Adaptive Designs: Lessons for Inflammatory Bowel Disease Trials
Abstract
1. Introduction
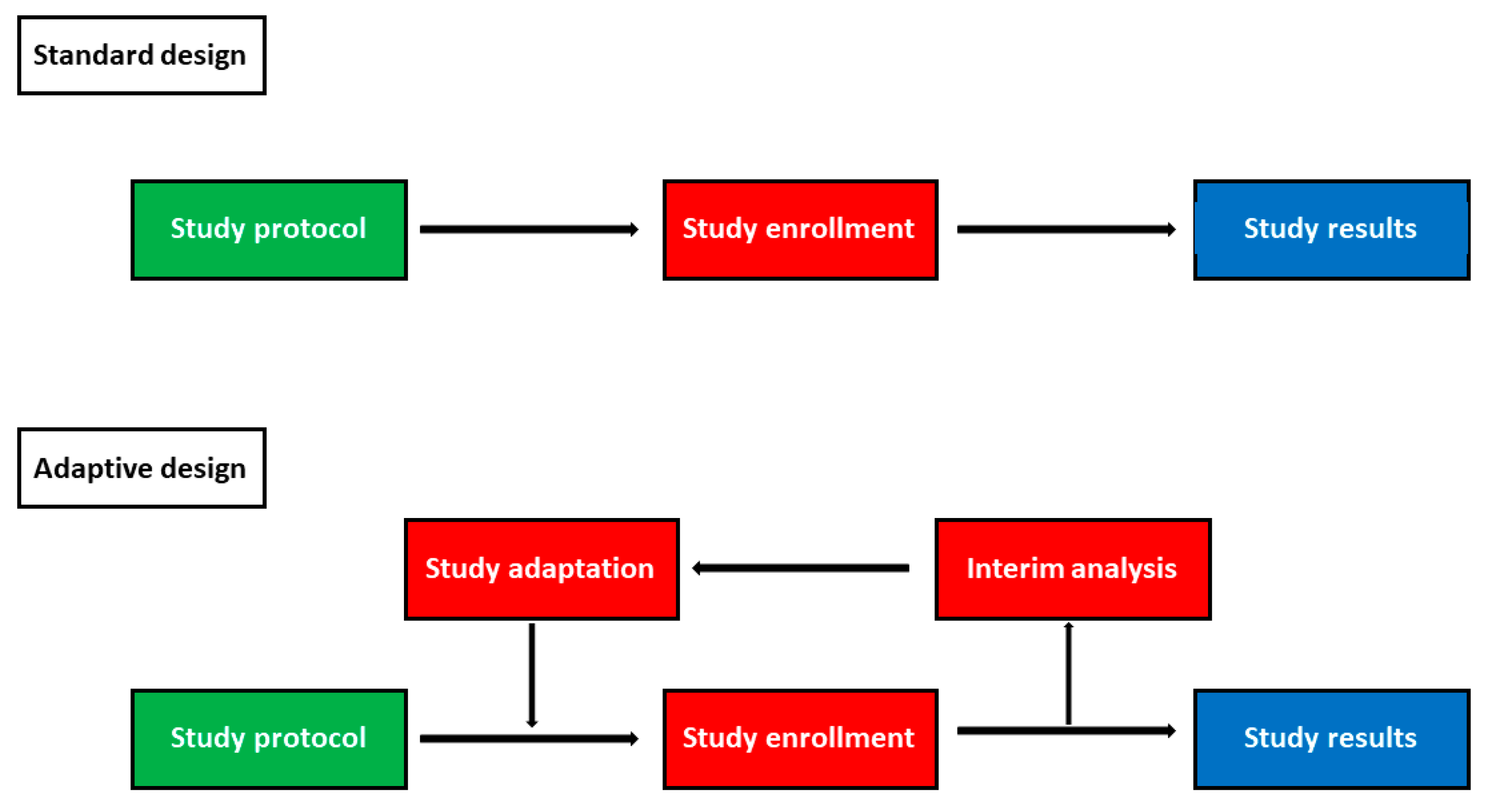
2. Types of Adaptive Designs
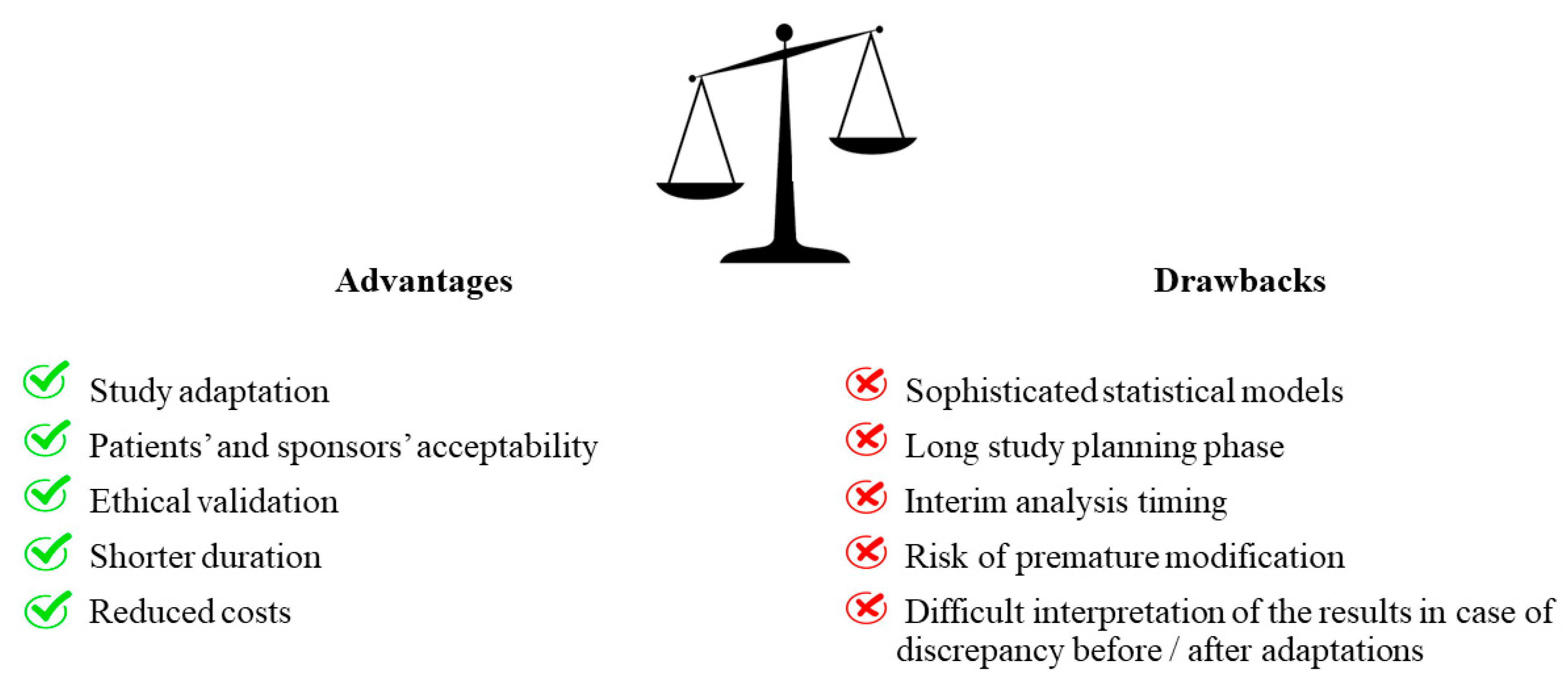
3. Advantages and Drawbacks of Adaptive Designs
4. Evolution of Adaptive Designs: the Regulatory Authorities’ Point of View
5. Lessons from Non-IBD Trials
6. Where Are We in IBD?
7. Outlook
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhatt, D.L.; Mehta, C. Adaptive Designs for Clinical Trials. N. Engl. J. Med. 2016, 375, 65–74. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Baumann, C.; Rousseau, H.; Danese, S.; Peyrin-Biroulet, L. Phase 1, 2 and 3 trials in inflammatory bowel diseases: A practical guide for the non-specialist. J. Crohn’s Colitis 2020. [Google Scholar] [CrossRef]
- Chudy-Onwugaje, K.O.; Christian, K.E.; Farraye, F.A.; Cross, R.K. A State-of-the-Art Review of New and Emerging Therapies for the Treatment of IBD. Inflamm. Bowel Dis. 2019, 25, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Cesana, B.M.; Biganzoli, E.M. Phase IV Studies: Some Insights, Clarifications, and Issues. Curr. Clin. Pharmacol. 2018, 13, 14–20. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Feagan, B.G.; Lichtenstein, G.R.; Zhang, H.; Strauss, R.; Szapary, P.; Johanns, J.; Panés, J.; Vermeire, S.; et al. Peficitinib, an Oral Janus Kinase Inhibitor, in Moderate-to-severe Ulcerative Colitis: Results From a Randomised, Phase 2 Study. J. Crohn’s Colitis 2018, 12, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Colombel, J.-F.; Sands, B.E.; Rutgeerts, P.; Targan, S.R.; Panaccione, R.; Bressler, B.; Geboes, K.; Schreiber, S.; Aranda, R.; et al. Abatacept for Crohn’s disease and ulcerative colitis. Gastroenterology 2012, 143, 62–69.e4. [Google Scholar] [CrossRef]
- Bloomgren, G.; Richman, S.; Hotermans, C.; Goelz, S.; Natarajan, A.; Scanlon, J.V.; Sandrock, A.; Bozic, C.; Subramanyam, M.; Lee, S.; et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N. Engl. J. Med. 2012, 366, 1870–1880. [Google Scholar] [CrossRef]
- Schuhmacher, A.; Gassmann, O.; Hinder, M. Changing R&D models in research-based pharmaceutical companies. J. Transl. Med. 2016, 14, 105. [Google Scholar]
- Gallo, P.; Chuang-Stein, C.; Dragalin, V.; Gaydos, B.; Krams, M.; Pinheiro, J. Adaptive designs in clinical drug development--an Executive Summary of the PhRMA Working Group. J. Biopharm. Stat. 2006, 16, 275–283. [Google Scholar] [CrossRef]
- Bothwell, L.E.; Avorn, J.; Khan, N.F.; Kesselheim, A.S. Adaptive design clinical trials: A review of the literature and ClinicalTrials.gov. BMJ Open 2018, 8, e018320. [Google Scholar] [CrossRef]
- Chow, S.-C. Adaptive clinical trial design. Annu. Rev. Med. 2014, 65, 405–415. [Google Scholar] [CrossRef]
- Bothwell, L.E.; Kesselheim, A.S. The Real-World Ethics of Adaptive-Design Clinical Trials. Hastings Cent. Rep. 2017, 47, 27–37. [Google Scholar] [CrossRef]
- Bent, R. Adaptive Designs for Clinical Trials of Drugs and Biologics. Available online: https://www.fda.gov/media/78495/download (accessed on 23 May 2020).
- Cox, E.; Borio, L.; Temple, R. Evaluating Ebola therapies—The case for RCTs. N. Engl. J. Med. 2014, 371, 2350–2351. [Google Scholar] [CrossRef]
- Sanchez-Kam, M.; Gallo, P.; Loewy, J.; Menon, S.; Antonijevic, Z.; Christensen, J.; Chuang-Stein, C.; Laage, T. A Practical Guide to Data Monitoring Committees in Adaptive Trials. Ther. Innov. Regul. Sci. 2014, 48, 316–326. [Google Scholar] [CrossRef]
- Miller, E.; Gallo, P.; He, W.; Kammerman, L.A.; Koury, K.; Maca, J.; Jiang, Q.; Walton, M.K.; Wang, C.; Woo, K.; et al. DIA’s Adaptive Design Scientific Working Group (ADSWG): Best Practices Case Studies for “Less Well-understood” Adaptive Designs. Ther. Innov. Regul. Sci. 2017, 51, 77–88. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Research C for DE and Adaptive Design Clinical Trials for Drugs and Biologics Guidance for Industry. 2019. Available online: http://www.fda.gov/regulatory-information/search-fda-guidance-documents/adaptive-design-clinical-trials-drugs-and-biologics-guidance-industry (accessed on 23 February 2020).
- Kola, I.; Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004, 3, 711–715. [Google Scholar] [CrossRef]
- Collignon, O.; Koenig, F.; Koch, A.; Hemmings, R.J.; Pétavy, F.; Saint-Raymond, A.; Papaluca-Amati, M.; Posch, M. Adaptive designs in clinical trials: From scientific advice to marketing authorisation to the European Medicine Agency. Trials 2018, 19, 642. [Google Scholar] [CrossRef]
- Sato, A.; Shimura, M.; Gosho, M. Practical characteristics of adaptive design in phase 2 and 3 clinical trials. J. Clin. Pharm. Ther. 2018, 43, 170–180. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; Hoeger, P.; Mazereeuw-Hautier, J.; Guibaud, L.; Baselga, E.; Posiunas, G.; Phillips, R.J.; Cáceres, H.; Gutiérrez, J.C.L.; Ballona, R.; et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N. Engl. J. Med. 2015, 372, 735–746. [Google Scholar] [CrossRef]
- Rugo, H.S.; Olopade, O.I.; DeMichele, A.; Yau, C.; Veer, L.J.V.T.; Buxton, M.B.; Hogarth, M.; Hylton, N.M.; Paoloni, M.; Perlmutter, J.; et al. Adaptive Randomization of Veliparib-Carboplatin Treatment in Breast Cancer. N. Engl. J. Med. 2016, 375, 23–34. [Google Scholar] [CrossRef]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Öhman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef]
- Kapur, J.; Elm, J.; Chamberlain, J.M.; Barsan, W.; Cloyd, J.; Lowenstein, D.; Shinnar, S.; Conwit, R.; Meinzer, C.; Cock, H.; et al. Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus. N. Engl. J. Med. 2019, 381, 2103–2113. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Stone, G.W.; Mahaffey, K.W.; Gibson, C.M.; Steg, P.G.; Hamm, C.; Price, M.; Leonardi, S.; Gallup, D.; Bramucci, E.; et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N. Engl. J. Med. 2013, 368, 1303–1313. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.-F.; Reinisch, W.; et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 85–95. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.-F.; Reinisch, W.; et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 96–109.e1. [Google Scholar] [CrossRef]
- Kay, J.; Matteson, E.L.; Dasgupta, B.; Nash, P.; Durez, P.; Hall, S.; Hsia, E.C.; Han, J.; Wagner, C.; Xu, Z.; et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: A randomized, double-blind, placebo-controlled, dose-ranging study. Arthr. Rheum. 2008, 58, 964–975. [Google Scholar] [CrossRef]
- Keystone, E.; Genovese, M.C.; Klareskog, L.; Hsia, E.; Hall, S.; Miranda, P.C.; Pazdur, J.; Bae, S.; Palmer, W.; Xu, S.; et al. Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: 52-week results of the GO-FORWARD study. Ann. Rheum. Dis. 2010, 69, 1129–1135. [Google Scholar] [CrossRef]
- Pantavou, K.; Yiallourou, A.I.; Piovani, D.; Evripidou, D.; Danese, S.; Peyrin-Biroulet, L.; Bonovas, S.; Nikolopoulos, G.K. Efficacy and safety of biologic agents and tofacitinib in moderate-to-severe ulcerative colitis: A systematic overview of meta-analyses. United Eur. Gastroenterol. J. 2019, 7, 1285–1303. [Google Scholar] [CrossRef]
- Singh, S. Evolution of Clinical Trials in Inflammatory Bowel Diseases. Curr. Gastroenterol. Rep. 2018, 20, 41. [Google Scholar] [CrossRef]
- Danese, S.; Schabel, E.; Ainsworth, M.A.; Peyrin-Biroulet, L. Challenges and opportunities for IBD drug development: From early stage to regulatory approval. Gut 2020, 69, 1157–1161. [Google Scholar] [CrossRef]
| Adaptive Designs | Main Characteristic |
|---|---|
| Adaptive dose-finding design | To modify patients’ allocations to identify the optimal drug dosage |
| Adaptive hypothesis designs | To modify the study hypothesis (e.g., non-inferiority, superiority) or endpoint (primary and/or secondary) |
| Adaptive group sequential design | To modify dose or treatment duration |
| Sample size re-estimation design | To re-estimate the sample size |
| Adaptive treatment-switching design | To switch a patient from a treatment group to another due to loss of response to initial therapy |
| Adaptive randomization design | To adjust randomization schedules increasing the number of patients randomized to the most beneficial groups |
| Pick-the-winner/drop-the loser design | To modify the study arms removing less effective ones or adding groups that seem effective |
| Biomarker adaptive design | To adapt patient allocation based on treatment response to biomarkers |
| Seamless phase II/III design | To combine phases II and III of clinical trials. |
| Multiple adaptive design | To combine multiple adaptive designs in the same study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D'Amico, F.; Danese, S.; Peyrin-Biroulet, L. Adaptive Designs: Lessons for Inflammatory Bowel Disease Trials. J. Clin. Med. 2020, 9, 2350. https://doi.org/10.3390/jcm9082350
D'Amico F, Danese S, Peyrin-Biroulet L. Adaptive Designs: Lessons for Inflammatory Bowel Disease Trials. Journal of Clinical Medicine. 2020; 9(8):2350. https://doi.org/10.3390/jcm9082350
Chicago/Turabian StyleD'Amico, Ferdinando, Silvio Danese, and Laurent Peyrin-Biroulet. 2020. "Adaptive Designs: Lessons for Inflammatory Bowel Disease Trials" Journal of Clinical Medicine 9, no. 8: 2350. https://doi.org/10.3390/jcm9082350
APA StyleD'Amico, F., Danese, S., & Peyrin-Biroulet, L. (2020). Adaptive Designs: Lessons for Inflammatory Bowel Disease Trials. Journal of Clinical Medicine, 9(8), 2350. https://doi.org/10.3390/jcm9082350






