Exhaled Biomarkers in Idiopathic Pulmonary Fibrosis—A Six-Month Follow-up Study in Patients Treated with Pirfenidone
Abstract
1. Introduction
2. Materials and Methods
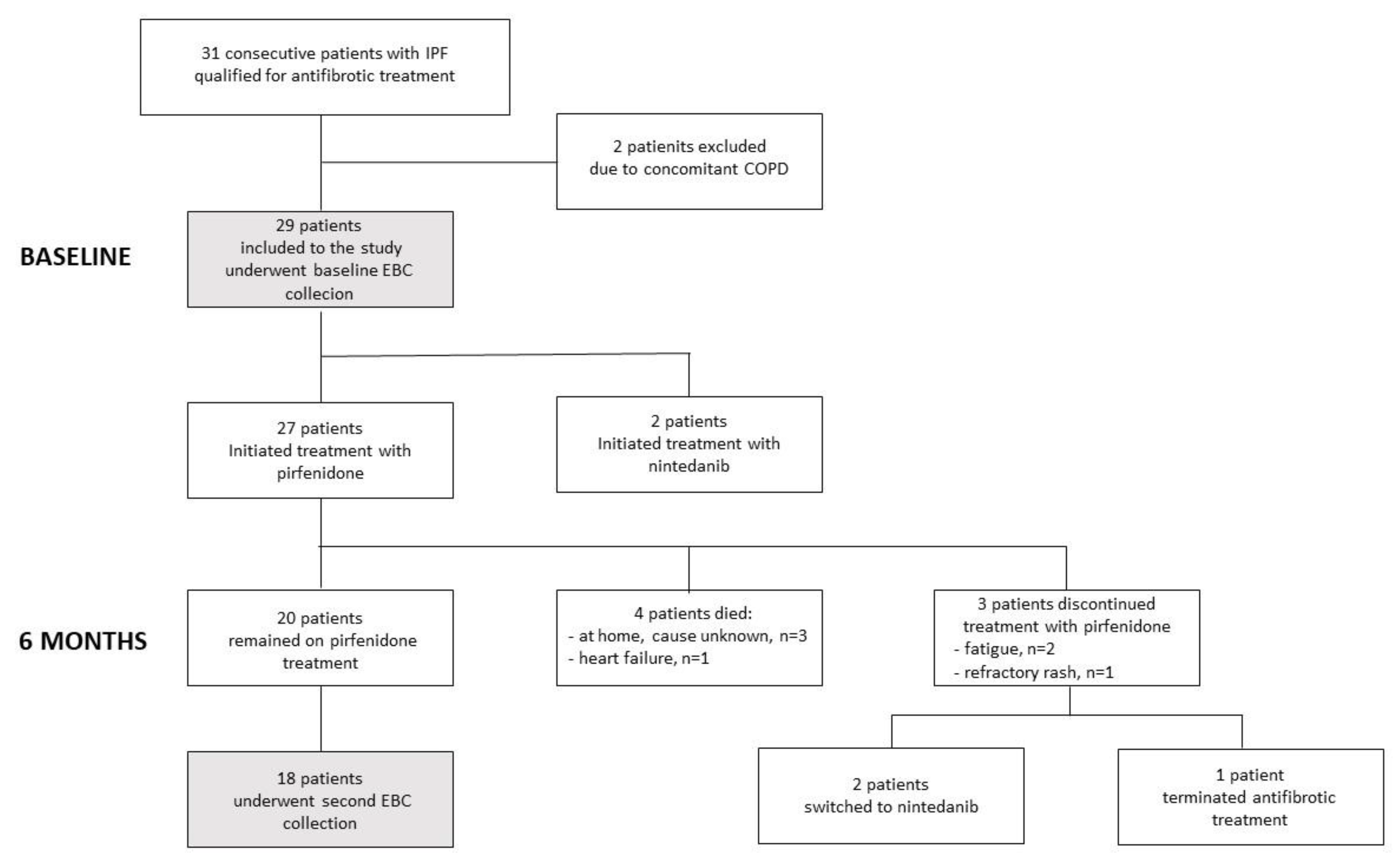
2.1. Study Design, Population and Definitions
2.2. Exhaled Breath Condensate Collection
2.3. Cytokine Measurements in EBC
2.4. Statistical Analysis
3. Results
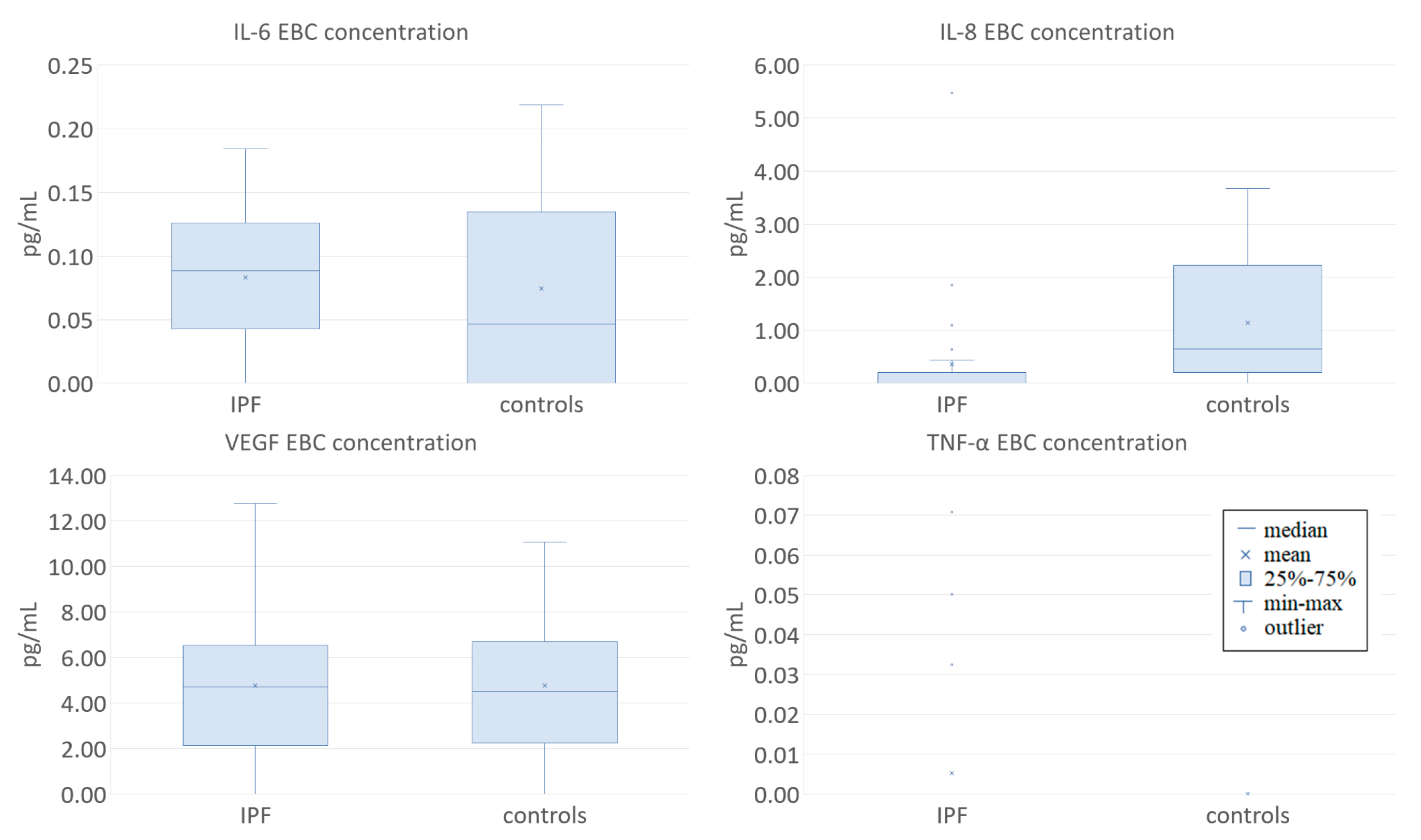
3.1. Clinical Characteristics of the Study Participants and EBC Cytokine Concentrations at Baseline
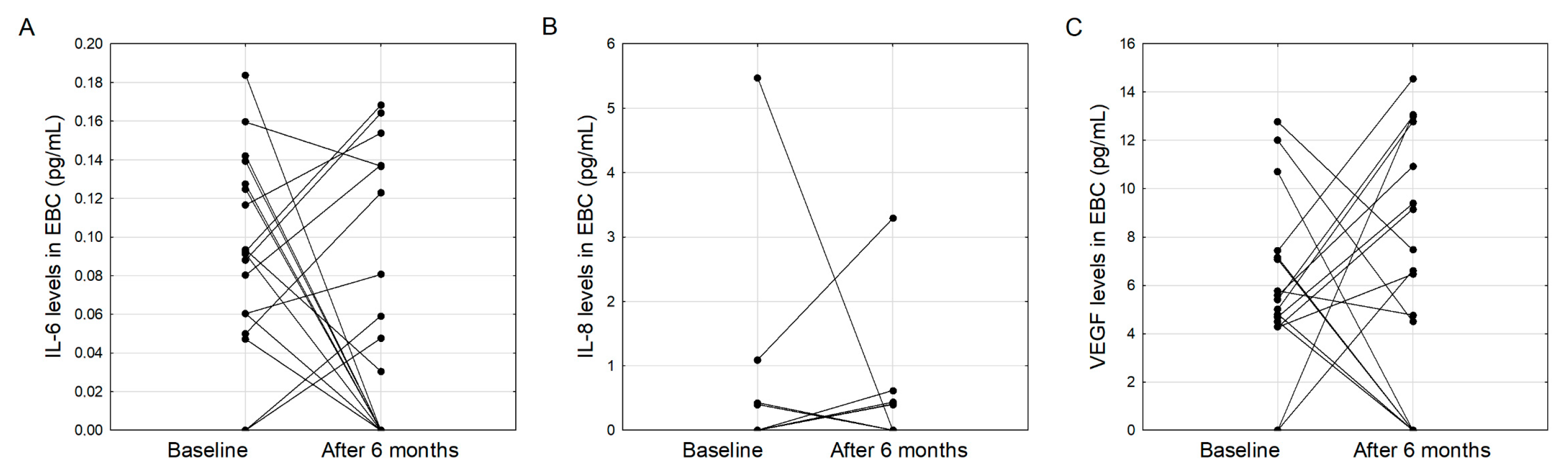
3.2. Clinical Characteristics of IPF Patients and EBC Cytokine Concentrations after Six Months of Treatment with Pirfenidone
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pantelidis, P.; Fanning, G.C.; Wells, A.U.; Welsh, K.I.; Du Bois, R.M. Analysis of tumor necrosis factor-alpha, lymphotoxin-alpha, tumor necrosis factor receptor II, and interleukin-6 polymorphisms in patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2001, 163, 1432–1436. [Google Scholar] [CrossRef] [PubMed]
- Desai, O.; Winkler, J.; Minasyan, M.; Herzog, E.L. The Role of Immune and Inflammatory Cells in Idiopathic Pulmonary Fibrosis. Front. Med. (Lausanne) 2018, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Sgalla, G.; Iovene, B.; Calvello, M.; Ori, M.; Varone, F.; Richeldi, L. Idiopathic pulmonary fibrosis: Pathogenesis and management. Respir. Res. 2018, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Ziora, D.; Jastrzębski, D.; Adamek, M.; Czuba, Z.; Kozielski, J.J.; Grzanka, A.; Kasperska-Zajac, A. Circulating concentration of markers of angiogenic activity in patients with sarcoidosis and idiopathic pulmonary fibrosis. BMC Pulm. Med. 2015, 15, 113. [Google Scholar] [CrossRef]
- Car, B.D.; Meloni, F.; Luisetti, M.; Semenzato, G.; Gialdroni-Grassi, G.; Walz, A. Elevated IL-8 and MCP-1 in the bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am. J. Respir. Crit. Care Med. 1994, 149, 655–659. [Google Scholar] [CrossRef]
- Guiot, J.; Henket, M.; Corhay, J.L.; Moermans, C.; Louis, R. Sputum biomarkers in IPF: Evidence for raised gene expression and protein level of IGFBP-2, IL-8 and MMP-7. PLoS ONE 2017, 12, e0171344. [Google Scholar] [CrossRef]
- Papiris, S.A.; Tomos, I.; Karakatsani, A.; Spathis, A.; Korbila, I.; Analitis, A.; Kolilekas, L.; Kagouridis, K.; Loukides, S.; Karakitsos, P.; et al. High levels of IL-6 and IL-8 characterize early-on idiopathic pulmonary fibrosis acute exacerbations. Cytokine 2018, 102, 168–172. [Google Scholar] [CrossRef]
- Richards, T.J.; Kaminski, N.; Baribaud, F.; Flavin, S.; Brodmerkel, C.; Horowitz, D.; Li, K.; Choi, J.; Vuga, L.J.; Lindell, K.O.; et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2012, 185, 67–76. [Google Scholar] [CrossRef]
- Idiopathic Pulmonary Fibrosis Clinical Research Network; Raghu, G.; Anstrom, K.J.; King, T.E., Jr.; Lasky, J.A.; Martinez, F.J. Prednisone, azathioprine, and N-Acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 2012, 366, 1968–1977. [Google Scholar] [CrossRef]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; du Bois, R.M.; Fagan, E.A.; Fishman, R.S.; Glaspole, I.; Glassberg, M.K.; Lancaster, L.; et al. Pirfenidone for idiopathic pulmonary fibrosis: Analysis of pooled data from three multinational phase 3 trials. Eur. Respir. J. 2015, 47, 243–253. [Google Scholar] [CrossRef]
- Graney, B.A.; Lee, J.S. Impact of novel antifibrotic therapy on patient outcomes in idiopathic pulmonary fibrosis: Patient selection and perspectives. Patient Relat. Outcome Meas. 2018, 9, 321–328. [Google Scholar] [CrossRef]
- Zurkova, M.; Section, I.; Kriegova, E.; Kolek, V.; Lostakova, V.; Sterclova, M.; Bartos, V.; Doubkova, M.; Binkova, I.; Svoboda, M.; et al. Effect of pirfenidone on lung function decline and survival: 5-yr experience from a real-life IPF cohort from the Czech EMPIRE registry. Respir. Res. 2019, 20, 16. [Google Scholar] [CrossRef]
- Collins, B.F.; Raghu, G. Antifibrotic therapy for fibrotic lung disease beyond idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2019, 28. [Google Scholar] [CrossRef]
- Lopez-de la Mora, D.A.; Sanchez-Roque, C.; Montoya-Buelna, M.; Sanchez-Enriquez, S.; Lucano-Landeros, S.; Macias-Barragan, J.; Armendariz-Borunda, J. Role and New Insights of Pirfenidone in Fibrotic Diseases. Int. J. Med Sci. 2015, 12, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.L.; Margolin, S.B.; Krakauer, T.; Roy, C.J.; Stiles, B.G. Pirfenidone Blocks the In Vitro and In Vivo Effects of Staphylococcal Enterotoxin B. Infect. Immun. 2002, 70, 2989–2994. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, S.; Veijola, A.; Karvonen, H.; Lappi-Blanco, E.; Sormunen, R.; Korpela, S.; Zagai, U.; Sköld, M.C.; Kaarteenaho, R. Pirfenidone and nintedanib modulate properties of fibroblasts and myofibroblasts in idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.N.; Hyde, D.M.; Giri, S.N. Anti-inflammatory effect of pirfenidone in the bleomycin-hamster model of lung inflammation. Inflammation 2000, 24, 477–491. [Google Scholar] [CrossRef]
- Ronan, N.; Bennett, D.M.; Khan, K.A.; McCarthy, Y.; Dahly, D.; Bourke, L.; Chelliah, A.; Cavazza, A.; O’Regan, K.; Moloney, F.; et al. Tissue and Bronchoalveolar Lavage Biomarkers in Idiopathic Pulmonary Fibrosis Patients on Pirfenidone. Lung 2018, 196, 543–552. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, Y.; Ye, Q. Risk factors for acute exacerbation of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Clin. Respir. J. 2018, 12, 1084–1092. [Google Scholar] [CrossRef]
- Majewski, S.; Szewczyk, K.; Bialas, A.J.; Miłkowska-Dymanowska, J.; Górski, P.; Piotrowski, W.J. Epithelial Alarmins in Serum and Exhaled Breath in Patients with Idiopathic Pulmonary Fibrosis: A Prospective One-Year Follow-Up Cohort Study. J. Clin. Med. 2019, 8, 1590. [Google Scholar] [CrossRef]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Ley, B.; Ryerson, C.J.; Vittinghoff, E.; Ryu, J.H.; Tomassetti, S.; Lee, J.S.; Poletti, V.; Buccioli, M.; Elicker, B.M.; Jones, K.D.; et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 2012, 156, 684–691. [Google Scholar] [CrossRef]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.C.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A European Respiratory Society technical standard: Exhaled biomarkers in lung disease. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef] [PubMed]
- Przysucha, N.; Gorska, K.; Paplinska, M.; Nejman-Gryz, P.; Proboszcz, M.; Krenke, R. Exhaled breath condensate—A new tool for assessment of idiopathic pulmonary fibrosis? Eur. Respir. J. 2018, 52, 29–31. [Google Scholar]
- Krauss, E.; Froehler, M.; Degen, M.; Mahavadi, P.; Dartsch, R.C.; Korfei, M.; Ruppert, C.; Seeger, W.; Günther, A. Exhalative Breath Markers Do Not Offer for Diagnosis of Interstitial Lung Diseases: Data from the European IPF Registry (eurIPFreg) and Biobank. J. Clin. Med. 2019, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Rindlisbacher, B.; Strebel, C.; Guler, S.; Kollár, A.; Geiser, T.; Fiedler, G.M.; Leichtle, A.B.; Bovet, C.; Funke-Chambour, M. Exhaled breath condensate as a potential biomarker tool for idiopathic pulmonary fibrosis—A pilot study. J. Breath Res. 2017, 12, 016003. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidi, E.M.; Lappas, A.S.; Tzortzi, A.S.; Behrakis, P.K. Exhaled Breath Condensate: Technical and Diagnostic Aspects. Sci. World J. 2015, 2015, 435160. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Herrera, J.; Gilbertsen, A.; Xia, H.; Smith, K.; Benyumov, A.; Bitterman, P.B.; Henke, C.A. IL-8 mediates idiopathic pulmonary fibrosis mesenchymal progenitor cell fibrogenicity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L127–L136. [Google Scholar] [CrossRef]
- Blandinières, A.; Gendron, N.; Bacha, N.; Bièche, I.; Chocron, R.; Nunes, H.; Nevo, N.; Rossi, E.; Crestani, B.; Lecourt, S.; et al. Interleukin-8 release by endothelial colony-forming cells isolated from idiopathic pulmonary fibrosis patients might contribute to their pathogenicity. Angiogenesis 2019, 22, 325–339. [Google Scholar] [CrossRef]
- Kobayashi, K.; Suzukawa, M.; Watanabe, K.; Arakawa, S.; Igarashi, S.; Asari, I.; Hebisawa, A.; Matsui, H.; Nagai, H.; Nagase, T.; et al. Secretory IgA accumulated in the airspaces of idiopathic pulmonary fibrosis and promoted VEGF, TGF-β and IL-8 production by A549 cells. Clin. Exp. Immunol. 2020, 199, 326–336. [Google Scholar] [CrossRef]
- Willems, S.; Verleden, S.E.; Vanaudenaerde, B.M.; Wynants, M.; Dooms, C.; Yserbyt, J.; Somers, J.; Verbeken, E.K.; Verleden, G.M.; Wuyts, W.A. Multiplex protein profiling of bronchoalveolar lavage in idiopathic pulmonary fibrosis and hypersensitivity pneumonitis. Ann. Thorac. Med. 2013, 8, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Górska, K.; Maskey-Warzechowska, M.; Nejman-Gryz, P.; Korczynski, P.; Prochorec-Sobieszek, M.; Krenke, R. Comparative study of periostin expression in different respiratory samples in patients with asthma and chronic obstructive pulmonary disease. Pol. Arch. Med. Wewn 2016, 126, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Von Scheele, I.; Bergström, J.; Billing, B.; Dahlén, B.; Lantz, A.-S.; Larsson, K.; Palmberg, L. Compartment differences of inflammatory activity in chronic obstructive pulmonary disease. Respir. Res. 2014, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, S.; Suzukawa, M.; Watanabe, K.; Kobayashi, K.; Matsui, H.; Nagai, H.; Nagase, T.; Ohta, K. Secretory immunoglobulin A induces human lung fibroblasts to produce inflammatory cytokines and undergo activation. Clin. Exp. Immunol. 2019, 195, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.M.; Nunes, H.; Juvin, K.; Bertil, S.; Valeyre, D.; Gaussem, P.; Israel-Biet, D. Increase in both angiogenic and angiostatic mediators in patients with idiopathic pulmonary fibrosis. Pathol. Boil. (Paris) 2014, 62, 391–394. [Google Scholar] [CrossRef]
- Barratt, S.L.; Flower, V.A.; Pauling, J.D.; Millar, A.B. VEGF (Vascular Endothelial Growth Factor) and Fibrotic Lung Disease. Int. J. Mol. Sci. 2018, 19, 1269. [Google Scholar] [CrossRef]
- Neighbors, M.; Cabanski, C.R.; Ramalingam, T.R.; Sheng, X.R.; Tew, G.W.; Gu, C.; Jia, G.; Peng, K.; Ray, J.M.; Ley, B.; et al. Prognostic and predictive biomarkers for patients with idiopathic pulmonary fibrosis treated with pirfenidone: Post-hoc assessment of the CAPACITY and ASCEND trials. Lancet Respir. Med. 2018, 6, 615–626. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Otsuka, M.; Chiba, H.; Ikeda, K.; Mori, Y.; Umeda, Y.; Nishikiori, H.; Kuronuma, K.; Takahashi, H. Surfactant protein A as a biomarker of outcomes of anti-fibrotic drug therapy in patients with idiopathic pulmonary fibrosis. BMC Pulm. Med. 2020, 20, 27. [Google Scholar] [CrossRef]
- Majewski, S.; Białas, A.J.; Buchczyk, M.; Gomółka, P.; Górska, K.; Jagielska-Len, H.; Jarzemska, A.; Jassem, E.; Jastrzębski, D.; Kania, A.; et al. A multicentre retrospective observational study on Polish experience of pirfenidone therapy in patients with idiopathic pulmonary fibrosis: The PolExPIR study. BMC Pulm. Med. 2020, 20, 122. [Google Scholar] [CrossRef]
- Cottin, V.; Koschel, D.; Günther, A.; Albera, C.; Azuma, A.; Sköld, C.M.; Tomassetti, S.; Hormel, P.; Stauffer, J.L.; Strombom, I.; et al. Long-term safety of pirfenidone: Results of the prospective, observational PASSPORT study. ERJ Open Res. 2018, 4. [Google Scholar] [CrossRef]
- Richeldi, L.; Du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- Murray, L.; Habiel, D.M.; Hohmann, M.S.; Camelo, A.; Shang, H.; Zhou, Y.; Coelho, A.L.; Peng, X.; Gulati, M.; Crestani, B.; et al. Antifibrotic role of vascular endothelial growth factor in pulmonary fibrosis. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Barratt, S.; Blythe, T.; Jarrett, C.; Ourradi, K.; Shelley-Fraser, G.; Day, M.J.; Qiu, Y.; Harper, S.; Maher, T.M.; Oltean, S.; et al. Differential Expression of VEGF-AxxxIsoforms Is Critical for Development of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 196, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Caminati, A.; Lonati, C.; Cassandro, R.; Elia, D.; Pelosi, G.; Torre, O.; Zompatori, M.; Uslenghi, E.; Harari, S. Comorbidities in idiopathic pulmonary fibrosis: An underestimated issue. Eur. Respir. Rev. 2019, 28. [Google Scholar] [CrossRef] [PubMed]
- Oldham, J.M.; Collard, H.R. Comorbid conditions in idiopathic pulmonary fibrosis: Recognition and management. Front. Med. 2017, 4, 123. [Google Scholar] [CrossRef] [PubMed]
| IPF n = 29 | Controls n = 13 | p | |
|---|---|---|---|
| Male gender | 24 (83%) | 9 (69%) | 0.323 |
| Age (years) | 73 (68–78) | 62 (61–76) | 0.058 |
| BMI (kg/m2) | 28.2 (25.8–29.7) | 27.9 (25.2–30.1) | 0.747 |
| Months since IPF diagnosis | 24 (13–37) | NA | - |
| Months since first symptoms | 23 (10–40) | NA | - |
| GAP score (points) | 4 (2–4) | NA | - |
| GAP stage I/II/III (number) | 12/17/0 | - | |
| Lung function | |||
| FVC (% of predicted) | 81.6 (70.7–101.2) | 119.0 (97.0–123.0) | <0.001 |
| TLC (% of predicted) | 79.3 (71.0–94.5) | NA | - |
| TLCO (% of predicted) | 57.7 (40.0–70.5) | NA | - |
| 6 MWD (meters) | 482.5 (420–520) | NA | - |
| Δ SpO2 in 6 MWT (%) | 9.5 (5–12.5) | NA | - |
| Smoking status | |||
| Current/ex/never smoker (number) | 0/25/4 | 2/7/4 | 0.029 |
| Time since smoking cessation (years) | 23 (15–31) | 28 (20–45) | 0.296 |
| Overall smoking exposure (pack-years) | 20 (12–47) | 10 (5–20) | 0.086 |
| Comorbidities n (%) | |||
| Diabetes mellitus | 12 (41%) | 1 (8%) | 0.024 |
| Arterial hypertension | 14 (48%) | 6 (46%) | 0.899 |
| Ischemic heart disease | 12 (41%) | 2 (15%) | 0.323 |
| Cardiac arrhythmias | 1 (3%) | 3 (23%) | 0.045 |
| GERD | 16 (55%) | 0 | <0.001 |
| At Baseline | After Six Months of Pirfenidone Treatment | p | |
|---|---|---|---|
| BMI (kg/m2) | 28.2 (26.0–29.6) | 26.5 (22.9–29.0) | 0.024 |
| FVC (% of predicted) | 81.4 (66.4–101.9) | 82.5 (67.9–103.7) | 0.393 |
| TLC (% of predicted) | 79.3 (71.4–90.3) | 71.1 (67.9–81.5) | 0.277 |
| TLCO (% of predicted) | 57.7 (40.0–70.5) | 52.2 (39.2–70.1) | 0.974 |
| 6 MWD (meters) | 485.0 (420.0–530.0) | 482.5 (430.0–540.0) | 0.958 |
| ΔSpO2 in 6 MWT | 8.5 (5.5–11.5) | 9.0 (7.0–13.0) | 0.793 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaskiewicz, K.; Mycroft, K.; Maskey-Warzechowska, M.; Paralusz, K.; Siemiez, N.; Nejman-Gryz, P.; Barnas, M.; Krenke, R.; Gorska, K. Exhaled Biomarkers in Idiopathic Pulmonary Fibrosis—A Six-Month Follow-up Study in Patients Treated with Pirfenidone. J. Clin. Med. 2020, 9, 2523. https://doi.org/10.3390/jcm9082523
Jaskiewicz K, Mycroft K, Maskey-Warzechowska M, Paralusz K, Siemiez N, Nejman-Gryz P, Barnas M, Krenke R, Gorska K. Exhaled Biomarkers in Idiopathic Pulmonary Fibrosis—A Six-Month Follow-up Study in Patients Treated with Pirfenidone. Journal of Clinical Medicine. 2020; 9(8):2523. https://doi.org/10.3390/jcm9082523
Chicago/Turabian StyleJaskiewicz, Kaja, Katarzyna Mycroft, Marta Maskey-Warzechowska, Karolina Paralusz, Natalia Siemiez, Patrycja Nejman-Gryz, Malgorzata Barnas, Rafal Krenke, and Katarzyna Gorska. 2020. "Exhaled Biomarkers in Idiopathic Pulmonary Fibrosis—A Six-Month Follow-up Study in Patients Treated with Pirfenidone" Journal of Clinical Medicine 9, no. 8: 2523. https://doi.org/10.3390/jcm9082523
APA StyleJaskiewicz, K., Mycroft, K., Maskey-Warzechowska, M., Paralusz, K., Siemiez, N., Nejman-Gryz, P., Barnas, M., Krenke, R., & Gorska, K. (2020). Exhaled Biomarkers in Idiopathic Pulmonary Fibrosis—A Six-Month Follow-up Study in Patients Treated with Pirfenidone. Journal of Clinical Medicine, 9(8), 2523. https://doi.org/10.3390/jcm9082523






