 (105) | 8.0 (40) | 37.3 (22) |
| Remnant score, Median (IQR), Mean (St.D.) (n = 218) | 3 (2, 6), 4.37 (3.52) | 6 (3, 8), 6 (4.53) | 0 (0, 2), 1.94 (3.5) | 3 (0, 9), 5.19 (5.2) |
| Stimulation method: | ||||
| THW | 81.4% (223) | 48.3% (260) | 30.7% (154) | 5.1% (3) |
| rh-TSH | 18.6% (51) | 51.7% (279) | 69.3% (347) | 94.9% (52) |
| Treatment Response Category | % (n) |
|---|---|
| ER (Excellent Response) | 37.7% (177) |
| BIR (Biochemical Incomplete Response) | 14.7% (69) |
| SIR (Structural Incomplete Response) | 21.7% (102) |
| IR (Indeterminate Response) | 26% (122) |
| Response to Treatment | ||||||
|---|---|---|---|---|---|---|
| BIR | ER | IR | SIR | |||
| n (%) | n (%) | n (%) | n (%) | |||
| Main Characteristics | Gender, n (%) | F | 59 (16.5) | 139 (38.9) | 87 (24.4) | 72 (20.2) |
| M | 10 (8.8) | 38 (33.6) | 35 (31) | 30 (26.5) | ||
| PTC, n (%) | No | 23 (14.2) | 56 (34.6) | 39 (24.1) | 44 (27.2) | |
| Yes | 46 (14.9) | 121 (39.3) | 83 (26.9) | 58 (18.8) | ||
| Follicular variant of PTC, n (%) | No | 48 (14.3) | 118 (35.2) | 96 (28.7) | 73 (21.8) | |
| Yes | 21 (15.6) | 59 (43.7) | 26 (19.3) | 29 (21.5) | ||
| F, n (%) | No | 68 (16) | 166 (39.1) | 107 (25.2) | 84 (19.8) | |
| Yes | 1 (2.2) | 11 (24.4) | 15 (33.3) | 18 (40) | ||
| HC, n (%) | No | 68 (14.8) | 174 (37.8) | 118 (25.7) | 100 (21.7) | |
| Yes | 1 (10) | 3 (30) | 4 (40) | 2 (20) | ||
| Insular, n (%) | No | 68 (14.6) | 177 (38) | 120 (25.8) | 101 (21.7) | |
| Yes | 1 (25) | 0 (0) | 2 (50) | 1 (25) | ||
| TVP, n (%) | No | 68 (14.7) | 174 (37.7) | 122 (26.4) | 98 (21.2) | |
| Yes | 1 (12.5) | 3 (37.5) | 0 (0) | 4 (50) | ||
| A, n (%) | No | 69 (14.8) | 176 (37.7) | 121 (25.9) | 101 (21.6) | |
| Yes | 0 (0) | 1 (33.3) | 1 (33.3) | 1 (33.3) | ||
| MTC, n (%) | No | 45 (14.4) | 139 (44.6) | 65 (20.8) | 63 (20.2) | |
| Yes | 2 (33.3) | 2 (33.3) | 1 (16.7) | 1 (16.7) | ||
| StatusN, n (%) | N0 | 29 (18.4) | 64 (40.5) | 35 (22.2) | 30 (19) | |
| N1 | 21 (11) | 72 (37.7) | 50 (26.2) | 48 (25.1) | ||
| Nx | 19 (15.8) | 40 (33.3) | 37 (30.8) | 24 (20) | ||
| StatusM, n (%) | M0 | 67 (15.1) | 177 (39.9) | 118 (26.6) | 82 (18.5) | |
| M1 | 2 (7.7) | 0 (0) | 4 (15.4) | 20 (76.9) | ||
| Μ0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| StageT_new, n (%) | Any T1 | 40 (16.1) | 107 (43.1) | 52 (21) | 49 (19.8) | |
| T2 | 10 (18.2) | 17 (30.9) | 14 (25.5) | 14 (25.5) | ||
| Any T3 | 19 (11.9) | 52 (32.7) | 53 (33.3) | 35 (22) | ||
| Any T4 | 0 (0) | 1 (12.5) | 3 (37.5) | 4 (50) | ||
| Stimulation method, n (%) | rhTSH | 25 (21.2) | 42 (35.6) | 30 (25.4) | 21 (17.8) | |
| THW | 23 (10.7) | 79 (36.9) | 64 (29.9) | 48 (22.4) | ||
| Age at diagnosis mean (St. D.) | 46.86 (14.5) | 46.18 (14.43) | 46.63 (14.26) | 45.3 (14.55) | ||
| Multifocality, % (n) | 56.5 (39) | 54.5 (96) | 48.4 (59) | 63.7 (65) | ||
| Invasiveness % (n) | 14.7 (28) | 33.0 (63) | 28.8 (55) | 23.6 (45) | ||
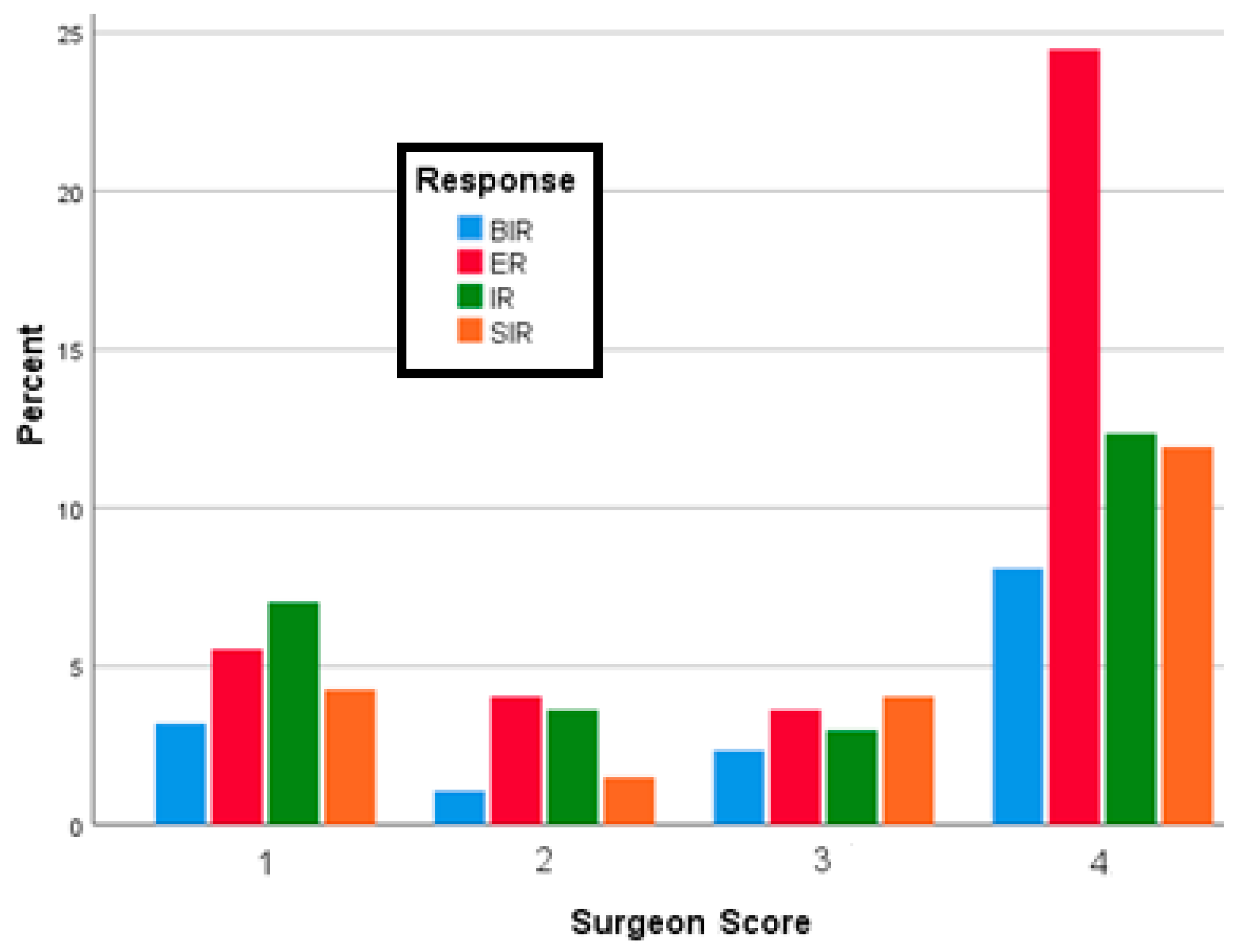
| Surgeons’score, % (n) | 1 | 21.7 (15) | 14.7 (26) | 27 (33) | 19.6 (20) | |
| 2 | 7.2 (5) | 10.7 (19) | 13.9 (17) | 6.9 (7) | ||
| 3 | 15.9 (11) | 9.6 (17) | 11.5 (14) | 18.6 (19) | ||
| 4 | 55.1 (38) | 65 (115) | 47.5 (58) | 54.9 (56) | ||
| RAI | Activity administrated, mean (St.d) | 96.30 (16.13) | 96.05 (11.29) | 95.57 (13.85) | 97.84 (19.68) | |
| remnant_score, mean (St.d) | 4.16 (3.08) | 3.08 (1.93) | 4.55 (3.19) | 7.09 (4.79) | ||
| Tg, mean (St.d) | 3.47 (3.87) | 2.86 (4.05) | 17.47 (87.77) | 141.37 (369.66) | ||
| Tg-Ab, mean (St.d) | 28.28 (29.83) | 39.38 (69.54) | 53.89 (214.17) | 155.87 (569.77) | ||
| TM, median (IQR) | 27.10 (9.81, 199.30) | 19.50 (13.56, 59.81) | 21.30 (9.93, 56.32) | 19.00 (11.31, 58.90) | ||
| WBS | remnant_score, mean (St.d) | 0.58 (0.34) | 0.74 (0.45) | 2.8 (2.95) | 5.6 (5.1) | |
| Tg, mean (St.d) | 1.15 (4.48) | 0.52 (0.79) | 1.8 (2.98) | 49.15 (152.42) | ||
| Tg-Ab, mean (St.d) | 42.06 (32.71) | 20.96 (20.04) | 57.98 (183.67) | 119.84 (439.81) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannoula, E.; Melidis, C.; Papadopoulos, N.; Bamidis, P.; Raftopoulos, V.; Iakovou, I. Dynamic Risk Stratification for Predicting Treatment Response in Differentiated Thyroid Cancer. J. Clin. Med. 2020, 9, 2708. https://doi.org/10.3390/jcm9092708
Giannoula E, Melidis C, Papadopoulos N, Bamidis P, Raftopoulos V, Iakovou I. Dynamic Risk Stratification for Predicting Treatment Response in Differentiated Thyroid Cancer. Journal of Clinical Medicine. 2020; 9(9):2708. https://doi.org/10.3390/jcm9092708
Chicago/Turabian StyleGiannoula, Evanthia, Christos Melidis, Nikitas Papadopoulos, Panagiotis Bamidis, Vasilios Raftopoulos, and Ioannis Iakovou. 2020. "Dynamic Risk Stratification for Predicting Treatment Response in Differentiated Thyroid Cancer" Journal of Clinical Medicine 9, no. 9: 2708. https://doi.org/10.3390/jcm9092708
APA StyleGiannoula, E., Melidis, C., Papadopoulos, N., Bamidis, P., Raftopoulos, V., & Iakovou, I. (2020). Dynamic Risk Stratification for Predicting Treatment Response in Differentiated Thyroid Cancer. Journal of Clinical Medicine, 9(9), 2708. https://doi.org/10.3390/jcm9092708







