Athlete’s Passport: Prevention of Infections, Inflammations, Injuries and Cardiovascular Diseases
Abstract
1. Introduction
2. Experimental Section
2.1. Ethical Approval
2.2. Participants
2.3. Experimental Approach
2.4. Procedures
2.5. Statistical Analysis
3. Results
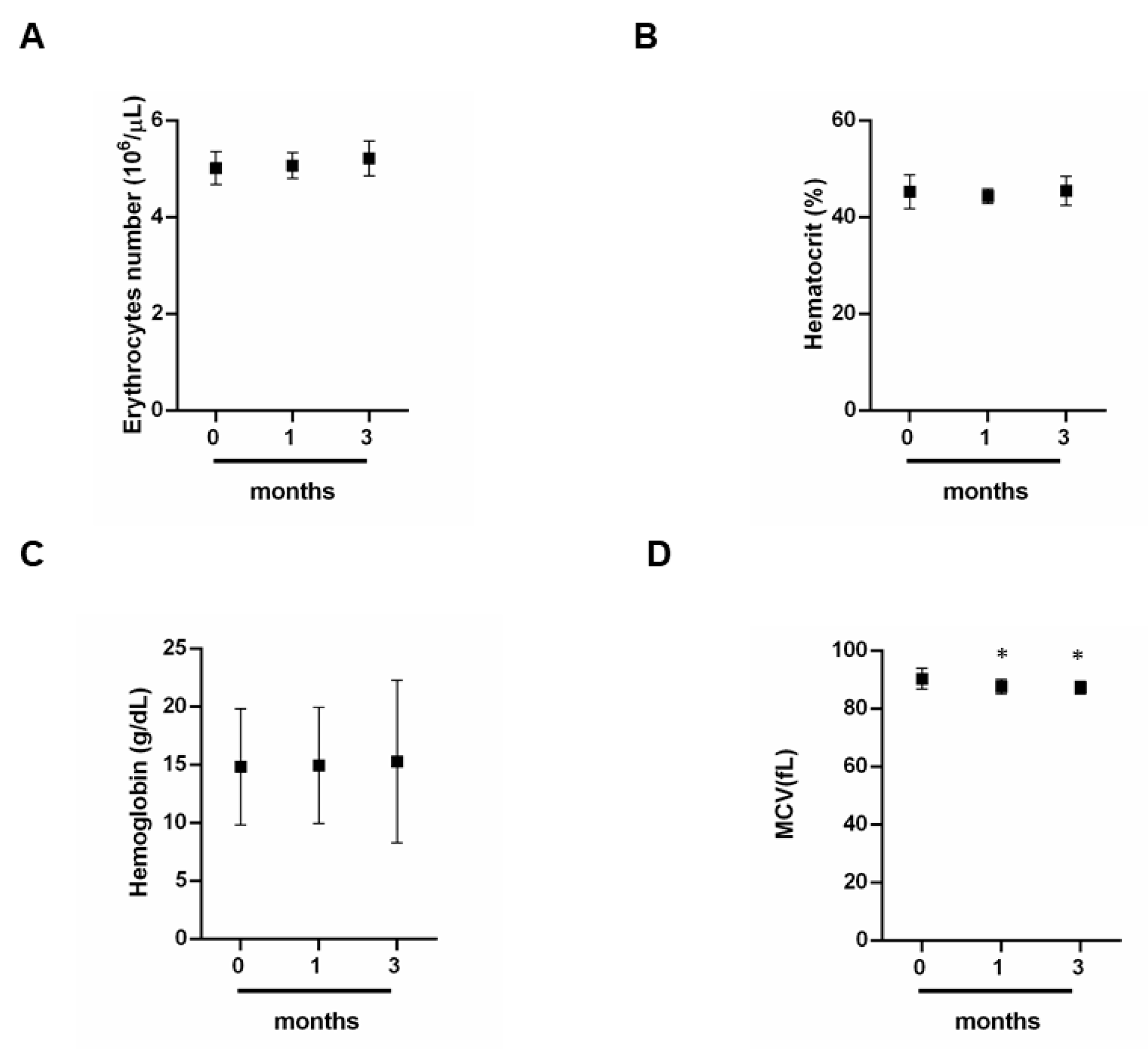
3.1. Effect of Exercise on Red Blood Cells
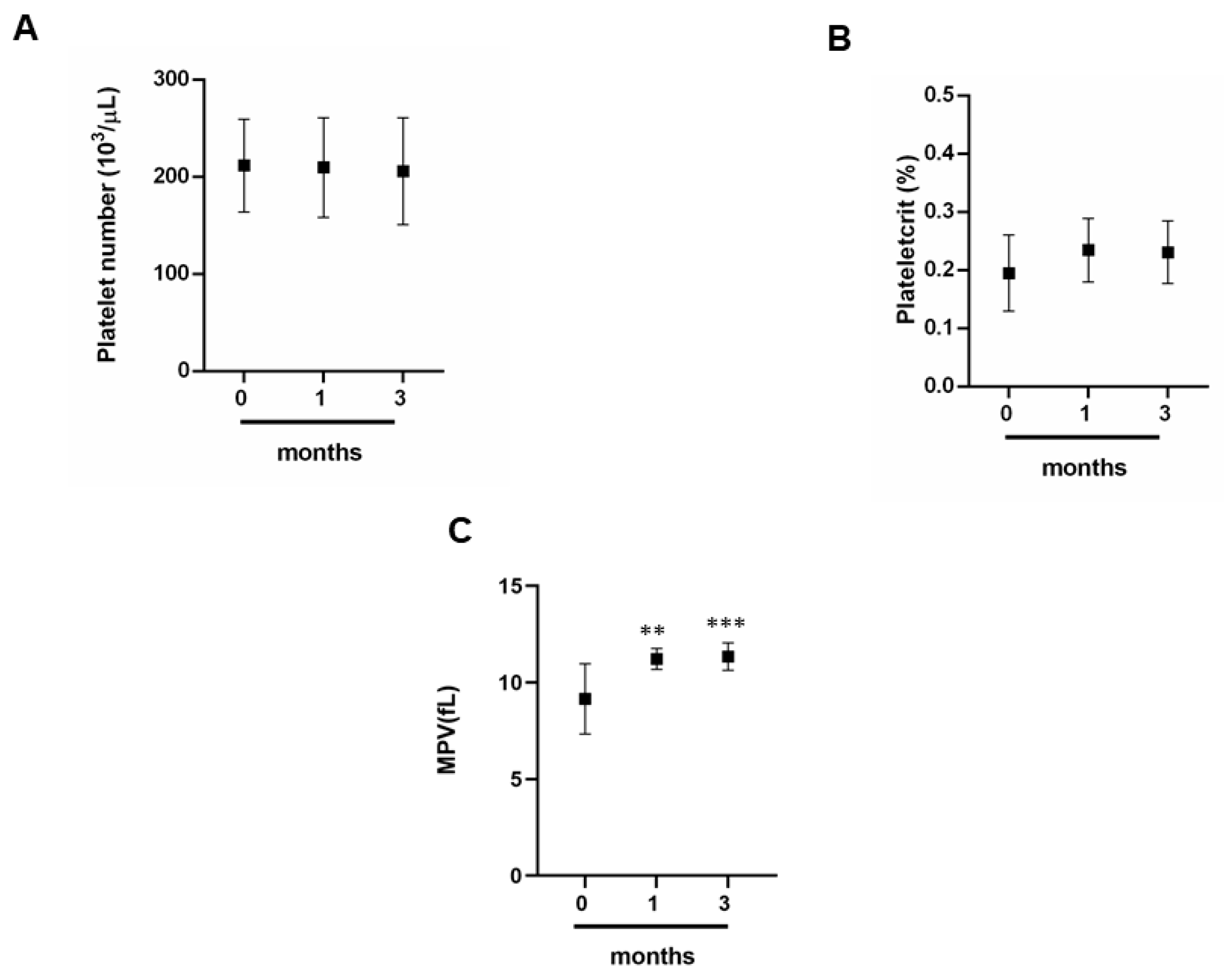
3.2. Exercise and Platelet Indices
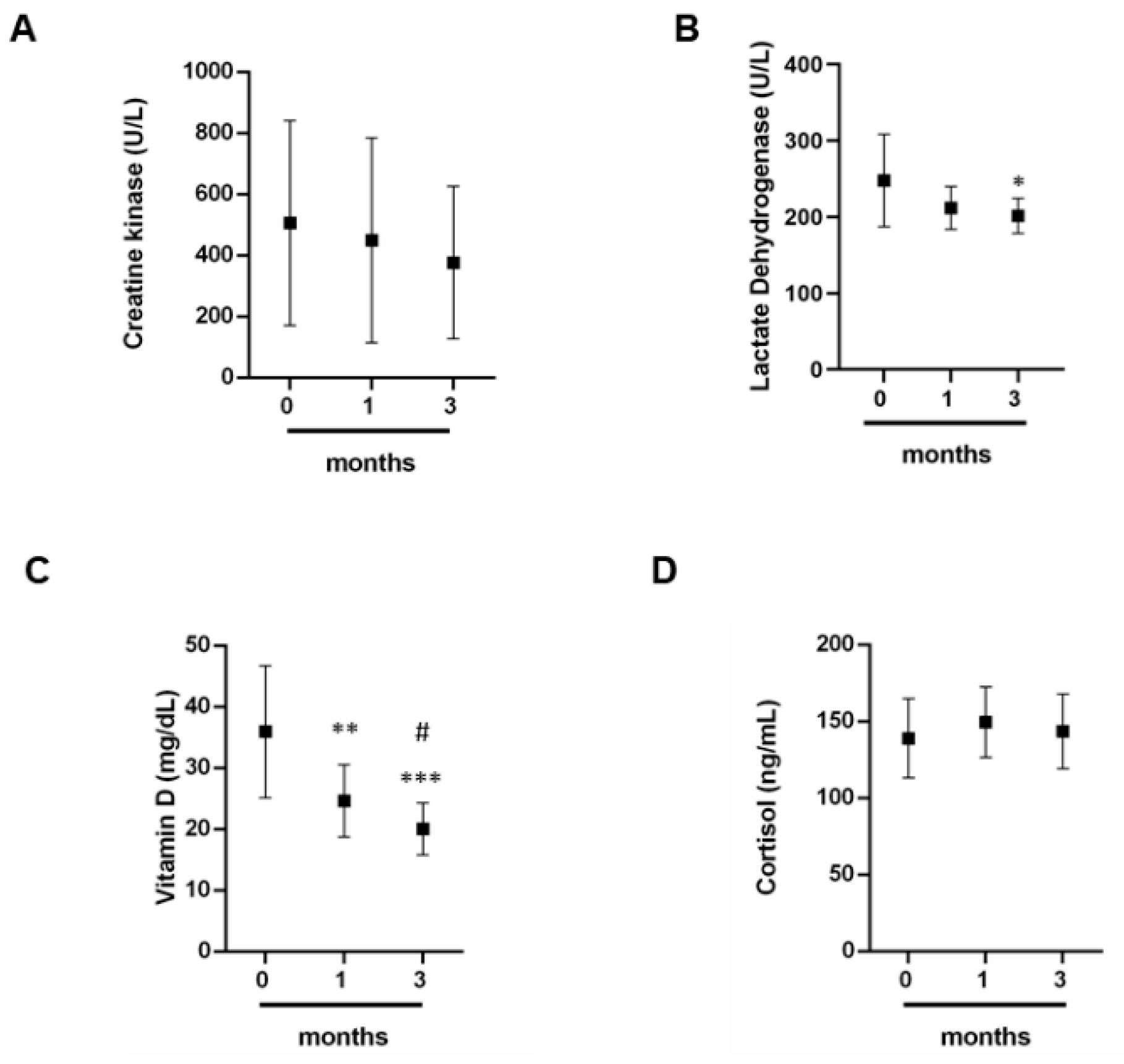
3.3. Dosage of CK, LDH, Vitamin D and Cortisol
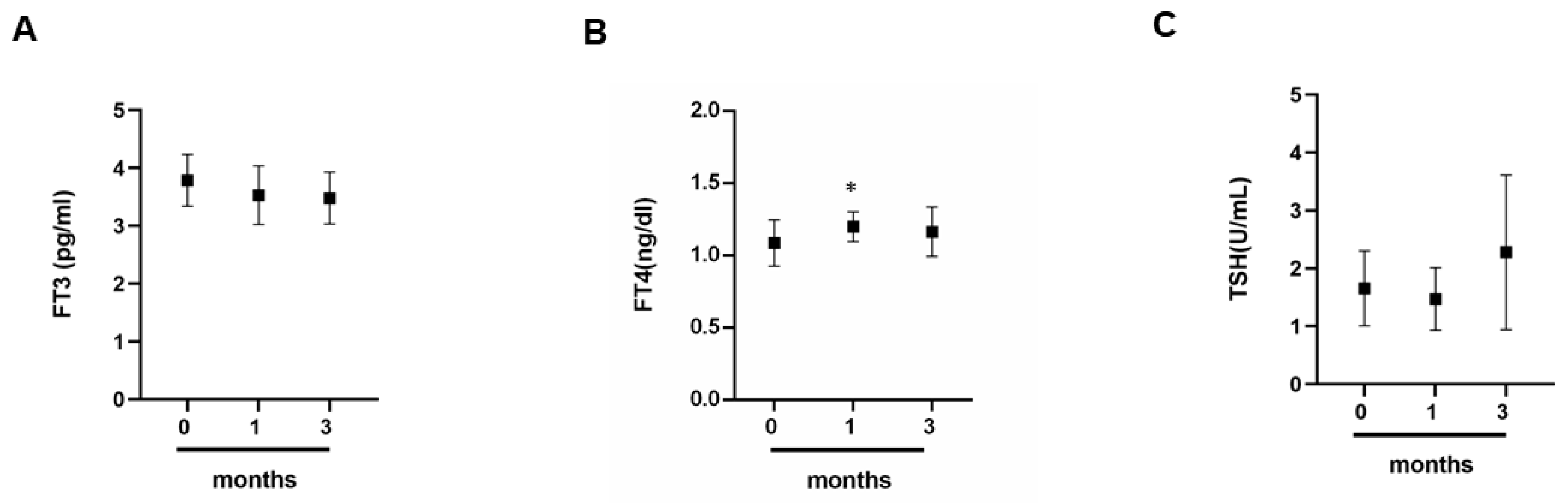
3.4. The Impact of Exercise on the Thyroid Gland
3.5. Comparison between Cortisol and Thyroid Hormones
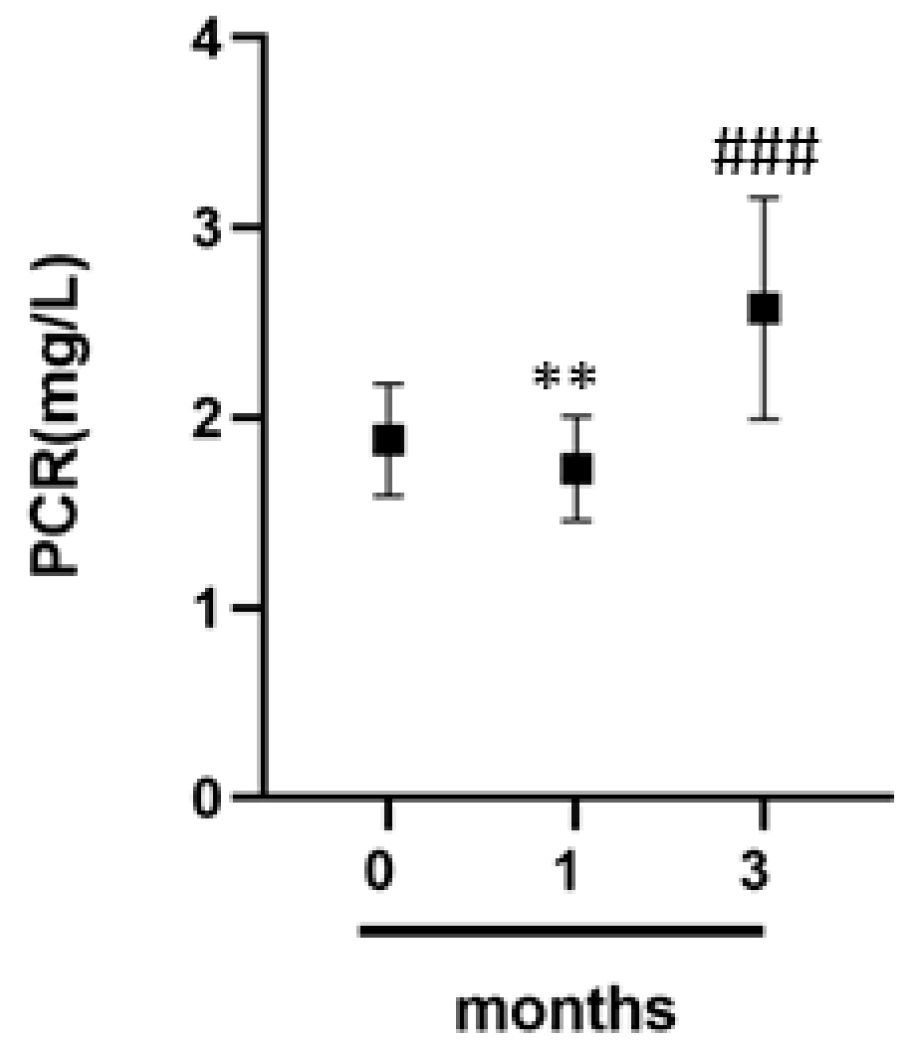
3.6. C-Reactive Protein and Inflammation in Athletes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lippi, G.; Banfi, G.; Botrè, F.; De La Torre, X.; De Vita, F.; Gomez-Cabrera, M.C.; Maffulli, N.; Marchioro, L.; Pacifici, R.; Sanchis-Gomar, F.; et al. Laboratory medicine and sports: Between Scylla and Charybdis. Clin. Chem. Lab. Med. 2012, 50, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Clénin, G.E.; Cordes, M. Laboratory analyses in sports medicine. Ther. Umsch. 2015, 72, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, B.; Izzo, V.; Terracciano, D.; Ranieri, A.; Mazzaccara, C.; Fimiani, F.; Cesaro, A.; Gentile, L.; Leggiero, E.; Pero, R.; et al. Laboratory medicine: Health evaluation in elite athletes. Clin. Chem. Lab. Med. 2019, 57, 1450–1473. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Colombini, A.; Lombardi, G.; Lubkowska, A. Metabolic markers in sports medicine. Adv. Clin. Chem. 2012, 56, 1–54. [Google Scholar]
- Shin, K.-A.; Park, K.D.; Ahn, J.; Park, Y.; Kim, Y.-J. Comparison of Changes in Biochemical Markers for Skeletal Muscles, Hepatic Metabolism, and Renal Function after Three Types of Long-distance Running: Observational Study. Medicine 2016, 95, e3657. [Google Scholar] [CrossRef]
- Noakes, T.D. Effect of Exercise on Serum Enzyme Activities in Humans. Sports Med. 1987, 4, 245–267. [Google Scholar] [CrossRef]
- Nowakowska, A.; Kostrzewa-Nowak, D.; Buryta, R.; Nowak, R. Blood Biomarkers of Recovery Efficiency in Soccer Players. Int. J. Environ. Res. Public Health 2019, 16, 3279. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81, 209–230. [Google Scholar] [CrossRef]
- Brancaccio, P.; Limongelli, F.M.; Maffulli, N. Monitoring of serum enzymes in sport. Br. J. Sports Med. 2006, 40, 96–97. [Google Scholar] [CrossRef]
- Tarpenning, K.M.; Wiswell, R.A.; Hawkins, S.A.; Marcell, T.J. Influence of weight training exercise and modification of hormonal response on skeletal muscle growth. J. Sci. Med. Sport 2001, 4, 431–446. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Copeland, J.L.; Van Helder, W. Effect of training status and exercise mode on endogenous steroid hormones in men. J. Appl. Physiol. 2004, 96, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Munck, A.; Guyre, P.M.; Holbrook, N.J. Physiological Functions of Glucocorticoids in Stress and Their Relation to Pharmacological Actions. Endocr. Rev. 1984, 5, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Duclos, M.; Gouarne, C.; Bonnemaison, D. Acute and chronic effects of exercise on tissue sensitivity to glucocorticoids. J. Appl. Physiol. 2003, 94, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A.; Hymer, W.C.; Nindl, B.C.; Fragala, M.S. Growth Hormone(s), Testosterone, Insulin-Like Growth Factors, and Cortisol: Roles and Integration for Cellular Development and Growth With Exercise. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef]
- Protzner, A.; Szmodis, M.; Udvardy, A.; Bosnyák, E.; Trájer, E.; Komka, Z.; Györe, I.; Toth, M. Hormonal Neuroendocrine and Vasoconstrictor Peptide Responses of Ball Game and Cyclic Sport Elite Athletes by Treadmill Test. PLoS ONE 2015, 10, e0144691. [Google Scholar] [CrossRef]
- Barbara, M.; Anna, K.; Agnieszka, Z.-L. The Impact of Professional Sports Activity on GH-IGF-I Axis in Relation to Testosterone Level. Am. J. Mens Health 2020, 14, 1557988319900829. [Google Scholar] [CrossRef]
- Barbas, I.; Fatouros, I.G.; Douroudos, I.I.; Chatzinikolaou, A.; Michailidis, Y.; Draganidis, D.; Jamurtas, A.Z.; Nikolaidis, M.G.; Parotsidis, C.; Theodorou, A.A.; et al. Physiological and performance adaptations of elite Greco-Roman wrestlers during a one-day tournament. Eur. J. Appl. Physiol. 2010, 111, 1421–1436. [Google Scholar] [CrossRef]
- Bartoszewska, M.; Kamboj, M.; Patel, D.R. Vitamin D, Muscle Function, and Exercise Performance. Pediatr. Clin. N. Am. 2010, 57, 849–861. [Google Scholar] [CrossRef]
- Abrams, G.D.; Feldman, D.; Safran, M.R. Effects of Vitamin D on Skeletal Muscle and Athletic Performance. J. Am. Acad. Orthop. Surg. 2018, 26, 278–285. [Google Scholar] [CrossRef]
- Larson-Meyer, E.; Willis, K.S. Vitamin D and Athletes. Curr. Sports Med. Rep. 2010, 9, 220–226. [Google Scholar] [CrossRef]
- Butscheidt, S.; Rolvien, T.; Ueblacker, P.; Amling, M.; Barvencik, F. Impact of Vitamin D in Sports: Does Vitamin D Insufficiency Compromise Athletic Performance? Sportverletz Sportschaden 2017, 31, 37–44. [Google Scholar] [PubMed]
- Owens, D.J.; Allison, R.J.; Close, G.L. Vitamin D and the Athlete: Current Perspectives and New Challenges. Sports Med. 2018, 48 (Suppl. 1), 3–16. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, G.; Trimarco, B. Gene–environment interactions and vitamin D effects on cardiovascular risk. BMC Med. 2019, 17, 166. [Google Scholar] [CrossRef] [PubMed]
- Querques, F.; Cantilena, B.; Cozzolino, C.; Esposito, M.T.; Passaro, F.; Parisi, S.; Lombardo, B.; Russo, T.; Pastore, L. Angiotensin receptor I stimulates osteoprogenitor proliferation through TGFβ-mediated signaling. J. Cell. Physiol. 2015, 230. [Google Scholar] [CrossRef]
- Clément, K.; Viguerie, N.; Diehn, M.; Alizadeh, A.A.; Barbe, P.; Thalamas, C.; Storey, J.D.; Brown, P.O.; Barsh, G.S.; Langin, D. In Vivo Regulation of Human Skeletal Muscle Gene Expression by Thyroid Hormone. Genome Res. 2002, 12, 281–291. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Dillmann, W.H. Thyroid Hormone Action in the Heart. Endocr. Rev. 2005, 26, 704–728. [Google Scholar] [CrossRef]
- Eichner, E.R. Sports medicine pearls and pitfalls: Anemia in athletes. Curr. Sports Med. Rep. 2007, 6, 2–3. [Google Scholar] [CrossRef]
- Pakarinen, A.; Häkkinen, K.; Alen, M. Serum thyroid hormones, thyrotropin and thyroxine binding globulin in elite athletes during very intense strength training of one week. J. Sports Med. Phys. Fit. 1991, 31, 142–146. [Google Scholar]
- Eichner, E.R. Sports Medicine Pearls and Pitfalls: Nature’s anticoagulant. Curr. Sports Med. Rep. 2009, 8, 2–3. [Google Scholar] [CrossRef]
- Zebisch, A.; Schulz, E.; Grosso, M.; Lombardo, B.; Acierno, G.; Sill, H.; Iolascon, A. Identification of a novel variant of epsilon-gamma-delta-beta thalassemia highlights limitations of next generation sequencing. Am. J. Hematol. 2015, 90, 52–54. [Google Scholar] [CrossRef]
- Hinton, P.S. Iron and the endurance athlete. Appl. Physiol. Nutr. Metab. 2014, 39, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kraft, P.; Hagan, K.A.; Harrington, L.B.; Lindstroem, S.; Kabrhel, C. Interaction of a genetic risk score with physical activity, physical inactivity, and body mass index in relation to venous thromboembolism risk. Genet Epidemiol. 2018, 42, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, G.; Whiteside, W.K.; Kanwisher, M. Venous Thrombosis in Athletes. J. Am. Acad. Orthop. Surg. 2013, 21, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Heber, S.; Volf, I. Effects of Physical (In)activity on Platelet Function. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Salvagno, G.L.; Danese, E.; Tarperi, C.; Guidi, G.C.; Schena, F. Variation of Red Blood Cell Distribution Width and Mean Platelet Volume after Moderate Endurance Exercise. Adv. Hematol. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Ahmadizad, S.; El-Sayed, M.S. The Effects of Graded Resistance Exercise on Platelet Aggregation and Activation. Med. Sci. Sports Exerc. 2003, 35, 1026–1032. [Google Scholar] [CrossRef][Green Version]
- Mold, C.; Gewurz, H.; Du Clos, T.W. Regulation of complement activation by C-reactive protein. Immunopharmacology 1999, 42, 23–30. [Google Scholar] [CrossRef]
- Black, S.; Kushner, I.; Samols, D. C-reactive Protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef]
- Sorriento, D.; Iaccarino, G. Inflammation and Cardiovascular Diseases: The Most Recent Findings. Int. J. Mol. Sci. 2019, 20, 3879. [Google Scholar] [CrossRef]
- Iossa, S.; Costa, V.; Corvino, V.; Auletta, G.; Barruffo, L.; Cappellani, S.; Ceglia, C.; Cennamo, G.; D’Adamio, A.P.; D’Amico, A.; et al. Phenotypic and genetic characterization of a family carrying two Xq21.1-21.3 interstitial deletions associated with syndromic hearing loss. Mol. Cytogenet. 2015, 8, 18. [Google Scholar] [CrossRef]
- Sanna, V.; Ceglia, C.; Tarsitano, M.; Lombardo, B.; Coppola, A.; Zarrilli, F.; Castaldo, G.; Di Minno, G. Aberrant F8 gene intron 1 inversion with concomitant duplication and deletion in a severe hemophilia A patient from Southern Italy. J. Thromb. Haemost. 2013, 11, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Girolami, F.; Frisso, G.; Benelli, M.; Crotti, L.; Iascone, M.; Mango, R.; Mazzaccara, C.; Pilichou, K.; Arbustini, E.; Tomberli, B.; et al. Contemporary genetic testing in inherited cardiac disease: Tools, ethical issues, and clinical applications. J. Cardiovasc. Med. (Hagerstown) 2018, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Detta, N.; Frisso, G.; Limongelli, G.; Marzullo, M.; Calabrò, R.; Salvatore, F. Genetic analysis in a family affected by sick sinus syndrome may reduce the sudden death risk in a young aspiring competitive athlete. Int. J. Cardiol. 2014, 170, 63–65. [Google Scholar] [CrossRef]
- Dufaux, B.; Order, U.; Geyer, H.; Hollmann, W. C-Reactive Protein Serum Concentrations in Well-Trained Athletes*. Int. J. Sports Med. 1984, 5, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Woloshin, S.; Schwartz, L.M. Distribution of C-Reactive Protein Values in the United States. N. Engl. J. Med. 2005, 352, 1611–1613. [Google Scholar] [CrossRef]
- Moreira, A.; Delgado, L.; Moreira, P.; Haahtela, T. Does exercise increase the risk of upper respiratory tract infections? Br. Med. Bull. 2009, 90, 111–131. [Google Scholar] [CrossRef]
- Scudiero, O.; Brancaccio, M.; Mennitti, C.; Laneri, S.; Lombardo, B.; De Biasi, M.G.; De Gregorio, E.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; et al. Human Defensins: A Novel Approach in the Fight against Skin Colonizing Staphylococcus aureus. Antibiotics 2020, 9, 198. [Google Scholar] [CrossRef]
- Pero, R.; Brancaccio, M.; Laneri, S.; De Biasi, M.G.; Lombardo, B.; Scudiero, O. A Novel View of Human Helicobacter pylori Infections: Interplay between Microbiota and Beta-Defensins. Biomolecules 2019, 9, 237. [Google Scholar] [CrossRef]
- Coretti, L.; Cuomo, M.; Florio, E.; Palumbo, D.; Keller, S.; Pero, R.; Chiariotti, L.; Lembo, F.; Cafiero, C. Subgingival dysbiosis in smoker and non-smoker patients with chronic periodontitis. Mol. Med. Rep. 2017, 15, 2007–2014. [Google Scholar] [CrossRef]
- Keller, S.; Angrisano, T.; Florio, E.; Pero, R.; Decaussin-Petrucci, M.; Troncone, G.; Capasso, M.; Lembo, F.; Fusco, A.; Chiariotti, L. DNA methylation state of the galectin-3 gene represents a potential new marker of thyroid malignancy. Oncol. Lett. 2013, 6, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Coretti, L.; Natale, A.; Cuomo, M.; Florio, E.; Keller, S.; Lembo, F.; Chiariotti, L.; Pero, R. The Interplay between Defensins and Microbiota in Crohn’s Disease. Mediat. Inflamm. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Errico, F.; Zarrilli, F.; Florio, E.; Punzo, D.; Mansueto, S.; Angrisano, T.; Pero, R.; Lembo, F.; Castaldo, G.; et al. DNA methylation state of BDNF gene is not altered in prefrontal cortex and striatum of schizophrenia subjects. Psychiatry Res. 2014, 220, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Angrisano, T.; Pero, R.; Brancaccio, M.; Coretti, L.; Florio, E.; Pezone, A.; Calabrò, V.; Falco, G.; Keller, S.; Lembo, F.; et al. Cyclical DNA Methylation and Histone Changes Are Induced by LPS to Activate COX-2 in Human Intestinal Epithelial Cells. PLoS ONE 2016, 11, e0156671. [Google Scholar] [CrossRef] [PubMed]
- Chiariotti, L.; Coretti, L.; Pero, R.; Lembo, F. Epigenetic Alterations Induced by Bacterial Lipopolysaccharides. Adv. Exp. Med. Biol. 2016, 879, 91–105. [Google Scholar] [PubMed]
- Pero, R.; Angrisano, T.; Brancaccio, M.; Falanga, A.; Lombardi, L.; Natale, F.; Laneri, S.; Lombardo, B.; Galdiero, S.; Scudiero, O. Beta-defensins and analogs in Helicobacter pylori infections: mRNA expression levels, DNA methylation, and antibacterial activity. PLoS ONE 2019, 14, e0222295. [Google Scholar] [CrossRef] [PubMed]
- Pero, R.; Coretti, L.; Nigro, E.; Lembo, F.; Laneri, S.; Lombardo, B.; Daniele, A.; Scudiero, O. β-Defensins in the Fight against Helicobacter pylori. Molecules 2017, 22, 424. [Google Scholar] [CrossRef]
- Colavita, I.; Nigro, E.; Sarnataro, D.; Scudiero, O.; Granata, V.; Daniele, A.; Zagari, A.; Pessi, A.; Salvatore, F. Membrane protein 4F2/CD98 is a cell surface receptor involved in the internalization and trafficking of human β-Defensin 3 in epithelial cells. Chem. Biol. 2015, 2, 217–228. [Google Scholar] [CrossRef]
- Falanga, A.; Valiante, S.; Galdiero, E.; Franci, G.; Scudiero, O.; Morelli, G.; Galdiero, S. Dimerization in tailoring uptake efficacy of the HSV-1 derived membranotropic peptide gH625. Sci. Rep. 2017, 7, 9434. [Google Scholar] [CrossRef]
- Ragozzino, E.; Brancaccio, M.; Di Costanzo, A.; Scalabrì, F.; Andolfi, G.; Wanderlingh, L.G.; Patriarca, E.J.; Minchiotti, G.; Altamura, S.; Summa, V.; et al. 6-Bromoindirubin-3′-oxime intercepts GSK3 signaling to promote and enhance skeletal muscle differentiation affecting miR-206 expression in mice. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Koistinen, P.; Martikkala, V.; Karpakka, J.; Vuolteenaho, O.; Leppäluoto, J. The effects of moderate altitude on circulating thyroid hormones and thyrotropin in training athletes. J. Sports Med. Phys. Fit. 1996, 36, 408–411. [Google Scholar]
- Ciloglu, F.; Peker, I.; Pehlivan, A.; Karacabey, K.; İlhan, N.; Saygin, O.; Ozmerdivenli, R. Exercise intensity and its effects on thyroid hormones. Neuro Endocrinol. Lett. 2005, 26, 830–834. [Google Scholar] [PubMed]
| Variables | ρ | ||
|---|---|---|---|
| 0 Month | 1 Month | 3 Months | |
| Cortisol/fT3 | −0.46 | 0.08 | −0.13 |
| Cortisol/fT4 | −019 | 0.20 | 0.04 |
| Cortisol/TSH | 0.11 | 0.05 | 0.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mennitti, C.; Brancaccio, M.; Gentile, L.; Ranieri, A.; Terracciano, D.; Cennamo, M.; La Civita, E.; Liotti, A.; D’Alicandro, G.; Mazzaccara, C.; et al. Athlete’s Passport: Prevention of Infections, Inflammations, Injuries and Cardiovascular Diseases. J. Clin. Med. 2020, 9, 2540. https://doi.org/10.3390/jcm9082540
Mennitti C, Brancaccio M, Gentile L, Ranieri A, Terracciano D, Cennamo M, La Civita E, Liotti A, D’Alicandro G, Mazzaccara C, et al. Athlete’s Passport: Prevention of Infections, Inflammations, Injuries and Cardiovascular Diseases. Journal of Clinical Medicine. 2020; 9(8):2540. https://doi.org/10.3390/jcm9082540
Chicago/Turabian StyleMennitti, Cristina, Mariarita Brancaccio, Luca Gentile, Annaluisa Ranieri, Daniela Terracciano, Michele Cennamo, Evelina La Civita, Antonietta Liotti, Giovanni D’Alicandro, Cristina Mazzaccara, and et al. 2020. "Athlete’s Passport: Prevention of Infections, Inflammations, Injuries and Cardiovascular Diseases" Journal of Clinical Medicine 9, no. 8: 2540. https://doi.org/10.3390/jcm9082540
APA StyleMennitti, C., Brancaccio, M., Gentile, L., Ranieri, A., Terracciano, D., Cennamo, M., La Civita, E., Liotti, A., D’Alicandro, G., Mazzaccara, C., Frisso, G., Pero, R., Lombardo, B., & Scudiero, O. (2020). Athlete’s Passport: Prevention of Infections, Inflammations, Injuries and Cardiovascular Diseases. Journal of Clinical Medicine, 9(8), 2540. https://doi.org/10.3390/jcm9082540












