Adaptation and Validation of the Diabetic Foot Ulcer Scale-Short Form in Spanish Subjects
Abstract
1. Introduction
2. Experimental Section
2.1. Sample and Settings
2.2. Instruments
2.2.1. Diabetic Foot Ulcer Scale-Short Form (DFS-SF)
2.2.2. 36-Item Short-Form Health Survey (SF-36)
2.2.3. EuroQoL 5D Health Utility Index (EQ-5D)
2.3. Transcultural Adaptation of the DFS-SF
2.4. Sample Size
2.5. Data Analysis
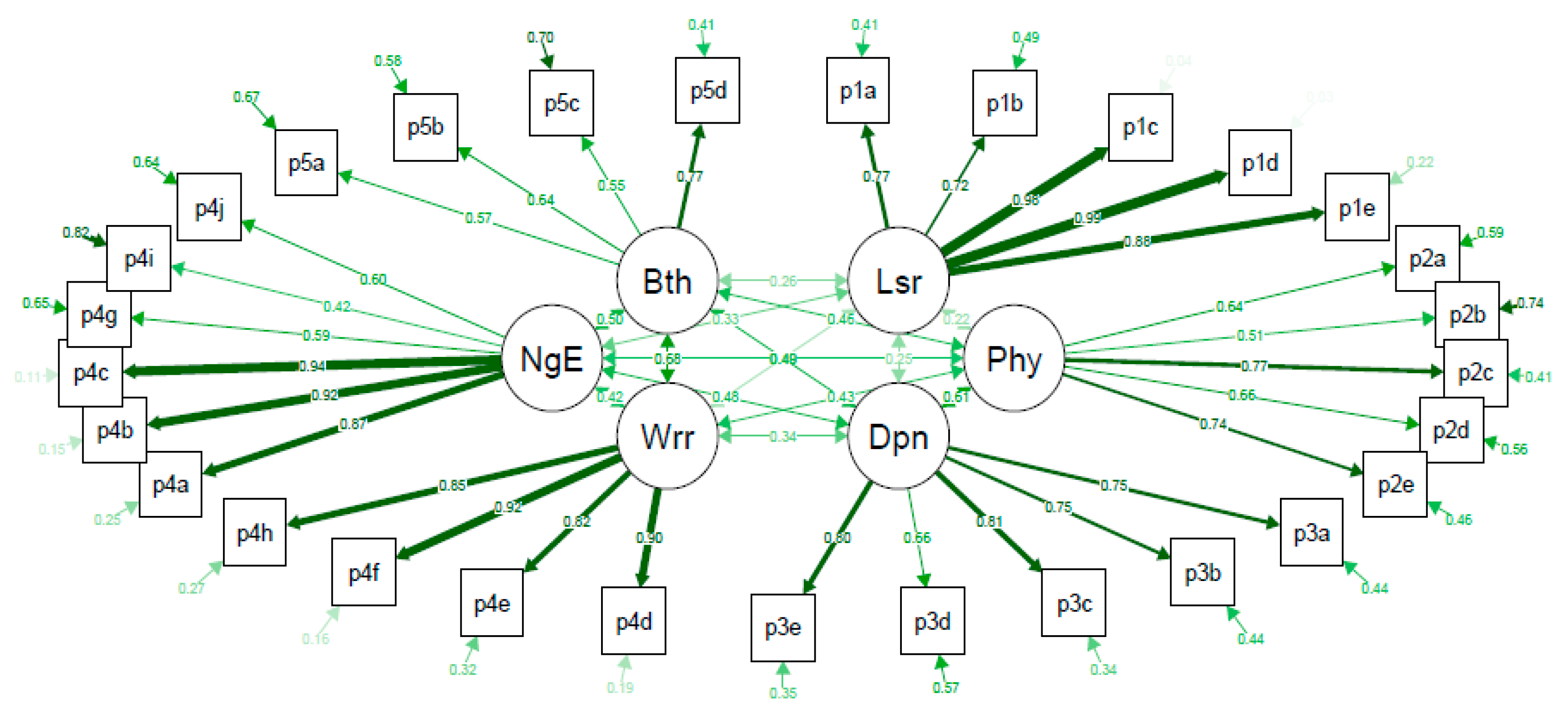
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hogg, F.R.A.; Peach, G.; Price, P.; Thompson, M.M.; Hinchliffe, R.J. Measures of health-related quality of life in diabetes-related foot disease: A systematic review. Diabetologia 2012, 55, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Brownrigg, J.R.W.; Davey, J.; Holt, P.J.; Davis, W.A.; Thompson, M.M.; Ray, K.K.; Hinchliffe, R.J. The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: A meta-analysis. Diabetologia 2012, 55, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Prompers, L.; Schaper, N.; Apelqvist, J.; Edmonds, M.; Jude, E.; Mauricio, D.; Uccioli, L.; Urbancic, V.; Bakker, K.; Holstein, P.; et al. Prediction of outcome in individuals with diabetic foot ulcers: Focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008, 51, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Jupiter, D.C.; Thorud, J.C.; Buckley, C.J.; Shibuya, N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. Int. Wound J. 2016, 13, 892–903. [Google Scholar] [CrossRef]
- Sekhar, M.S.; Thomas, R.R.; Unnikrishnan, M.K.; Vijayanarayana, K.; Rodrigues, G.S. Impact of diabetic foot ulcer on health-related quality of life: A cross-sectional study. Semin. Vasc. Surg. 2015, 28, 165–171. [Google Scholar] [CrossRef]
- Sämann, A.; Tajiyeva, O.; Müller, N.; Tschauner, T.; Hoyer, H.; Wolf, G.; Müller, U.A. Prevalence of the diabetic foot syndrome at the primary care level in Germany: A cross-sectional study. Diabetes Med. 2008, 25, 557–563. [Google Scholar] [CrossRef]
- Spanos, K.; Saleptsis, V.; Athanasoulas, A.; Karathanos, C.; Bargiota, A.; Chan, P.; Giannoukas, A.D. Factors Associated with Ulcer Healing and Quality of Life in Patients with Diabetic Foot Ulcer. Angiology 2017, 68, 242–250. [Google Scholar] [CrossRef]
- Ribu, L.; Rustøen, T.; Birkeland, K.; Hanestad, B.R.; Paul, S.M.; Miaskowski, C. The Prevalence and Occurrence of Diabetic Foot Ulcer Pain and Its Impact on Health-Related Quality of Life. J. Pain 2006, 7, 290–299. [Google Scholar] [CrossRef]
- Valensi, P.; Girod, I.; Baron, F.; Moreau-Defarges, T.; Guillon, P. Quality of life and clinical correlates in patients with diabetic foot ulcers. Diabetes Metab. 2005, 31, 263–271. [Google Scholar] [CrossRef]
- Wukich, D.K.; Raspovic, K.M. Assessing Health-Related Quality of Life in Patients With Diabetic Foot Disease: Why Is It Important and How Can We Improve? The 2017 Roger E. Pecoraro Award Lecture. Diabetes Care 2018, 41, 391–397. [Google Scholar] [CrossRef]
- Boutoille, D.; Féraille, A.; Maulaz, D.; Krempf, M. Quality of life with diabetes-associated foot complications: Comparison between lower-limb amputation and chronic foot ulceration. Foot Ankle Int. 2008, 29, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Abetz, L.; Sutton, M.; Brady, L.; McNulty, P.; Gagnon, D.D. The Diabetic Foot Ulcer Scale (DFS): A quality of life instrument for use in clinical trials. Pract. Diabetes Int. 2002, 19, 167–175. [Google Scholar] [CrossRef]
- Bann, C.M.; Fehnel, S.E.; Gagnon, D.D. Development and Validation of the Diabetic Foot Ulcer Scale-Short Form (DFS-SF). Pharmacoeconomics 2003, 21, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.F.; Yee-Tak Fong, D.; Yam, M.; Yuk Ip, W. Translation and validation of the chinese diabetic foot ulcer scale - short form. Patient 2008, 1, 137–145. [Google Scholar] [CrossRef]
- Kontodimopoulos, N.; Veniou, A.; Tentolouris, N.; Niakas, D. Validity and reliability of the greek version of the diabetic foot ulcer scale-short form (DFS-SF). Hormones 2016, 15, 394–403. [Google Scholar] [CrossRef]
- Macioch, T.; Sobol, E.; Krakowiecki, A.; Mrozikiewicz-Rakowska, B.; Kasprowicz, M.; Hermanowski, T. Health related quality of life in patients with diabetic foot ulceration - translation and Polish adaptation of Diabetic Foot Ulcer Scale short form. Health Qual. Life Outcomes 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Bakker, K.; Apelqvist, J.; Lipsky, B.A.; Van Netten, J.J.; Schaper, N.C. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: Development of an evidence-based global consensus. Diabetes. Metab. Res. Rev. 2016, 32, 2–6. [Google Scholar] [CrossRef]
- Dòria, M.; Rosado, V.; Pacheco, L.R.; Hernández, M.; Betriu, À.; Valls, J.; Franch-Nadal, J.; Fernández, E.; Mauricio, D. Prevalence of diabetic foot disease in patients with diabetes mellitus under renal replacement therapy in Lleida, Spain. Biomed Res. Int. 2016, 2016, 7217586. [Google Scholar] [CrossRef]
- Alcubierre, N.; Rubinat, E.; Traveset, A.; Martinez-Alonso, M.; Hernandez, M.; Jurjo, C.; Mauricio, D. A prospective cross-sectional study on quality of life and treatment satisfaction in type 2 diabetic patients with retinopathy without other major late diabetic complications. Health Qual. Life Outcomes 2014, 12, 131. [Google Scholar] [CrossRef]
- Doria, M.; Viadé, J.; Palomera, E.; Pérez, R.; Lladó, M.; Costa, E.; Huguet, T.; Reverter, J.L.; Serra-Prat, M.; Franch-Nadal, J.; et al. Short-term foot complications in Charcot neuroarthropathy: A retrospective study in tertiary care centres in Spain treatment. Endocrinol. Diabetes y Nutr. 2018, 65, 479–485. [Google Scholar] [CrossRef]
- Hinchliffe R., J.; Brownrigg, J.R.W.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Mills, J.L.; Reekers, J.; Shearman, C.P.; Zierler, R.E.; Schaper, N.C.; et al. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab. Res. Rev. 2016, 32, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.; Kono, S.; Lavery, L.; Senneville, E.; Urbančič-Rovan, V.; Van Asten, S.; Peters, E.J.G.; et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab. Res. Rev. 2016, 32, 45–74. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.; Snow, K.; Kosinski, M.; Gandek, B. SF-36 Health Survey Manual and Interpretation Guide; The Health Institute, New England Medical Center: Boston, MA, USA, 1993; pp. 1–29. [Google Scholar]
- Ragnarson, G.; Apelqvist, J. Health-related quality of life in patients with diabetes mellitus and foot ulcers. J. Diabetes Complicat. 2000, 14, 235–241. [Google Scholar] [CrossRef]
- Cronbach, L.J. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 297–334. [Google Scholar] [CrossRef]
- Liang, M.H.; Lew, R.A.; Stucki, G.; Fortin, P.R.; Daltroy, L. Measuring clinically important changes with patient-oriented questionnaires. Med. Care 2002, 40, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.H. Longitudinal construct validity: Establishment of clinical meaning in patient evaluative instruments. Med. Care 2000, 38, 84–90. [Google Scholar] [CrossRef]
- R Core Team R: A language and environment for statistical computing. Available online: https://www.r-project.org (accessed on 3 June 2020).
- Ribu, L.; Birkeland, K.; Hanestad, B.R.; Moum, T.; Rustoen, T. A longitudinal study of patients with diabetes and foot ulcers and their health-related quality of life: Wound healing and quality-of-life changes. J. Diabetes Complicat. 2008, 22, 400–407. [Google Scholar] [CrossRef]
| Characteristics | Study Group (n = 141) |
|---|---|
| Age (years) | 68.3 (13.3) |
| Male (sex) | 95 (67.4) |
| Race (Caucasian) | 140 (99.3) |
| Educational level | |
| Not even primary | 57 (40.4) |
| Complete primary | 47 (33.3) |
| Secondary high cycle | 28 (19.9) |
| Graduate or higher | 9 (6.4) |
| Employed | 24 (17.0) |
| Smoking | |
| Never | 63 (44.7) |
| Current or former | 78 (55.4) |
| Type 2 diabetes | 134 (95.0) |
| BMI (kg/m2) | 29.0 (4.9) |
| HbA1c (%) | 7.5 (1.6) |
| Hypertension | 116 (82.3) |
| Dyslipidemia | 87 (61.7) |
| Microvascular complications | |
| Retinopathy | 96 (68.1) |
| Nephropathy | 51 (36.2) |
| Neuropathy | 131 (92.9) |
| Cardiovascular disease 1 | 126 (89.4) |
| Diabetes therapy | |
| OAD | 41 (29.1) |
| OAD + insulin | 57 (40.4) |
| Insulin | 36 (25.5) |
| Diet | 7 (5.0) |
| Antiplatelet agents | 94 (66.7) |
| Dialysis | 8 (5.7) |
| Ulcer type | |
| Neuropathic | 87 (61.7) |
| Ischemic | 9 (6.4) |
| Neuroischemic | 45 (31.9) |
| Infected ulcer | 83 (58.9) |
| Previous amputation | |
| Minor | 41 (29.1) |
| Major | 2 (1.4) |
| Charcot foot disease | 9 (6.4) |
| Dfs-Sf Subscales | Number of Items 1 | Range of Correlations 2 | Average Inter-Item Correlation | Cronbach’s Alpha | Reproducibility (ICC [95%CI]) |
|---|---|---|---|---|---|
| Leisure | 5 | 0.678–0.966 | 0.786 | 0.948 | 0.87 [0.82, 0.91] |
| Physical health | 5 | 0.195–0.641 | 0.433 | 0.792 3 | 0.78 [0.70, 0.84] |
| Worried about ulcers/feet | 4 | 0.678–0.818 | 0.760 | 0.927 | 0.92 [0.89, 0.94] |
| Dependence/daily life | 5 | 0.417–0.662 | 0.566 | 0.867 | 0.77 [0.68, 0.83] |
| Negative emotions | 6 | 0.310–0.886 | 0.553 | 0.881 4 | 0.84 [0.79, 0.89] |
| Bothered by ulcer care | 4 | 0.304–0.537 | 0.391 | 0.720 | 0.77 [0.69, 0.83] |
| Domains. | DFS-SF Subscales | |||||
|---|---|---|---|---|---|---|
| Leisure | Physical Health | Dependence/Daily Life | Worried about Ulcers/Feet | Negative Emotions | Bothered by Ulcer Care | |
| SF-36 subscales | ||||||
| Physical functioning | 0.052 | 0.473 ** | 0.737 ** | 0.220 * | 0.398 ** | 0.370 ** |
| Role physical | 0.135 | 0.413 ** | 0.558 ** | 0.258 * | 0.445 ** | 0.376 ** |
| Bodily pain | 0.116 | 0.438 ** | 0.403 ** | 0.224 * | 0.395 ** | 0.277 * |
| General health | 0.057 | 0.165 | 0.139 | 0.183 * | 0.350 ** | 0.198 * |
| Vitality | 0.126 | 0.327 ** | 0.418 ** | 0.245 * | 0.383 ** | 0.345 ** |
| Social functioning | 0.358 ** | 0.401 ** | 0.453 ** | 0.321 ** | 0.526 ** | 0.336 ** |
| Role emotional | 0.140 | 0.271 ** | 0.221 * | 0.187 * | 0.365 ** | 0.288 * |
| Mental health | 0.079 | 0.204 * | 0.174 * | 0.256 * | 0.484 ** | 0.199 * |
| Overall physical component 1 | 0.082 | 0.482 ** | 0.674 ** | 0.229 * | 0.394 ** | 0.368 ** |
| Overall mental component 2 | 0.178 * | 0.195 * | 0.110 | 0.248 * | 0.449 ** | 0.235 * |
| EQ-5D subscales | ||||||
| VAS | 0.062 | 0.166 * | 0.223 * | 0.240 * | 0.306 ** | 0.183 * |
| EQ-5D index value | 0.039 | 0.445 ** | 0.454 ** | 0.204 * | 0.324 ** | 0.184 * |
| Variables | Unhealed 1 (n = 34) | Healed (n = 107) | p-Overall |
|---|---|---|---|
| Change in DFS Leisure from baseline | 0.00 [0.00; 8.75] | 5.00 [0.00; 25.00] | 0.014 |
| Change in DFS Physical Health from baseline | 0.00 [−10.00; 0.00] | 20.00 [5.00; 30.00] | <0.001 |
| Change in DFS Dependence from baseline | 0.00 [0.00; 3.75] | 10.00 [0.00; 25.00] | <0.001 |
| Change in DFS Negative Emotions from baseline | 0.00 [0.00; 4.17] | 8.34 [0.00; 25.00] | 0.001 |
| Change in DFS Worried about ulcers/feet from baseline | 0.00 [0.00; 18.80] | 31.2 [9.38; 50.00] | <0.001 |
| Change in DFS Bothered by ulcer care from baseline | 0.00 [0.00; 6.25] | 18.80 [0.00; 37.50] | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Gonzalez, D.; Dòria, M.; Martínez-Alonso, M.; Alcubierre, N.; Valls, J.; Verdú-Soriano, J.; Granado-Casas, M.; Mauricio, D. Adaptation and Validation of the Diabetic Foot Ulcer Scale-Short Form in Spanish Subjects. J. Clin. Med. 2020, 9, 2497. https://doi.org/10.3390/jcm9082497
Martinez-Gonzalez D, Dòria M, Martínez-Alonso M, Alcubierre N, Valls J, Verdú-Soriano J, Granado-Casas M, Mauricio D. Adaptation and Validation of the Diabetic Foot Ulcer Scale-Short Form in Spanish Subjects. Journal of Clinical Medicine. 2020; 9(8):2497. https://doi.org/10.3390/jcm9082497
Chicago/Turabian StyleMartinez-Gonzalez, Dolores, Montserrat Dòria, Montserrat Martínez-Alonso, Nuria Alcubierre, Joan Valls, José Verdú-Soriano, Minerva Granado-Casas, and Didac Mauricio. 2020. "Adaptation and Validation of the Diabetic Foot Ulcer Scale-Short Form in Spanish Subjects" Journal of Clinical Medicine 9, no. 8: 2497. https://doi.org/10.3390/jcm9082497
APA StyleMartinez-Gonzalez, D., Dòria, M., Martínez-Alonso, M., Alcubierre, N., Valls, J., Verdú-Soriano, J., Granado-Casas, M., & Mauricio, D. (2020). Adaptation and Validation of the Diabetic Foot Ulcer Scale-Short Form in Spanish Subjects. Journal of Clinical Medicine, 9(8), 2497. https://doi.org/10.3390/jcm9082497







