1. Introduction
Cystic fibrosis, a genetic disorder observed in people of all races and ethnicities, affects approximately 80,000 people worldwide. It is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which leads to abnormal ion transport in mucous membranes throughout the body. The resultant thick, dehydrated, pH imbalanced mucus adversely impacts the function of the respiratory, gastrointestinal, and reproductive tracts.
Since the first clinical and pathological descriptions of the disease in the 1930s [
1,
2], advances in care and the development of CF-specific therapies have led to an increase in survival. With the median predicted survival in the US now at 46 years, more than half of the people with CF are over the age of 18 years [
3]. Not only has the quantity of people’s lives increased, but so has the quality. Thus, more women are expressing the desire to become pregnant [
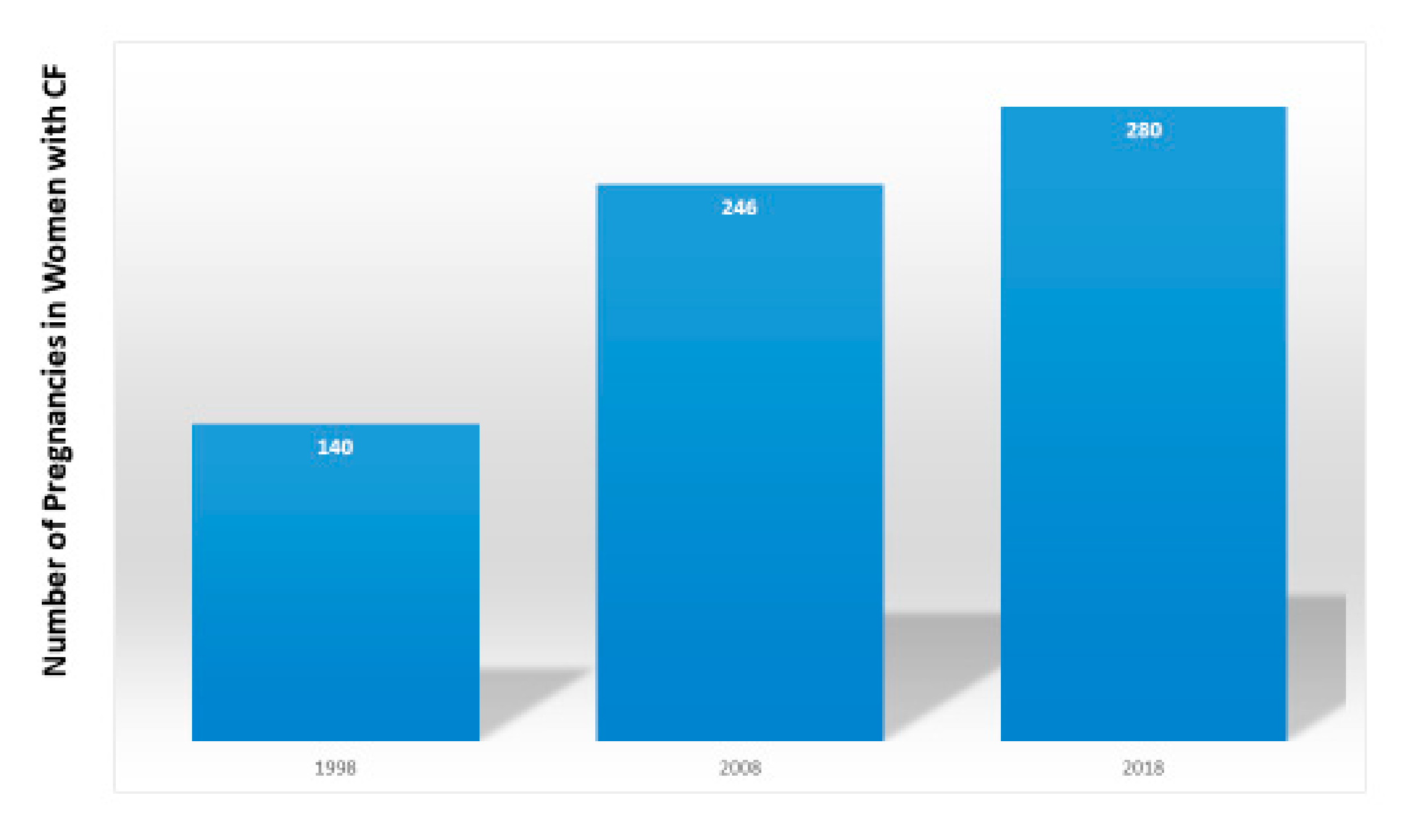
4]. The number of women with CF in the United States who became pregnant doubled from 140 in 1998 to 280 in 2018 [
3] (
Figure 1).
The first pregnancy in a woman with CF was reported in the literature in 1960 [
5]. Although the 20-year old carried the pregnancy to 34 weeks and delivered a healthy infant, she unfortunately died from her CF 6 weeks after the infant’s birth. Subsequently, multiple case series and single site retrospective studies suggested that, in spite of the multitude of consequences of CFTR dysfunction, some women with CF could successfully navigate pregnancy and motherhood, although lower lung function (as measured by percent-predicted forced expiratory volume in 1 s [ppFEV
1]), infection with certain gram negative infections, such as the
Burkholderia cepacia complex, and the complication of cystic fibrosis related diabetes (CFRD), were associated with increased risk [
6,
7,
8,
9,
10,
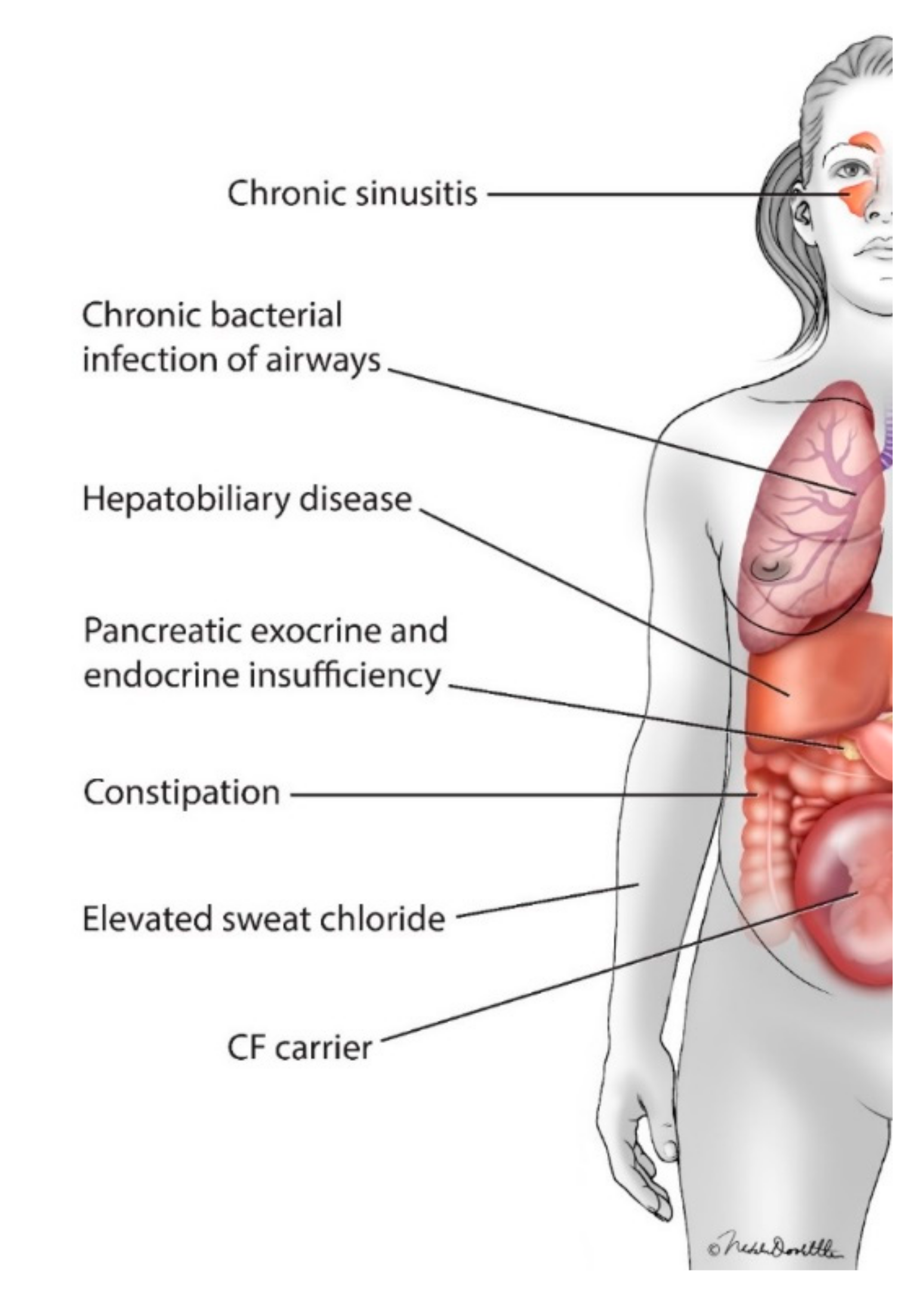
11] (
Figure 2). More recent registry-based data suggests that pregnant women with CF and their infants experience more complications than pregnant women without CF [
12,
13]. Registry-based/large cohort studies demonstrated that, although pregnant women with CF may have increased illness-related visits and decreased quality of life, their survival was not decreased compared to that of women who did not become pregnant [
14,
15].
In addition to the potential risk of the common complications of CF to the health of women with CF and to that of their infants (including pancreatic endocrine and exocrine insufficiency and infectious sinus and pulmonary exacerbations;
Figure 2), women with CF and their providers must also consider the potential risks of the many medications women with CF must take to maintain their health [
16,
17] versus the risk to the mother’s health of discontinuing the therapy [
18,
19]. Of particular importance for the care of women with CF is the use of the new class of pharmaceuticals, for which up to 90% of women with CF may be eligible: CFTR modulators.
CFTR modulators are the first pharmaceutical class designed specifically to alter the basic defect in CF by either improving the function of CFTR protein present at the cell surface and/or improving the trafficking of the CFTR to the cell surface [
20,
21]. The first approved CFTR modulator, ivacaftor, improved ppFEV
1 by an average of 10.6% for the approximately 4% of people with the G551D (Gly551Asp) mutation [
22,
23]. Two more modestly effective modulator combinations were approved based on their ability to improve ppFEV
1 and/or decrease CF pulmonary exacerbations [
24,
25,
26]. In October 2019, the triple combination therapy, elexacaftor/tezacaftor/ivacaftor, which improved lung function by an average of 13.8% in people with one copy of F508del (Phe508del) and by 10% in people with CF already treated with tezacaftor/ivacaftor, was approved in the U.S. to treat people with CF with at least one copy of F508del [
27,
28].
This review summarizes what is currently known about the impact of use of CFTR modulators on fertility, pregnancy, and lactation in women with CF.
2. Impact of CFTR Modulators on the Sexual and Reproductive Health (SRH) of Women with CF
2.1. Impact of Modulators on Fertility
Tizzano and colleagues reported that there are variable levels of expression of CFTR in the cervical epithelium and fallopian tubes, but that post-puberty, the endometrial epithelium and glands express CFTR at high levels [
29]. Women with CF generally have reproductive anatomy that is similar to that of women without CF, but abnormal CFTR function can lead to viscous, pH imbalanced cervical secretions that lead to subfertility in some women with CF [
30]. Delayed puberty and ovulation, and suboptimal nutrition status may also play a role in decreased fertility. Recently, Shteinberg, et al. found older maternal age and exocrine pancreatic insufficiency to be risk factors for subfertility in women with CF [
31].
Perhaps because of a personal history of infertility in the setting of poor health that later improved, or possibly because of misperceptions about female fertility based on the 97–98% incidence of male infertility in CF [
32], 25–50% of pregnancies in women with CF are unplanned [
33,
34]. CFTR modulators in animal models showed no adverse impact on fertility when given at normal human doses [
35,
36,
37,
38] (
Table 1). In people using CFTR modulators and consequent systemic improvement in their CFTR function (leading to improvements in nutritional status, and possibly improving cervical mucus consistency and pH) [
30], there have been reports of both improved fertility (7/12 women who previously reported infertility became pregnant with an average time to conception, following ivacaftor initiation of 3.2 months [range 1–8.5]) and unplanned pregnancies [
39,
40,
41]. In the phase 3 trial of ivacaftor, in which participants agreed to use contraception throughout the trial [
23], approximately 2% of the women became pregnant [
39]. It was not reported whether the pregnancies represented failure of a particular contraceptive method or failure to use contraception. To avoid unplanned pregnancies, CF teams should routinely counsel women who are starting CFTR modulators about the potential for increased fertility.
2.2. Impact of Modulators on Pregnancy
In pregnancy, the benefit of any medication to the mother must be weighed against the risk of administration to the fetus. While many medications used in people with CF have sufficient history of use so that they are deemed safe for use during pregnancy, CFTR modulators are not amongst them [
16,
17]. However, unlike other medications used in the chronic treatment of people with CF, acute destabilization [
18,
19] and one incident of death [
18] have been reported with abrupt discontinuation of CFTR modulators [
18,
19].
Prior to 2015, the federal drug administration (FDA) categorized the risk of drugs during pregnancy as A-D or X based on the combination of data from animal reproduction studies and studies in pregnant women. However, in order to facilitate an informed discussion between providers and pregnant women, the FDA now requires that prescription drugs submitted for FDA approval after 30 June 2015, must follow the new pregnancy and lactation labeling rule (PLLR) [
42]. Rather than reporting risk categories, information is based on the required sponsor information stating how much drug was administered to the animals used in reproduction models compared to the maximum recommended human dose (MRHD), and what happened to the fetus as a result.
In animals, placental transfer of individual modulators (none have been tested in combination) has been established [
35,
36,
37,
38]. While ivacaftor, tezacaftor, and elexacaftor, given at maternally toxic doses, caused minor abnormalities (decreased body weight in rats given ivacaftor at 7 times the MRHD, decreased body weight, delayed pinna attachment and eye opening in rabbits given tezacaftor at 1 times the MRHD, and decreased body weight in rats given ≥4 times the MRHD), none of the approved modulators impacted organogenesis at normal human doses [
35,
36,
37,
38] (
Table 1). Although this animal data is reassuring, because of the lack of data in pregnant women, caution is advised for use in pregnancy, and some clinicians have advised women to discontinue use of modulators during pregnancy [
40,
41,
43,
44,
45].
In addition to describing results of administration of drugs in animal reproduction models, sponsors are required to state whether there are adequate and well-controlled studies of the agent in pregnant women to determine if there is a drug-associated risk of major birth defects or miscarriage. As the impacts of drugs in pregnancy are evaluated, it is important to know baseline rates of major birth defects and miscarriages in the population being studied. Based on a large database study conducted in California between 2005–2008, the research showed that pregnancies of women with CF were more likely to be affected by congenital anomalies (14.3% vs. 6.4%,
p = 0.005); in particular, a trend towards the occurrence of cardiac anomalies in infants of women with CF at a rate 7 times higher than in pregnancies of mothers without CF was observed (3.9% vs. 0.5%,
p = 0.08). Because the first CFTR modulator was not approved until 2012 [
35], this cohort preceded the modulator era and thus cannot be used to speculate about the impact of CFTR modulators on the occurrence of congenital anomalies. Although the U.S. Cystic Fibrosis Foundation Patient Registry (CFFPR) collects data regarding the use of CFTR modulators, it does not collect data on infants born to mothers of CF; thus, it is not possible to determine from the U.S. CFFPR [
3] the rate of congenital malformations in infants born to women with CF in general nor the rate of congenital malformation in infants in association with CFTR modulator use.
There are no studies of the use of CFTR modulators in pregnant women with CF; however, several case reports have appeared in the literature that include women who unintentionally and intentionally became pregnant while being treated with ivacaftor and lumacaftor/ivacaftor [
39,
40,
41,
43,
44,
45,
46] (
Table 2). Of the six pregnancies reported in five women using ivacaftor, four of the infants were exposed to ivacaftor in all trimesters (the number of trimesters of exposure was not reported in one case, and in one case, a woman discontinued ivacaftor when it was determined that she was pregnant) [
39,
40,
43,
46]. No infant abnormalities were reported for any of the pregnancies. Infants were full term in three of the pregnancies (infant gestational age of twins born to one mother was not reported). For a mother with severe lung dysfunction, whose infants were exposed to ivacaftor during all three trimesters, both pregnancies resulted in the birth of premature infants (34–36 weeks of age) [
43]. In the two reports of infants born to mothers who used lumacaftor/ivacaftor during all three trimesters, infants were born at 38 weeks (maternal ppFEV
1 90%) and 35 weeks (maternal ppFEV
1 43–46%) [
44,
45]. One infant had mild hyperbilirubinemia in the neonatal period, which resolved spontaneously [
44]. In a third report of two infants born to the same mother, both infants were exposed to lumacaftor/ivacaftor throughout both pregnancies; no adverse impacts on the infants were reported. [
41] Of the eleven infants exposed to modulators during pregnancy, only four had formal ophthalmologic exams; all were normal. Importantly, three attempts to discontinue CFTR modulators because of unknown safety in pregnancy resulted in pulmonary decline in three women, leading to resumption of therapy [
43,
44,
45].
In addition to the case reports, which provided reassurance for normal infant outcomes following modulator exposure during pregnancy, Nash et al. recently reported the results of an international survey of CF center physicians regarding modulator use in women with CF during pregnancy [
47]. Results from a total of 64 pregnancies in 61 women were summarized (31 pregnancies with ivacaftor exposure, 26 pregnancies with lumacaftor/ivacaftor exposure, and 7 pregnancies with tezacaftor/ivacaftor exposure). For maternal and infant complications, the physician providing care for the mother was asked to provide his/her opinion regarding whether the complication was related to modulator use. Use of CFTR modulators during part or all of the pregnancy resulted in two maternal complications that were deemed related to CFTR modulator therapy (one pulmonary exacerbation and one incident of acute myelocytic leukemia [AML] were attributed to lumacaftor-ivacaftor; there are no other reports in the literature of AML in association with CFTR modulator use). Critically, cessation of modulator therapy (based on the unknown risk to the fetus of modulators in pregnancy) resulted in clinical decline in nine women, prompting resumption of therapy during pregnancy. More than half of the infants in the study were exposed to modulators for all three trimesters. No modulator-related complications were reported in infants exposed in utero. The miscarriage rate for women with CF on modulator therapy was 4.7% [
47], which was lower than the reported 10–15% miscarriage rate expected in the general population [
48].
2.3. Impact of Modulators on Lactation
Although breast feeding by women with CF was historically discouraged because of concerns about sodium content and milk nutrient composition, subsequent work demonstrated normal electrolyte and protein content [
49]. Currently, the major factors determining recommendations for lactation for women with CF are related to a woman’s ability to maintain her weight in spite of the high caloric demands of breast feeding and whether re-initiation of medications of unknown risk or potential harm to the infant are indicated for the mother’s health [
50]. Although the CFTR modulator ivacaftor is now approved for infants with CF as young as 6 months of age [
35], there is minimal human data demonstrating safety in lactation. Thus, caution is advised for use of all CFTR modulators during lactation [
35,
36,
37,
38].
Animal reproduction models have demonstrated the presence of individual modulators in breast milk [
35,
36,
37,
38] (
Table 1). Importantly, although ivacaftor did not cause congenital anomalies when administered to rats at normal human doses, at doses resulting in the exposure of 0.25 times the MRHD, ivacaftor administration in 7 to 35-day old rat pups led to the development of non-congenital lens opacities (cataracts) [
35]. Because cases of non-congenital lens opacities have been reported in pediatric patients treated with CFTR modulators, it is recommended that pediatric patients who are treated with these agents undergo baseline and follow-up ophthalmological examinations [
35,
36,
37]. Thus, it is advisable that infants exposed to CFTR modulators
in utero or through breast milk undergo formal examination for cataracts.
As noted above, there is limited human data on the use of CFTR modulators during lactation. In a woman taking lumacaftor/ivacaftor during pregnancy, Trimble et al. measured concentrations of both drugs in maternal and infant plasma, cord blood, and breast milk [
44]. Both drugs were present in maternal plasma at expected concentrations, but were also present in cord blood (in cord blood, the presence of lumacaftor was higher than in maternal plasma and at equivalent levels to maternal plasma for ivacaftor), infant plasma, and breast milk (at low levels). Although there were fluctuations in the infant’s liver function test values, it was not clear whether the occasional mild elevations resulted from the low levels of lumacaftor and ivacaftor exposure from breast feeding or from a normal variation. The infant had a normal ophthalmologic exam. Similarly, all four infants who had formal ophthalmologic exams in the international survey reported by Nash et al. (
n = total 27 infants exposed to modulators during lactation: iva,
n = 13; lum/iva,
n = 9; tez/iva
n = 5) had normal exams [
47].
3. Patient Perspective
I am a 28-year-old woman who is homozygous for F508del. I was diagnosed with CF at 10 months old due to failure to thrive and pneumonia. My first hospitalization occurred when I was 8 years old and, by high school, I was hospitalized every 6 months for pulmonary exacerbations. My health was declining, and I remember my doctor at the time guessing that I would have 10–15 years before I would need a lung transplant.
My whole life, I had hoped to become a mother, but I never counted on that being my reality. In August 2015, I was in the open-label portion of the Orkambi® study. Much to the surprise of me and my husband, we found out we were pregnant. Because I was in the Orkambi® study, I had to withdraw from the study once I became pregnant, and, based on a lack of data, my CF team chose not to start commercial Orkambi®. As a result of discontinuing Orkambi®, my lung function initially dropped but rebounded some. My health remained relatively stable and I only needed oral antibiotics a couple of times during the pregnancy. After being induced 10 days early and undergoing a thankfully non-eventful labor and delivery, our son was born at a healthy 6 lbs.
I didn’t feel strongly about breastfeeding when I was pregnant, but when my son was born, I wanted to try. Based on the advice of my CF team, I stayed off Orkambi® to minimize risks. However, when my son was 9 months old, I experienced a pulmonary exacerbation, requiring intravenous antibiotics. After weeks of deliberating with my team, my son’s pediatricians (not all of whom agreed with me), and painstakingly scouring whatever little research existed, I decided to continue breastfeeding while on Orkambi®. We chose to check my son’s liver enzyme levels for a couple of months (which were normal). Eventually, I switched to Symdeko® when it was approved, and my son weaned himself just before his third birthday.
When Trikafta® was approved ahead of schedule in October 2019, I was thrilled but torn, as my husband and I had discussed the possibility of growing our family. I let my miracle drug wait in the cupboard for a couple of months while we continued to try for baby #2 because I didn’t want to start the drug to see its great gains only to have to stop when I got pregnant. However, in January 2020, after a viral illness and associated decrease in lung function, my CF team and I decided it was time to start Trikafta®. After starting, my lung function increased by 20%. At that point, I had almost no cough, a lot of energy, and my quality of life improved greatly.
After discussing my health and impact of Trikafta® with my obstetrics and CF teams, we agreed that the benefits of Trikafta outweighed any potential unknown risk to the baby. My husband and I were given the go-ahead to continue trying for baby #2. Less than one month after starting Trikafta®, we found out we were pregnant. I am currently 28 weeks into this pregnancy and overall feeling great. I think, due to being on Trikafta®, I have much more energy and much less mucus. Navigating a high-risk pregnancy with a toddler in the midst of a pandemic has been a challenging experience to say the least. Although there will always be unknowns when journeying through CF and pregnancy and breastfeeding, I have peace knowing that we made the best decision with what information we have right now. I am grateful for the research and conversations that continue to happen in the CF community that will help people after me to make these huge and important decisions.
4. Conclusions
With the substantial gains in health experienced by people with CF over the last 20 years, the number of women who desire families and are becoming pregnant is increasing. It is likely that CFTR modulators increase fertility in women with CF, but the safety of their use in pregnancy and lactation is understudied. Animal reproduction models do not show alarming signals, and the sum of data from case reports and case series of use of modulators during pregnancy provides additional encouragement about the safety of modulators during pregnancy. Because of reports of acute deterioration in health following cessation of modulators, risks to the mother’s health due to discontinuation of modulators during pregnancy must be weighed carefully against the unknown risks to the fetus. If a mother chooses to continue using CFTR modulators during pregnancy and lactation, the development of non-congenital cataracts in juvenile rats and case reports in pediatric patients treated with modulators suggest the need for infant ophthalmologic exams. A prospective study of the use of CFTR modulators during pregnancy and lactation is greatly needed so that providers can offer informed discussions to women with CF who must make this difficult choice to continue or discontinue CFTR modulators prior to attempting pregnancy or during pregnancy. A Cystic Fibrosis Foundation Therapeutics funded study, maternal and fetal outcomes in the era of CFTR modulators (MAYFLOWERS) will prospectively evaluate the impact of the use of this class of drugs on the health of women with CF and their infants.
Funding
J.L.T.-C. receives funding from the Cystic Fibrosis Foundation for her role as Chair of the Women’s Health Research Working group (TAYLOR19Y3) and as Co-Investigator of the Evaluation of Predictors of Maternal-Fetal Outcomes in Cystic Fibrosis study (JAIN19A0). She also receives private donation funding paid to the institution from the Asher Fund.
Acknowledgments
The author wishes to thank Gillian Mocek for providing the patient perspective.
Conflicts of Interest
The funders of Dr. J.L.T.-C.’s work in women’s health had no role in the writing of the manuscript or in the decision to publish this review. Dr. J.L.T.-C. reports the following personal financial relationships with commercial interests relevant to medicine within the past 3 years: As faculty in an institution that is part of the CF Therapeutics Development Network, she has been site Principal Investigator on studies for Vertex, Bayer, Celtaxys, Nivalis, and Proteostasis. She received a career award from Gilead Pharmaceuticals. She has been on advisory boards for Genentech, Gilead, Novartis, Proteostasis, Protalix, Vertex, and Polarean. She has done consulting/provided clinical trial design advice for Vertex, Celtaxys, Proteostasis, Santhera, 4DMT, and Polarean. She has reported personal financial support from a non-commercial source relevant to medicine within the past 3 years. She has received grant funding from the CFF. She has had no personal relationships with tobacco industry entities within the last 3 years. Her professional memberships include membership on the CFF Clinical Research Executive Committee, Chair of the CFF Women’s Health Research Working Group, and Chair of the American Thoracic Society’s Clinical Problems Assembly Programming Committee.
References
- Andersen, D.H. Cystic fibrosis of the pancreas and its relation to celiac disease: A clinical and pathologic study. Am. J. Dis. Child. 1938, 56, 344–399. [Google Scholar] [CrossRef]
- Fanconi, G.; Uehlinger, E.; Knaver, C. Das Coeliac syndrome bei Angeborenerzysticherpankre as fi bromatose und bronchiektasen. Med. Wochenschr. 1936, 86, 753–756. [Google Scholar]
- Cystic Fibrosis Foundation Patient Registry. 2018 Annual Data Report. Available online: https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2018-Patient-Registry-Annual-Data-Report.pdf (accessed on 18 July 2020).
- Kazmerski, T.M.; Gmelin, T.; Slocum, B.; Borrero, S.; Miller, E. Attitudes and Decision Making Related to Pregnancy Among Young Women with Cystic Fibrosis. Matern. Child Health J. 2017, 21, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Siegel, B.; Siegel, S. Pregnancy and Delivery in a Patient with Cystic Fibrosis of the Pancreas: Report of a case. Obstet. Gynecol. 1960, 16, 438–440. [Google Scholar]
- Cohen, L.F.; di Sant’Agnese, P.A.; Friedlander, J. Cystic fibrosis and pregnancy. A national survey. Lancet 1980, 2, 42–44. [Google Scholar]
- Kent, N.E.; Farquharson, D.F. Cystic fibrosis in pregnancy. CMAJ 1993, 149, 809–813. [Google Scholar] [CrossRef][Green Version]
- FitzSimmons, S.C.; Fitzpatrick, S.; Thompson, B.; Aitkin, M.; Fiel, S.; Winnie, G.; Hilman, B. A longitudinal study of the effects of pregnancy on 325 women with cystic fibrosis. Pediatr. Pulmonol. 1996, 13, 99–101. [Google Scholar]
- Gilljam, M.; Antoniou, M.; Shin, J.; Dupuis, A.; Corey, M.; Tullis, D.E. Pregnancy in cystic fibrosis. Fetal and maternal outcome. Chest 2000, 118, 85–91. [Google Scholar] [CrossRef]
- Edenborough, F.P.; Stableforth, D.E.; Webb, A.K.; Mackenzie, W.E.; Smith, D.L. Outcome of pregnancy in women with cystic fibrosis. Thorax 1995, 50, 170–174. [Google Scholar] [CrossRef]
- Gillet, D.; de Braekeleer, M.; Bellis, G.; Durieu, I. Cystic fibrosis and pregnancy. Report from French data (1980–1999). BJOG 2002, 109, 912–918. [Google Scholar]
- Jelin, A.C.; Sharshiner, R.; Caughey, A.B. Maternal co-morbidities and neonatal outcomes associated with cystic fibrosis. J. Matern. Fetal Neonatal Med. 2017, 30, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Patel, E.M.; Swamy, G.K.; Heine, R.P.; Kuller, J.A.; James, A.H.; Grotegut, C.A. Medical and obstetric complications among pregnant women with cystic fibrosis. Am. J. Obstet. Gynecol. 2015, 212, 98.e1–98.e9. [Google Scholar] [CrossRef] [PubMed]
- Goss, C.H.; Rubenfeld, G.D.; Otto, K.; Aitken, M.L. The effect of pregnancy on survival in women with cystic fibrosis. Chest 2003, 124, 1460–1468. [Google Scholar] [CrossRef]
- Schechter, M.S.; Quittner, A.L.; Konstan, M.W.; Millar, S.J.; Pasta, D.J.; McMullen, A. Long-term effects of pregnancy and motherhood on disease outcomes of women with cystic fibrosis. Ann. Am. Thorac. Soc. 2013, 10, 213–219. [Google Scholar] [CrossRef]
- Kroon, M.; Akkerman-Nijland, A.M.; Rottier, B.L.; Koppelman, G.H.; Akkerman, O.W.; Touw, D.J. Drugs during pregnancy and breast feeding in women diagnosed with Cystic Fibrosis—An update. J. Cyst. Fibros. 2018, 17, 17–25. [Google Scholar] [CrossRef]
- Middleton, P.G.; Gade, E.J.; Aguilera, C.; MacKillop, L.; Button, B.M.; Coleman, C.; Johnson, B.; Albrechtsen, C.; Edenborough, F.; Rigau, D.; et al. ERS/TSANZ Task Force Statement on the management of reproduction and pregnancy in women with airways diseases. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef]
- Trimble, A.T.; Donaldson, S.H. Ivacaftor withdrawal syndrome in cystic fibrosis patients with the G551D mutation. J. Cyst. Fibros. 2018, 17, e13–e16. [Google Scholar] [CrossRef]
- Carpino, E.A.; Fowler, R.E.; Uluer, A.Z.; Sawicki, G.S. Acute Clinical Outcomes Following Participation in Short-Term CFTR Modulator Trials in Adults with Cystic Fibrosis: A Retrospective Chart Review. Pediatric Pulmonol. 2018, 53, 260–261. [Google Scholar]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.; Burton, B.; Cao, D.; Neuberger, T.; Turnbull, A.; Singh, A.; Joubran, J.; Hazlewood, A.; et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. USA 2009, 106, 18825–18830. [Google Scholar] [CrossRef]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.; Burton, B.; Stack, J.H.; Straley, K.S.; Decker, C.J.; Miller, M.; McCartney, J.; Olson, E.R.; et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. USA 2011, 108, 18843–18848. [Google Scholar] [CrossRef]
- Accurso, F.J.; Rowe, S.M.; Clancy, J.P.; Boyle, M.P.; Dunitz, J.M.; Durie, P.R.; Sagel, S.D.; Hornick, D.B.; Konstan, M.W.; Donaldson, S.H.; et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N. Engl. J. Med. 2010, 363, 1991–2003. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, B.W.; Davies, J.; McElvaney, N.G.; Tullis, E.; Bell, S.C.; Dřevínek, P.; Griese, M.; McKone, E.F.; Wainwright, C.E.; Konstan, M.W.; et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011, 365, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 373, 1783–1784. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Munck, A.; McKone, E.F.; Van Der Ent, C.K.; Moeller, A.; Simard, C.; Wang, L.T.; Ingenito, E.P.; McKee, C.; Lu, Y.; et al. Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N. Engl. J. Med. 2017, 377, 2013–2023. [Google Scholar] [CrossRef]
- Rowe, S.M.; Daines, C.; Ringshausen, F.C.; Kerem, E.; Wilson, J.; Tullis, E.; Nair, N.; Simard, C.; Han, L.; Ingenito, E.P.; et al. Tezacaftor-Ivacaftor in Residual-Function Heterozygotes with Cystic Fibrosis. N. Engl. J. Med. 2017, 377, 2024–2035. [Google Scholar] [CrossRef]
- Heijerman, H.G.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef]
- Tizzano, E.F.; Silver, M.M.; Chitayat, D.; Benichou, J.C.; Buchwald, M. Differential cellular expression of cystic fibrosis transmembrane regulator in human reproductive tissues. Clues for the infertility in patients with cystic fibrosis. Am. J. Pathol. 1994, 144, 906–914. [Google Scholar]
- Ahmad, A.; Ahmed, A.; Patrizio, P. Cystic fibrosis and fertility. Curr. Opin. Obstet. Gynecol. 2013, 25, 167–172. [Google Scholar] [CrossRef]
- Shteinberg, M.; Lulu, A.B.; Downey, D.G.; Blumenfeld, Z.; Rousset-Jablonski, C.; Perceval, M.; Colombo, A.; Stein, N.; Livnat, G.; Gur, M.; et al. Failure to conceive in women with CF is associated with pancreatic insufficiency and advancing age. J. Cyst. Fibros. 2019, 18, 525–529. [Google Scholar] [CrossRef]
- Kaplan, E.; Shwachman, H.; Perlmutter, A.D.; Rule, A.; Khaw, K.T.; Holsclaw, D.S. Reproductive failure in males with cystic fibrosis. N. Engl. J. Med. 1968, 279, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, E.M.; Mody, S.; Schwartz, M.R.; Heltshe, S.L.; Taylor-Cousar, J.L.; Jain, R.; Sufian, S.; Josephy, T.; Aitken, M.L. Contraceptive use among women with cystic fibrosis: A pilot study linking reproductive health questions to the Cystic Fibrosis Foundation National Patient Registry. Contraception 2020, 101, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Roe, A.H.; Traxler, S.A.; Hadjiliadis, D.; Sammel, M.D.; Schreiber, C.A. Contraceptive choices and preferences in a cohort of women with cystic fibrosis. Respir. Med. 2016, 121, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Vertex Pharmaceuticals Incorporated. Ivacaftor (Kalydeco) United States Prescribing Information. Available online: https://pi.vrtx.com/files/uspi_ivacaftor.pdf (accessed on 18 July 2020).
- Vertex Pharmaceuticals Incorporated. Lumacaftor/ivacaftor (Orkambi) United States Prescribing Information. Available online: https://pi.vrtx.com/files/uspi_lumacaftor_ivacaftor.pdf (accessed on 18 July 2020).
- Vertex Pharmaceuticals Incorporated. Tezacaftor/ivacaftor (Symdeko) United States Prescribing Information. Available online: https://pi.vrtx.com/files/uspi_tezacaftor_ivacaftor.pdf (accessed on 18 July 2020).
- Vertex Pharmaceuticals Incorporated. Elexacaftor/tezacaftor/ivacaftor (Trikafta) United States Prescribing Information. Available online: https://pi.vrtx.com/files/uspi_elexacaftor_tezacaftor_ivacaftor.pdf (accessed on 18 July 2020).
- Jones, G.H.; Walshaw, M.J. Potential impact on fertility of new systemic therapies for cystic fibrosis. Paediatr. Respir. Rev. 2015, 16 (Suppl. 1), 25–27. [Google Scholar] [CrossRef] [PubMed]
- Ladores, S.; Kazmerski, T.M.; Rowe, S.M. A Case Report of Pregnancy During Use of Targeted Therapeutics for Cystic Fibrosis. J. Obstet. Gynecol. Neonatal Nurs. 2017, 46, 72–77. [Google Scholar] [CrossRef]
- Ladores, S.; Bray, L.A.; Brown, J. Two Unanticipated Pregnancies While on Cystic Fibrosis Gene-Specific Drug Therapy. J. Patient Exp. 2020, 7, 4–7. [Google Scholar] [CrossRef]
- Federal Drug Administration. Pregnancy and Lactation Labeling Rule. Available online: https://www.fda.gov/drugs/labeling-information-drug-products/pregnancy-and-lactation-labeling-drugs-final-rule (accessed on 18 July 2020).
- Vekaria, S.; Popowicz, N.; White, S.W.; Mulrennan, S. To be or not to be on CFTR modulators during pregnancy: Risks to be considred. J. Cyst. Fibros. 2019. [Google Scholar] [CrossRef]
- Trimble, A.; McKinzie, C.; Terrell, M.; Stringer, E.; Esther, C.R., Jr. Measured fetal and neonatal exposure to Lumacaftor and Ivacaftor during pregnancy and while breastfeeding. J. Cyst. Fibros. 2018, 17, 779–782. [Google Scholar] [CrossRef]
- Mainz, J.G.; Michl, R.K.; Beiersdorf, N.; Lorenz, M.; Schneider, U.; Groten, T.; Jaudszus, A. Successful Pregnancy of a Patient with Cystic Fibrosis Genotype F508del/F508del and Progressed Pulmonary Destruction on lumacaftor/ivacaftor. Klin. Padiatr. 2019, 231, 271–273. [Google Scholar] [CrossRef]
- Kaminski, R.; Nazareth, D. A successful uncomplicated CF pregnancy while remaining on Ivacaftor. J. Cyst. Fibros. 2016, 15, 133–134. [Google Scholar] [CrossRef]
- Nash, E.F.; Middleton, P.G.; Taylor-Cousar, J.L. Outcomes of pregnancy in women with cystic fibrosis (CF) taking CFTR modulators—An international survey. J. Cyst. Fibros. 2020, 19, 521–526. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Office on Women’s Health: Pregnancy Loss. Available online: https://www.womenshealth.gov/pregnancy/youre-pregnant-now-what/pregnancy-loss (accessed on 18 July 2020).
- Shiffman, M.L.; Seale, T.W.; Flux, M.; Rennert, O.R.; Swender, P.T. Breast-milk composition in women with cystic fibrosis: Report of two cases and a review of the literature. Am. J. Clin. Nutr. 1989, 49, 612–617. [Google Scholar]
- Edenborough, F.P.; Borgo, G.; Knoop, C.; Lannefors, L.; Mackenzie, W.E.; Madge, S.; Morton, A.M.; Oxley, H.C.; Touw, F.P.; Benham, M.; et al. Guidelines for the management of pregnancy in women with cystic fibrosis. J. Cyst. Fibros. 2008, 7 (Suppl. 1), S2–S32. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).