A Cross-Sectional Retrospective Study of Non-Splenectomized and Never-Treated Patients with Type 1 Gaucher Disease
Abstract
1. Introduction
2. Patients and Methods
2.1. The French Gaucher Disease Registry (FGDR)
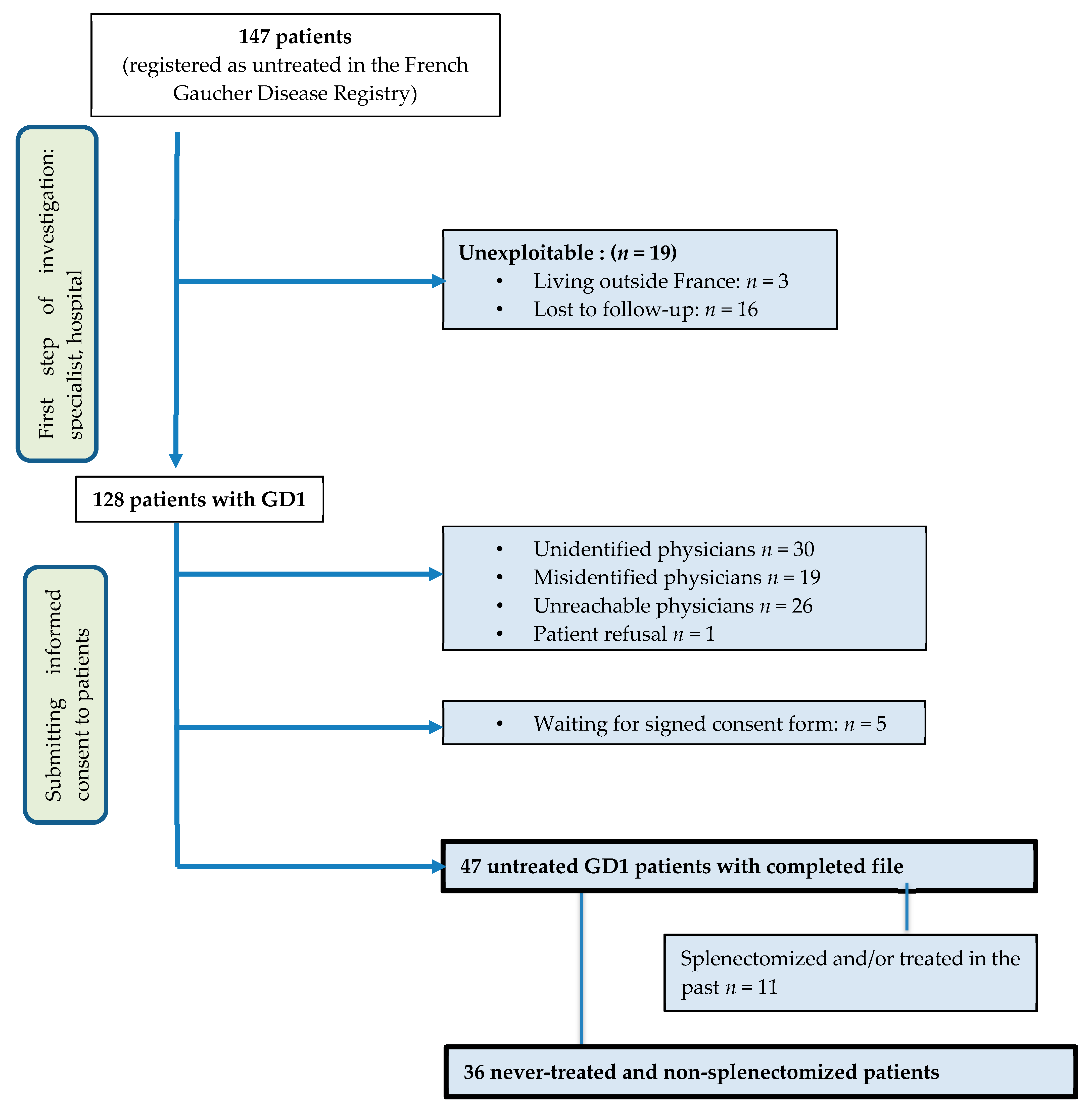
2.2. Patients’ Selection
2.3. Statistical Analyses
2.4. Data Analyzed
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brady, R.O.; Kanfer, J.N.; Shapiro, D. Metabolism of Glucocerebrosides. II. Evidence of an Enzymatic Deficiency in Gaucher’s Disease. Biochem. Biophys. Res. Commun. 1965, 18, 221–225. [Google Scholar] [CrossRef]
- Tamargo, R.J.; Velayati, A.; Goldin, E.; Sidransky, E. The role of saposin C in Gaucher disease. Mol. Genet. Metab. 2012, 106, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Stirnemann, J.; Vigan, M.; Hamroun, D.; Heraoui, D.; Rossi-Semerano, L.; Berger, M.G.; Rose, C.; Camou, F.; De Roux-Serratrice, C.; Grosbois, B.; et al. The French Gaucher’s disease registry: Clinical characteristics, complications and treatment of 562 patients. Orphanet J. Rare Dis. 2012, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.S.; Topol, J.; Kolodny, E.H. Leukocyte β-glucosidase in homozygotes and heterozygotes for Gaucher disease. Am. J. Hum. Genet. 1980, 32, 158–173. [Google Scholar]
- Mistry, P.K.; Belmatoug, N.; Dahl, S.V.; Giugliani, R. Understanding the natural history of Gaucher disease. Am. J. Hematol. 2015, 90, S6–S11. [Google Scholar] [CrossRef]
- Arends, M.; Van Dussen, L.; Biegstraaten, M.; Hollak, C.E.M. Malignancies and monoclonal gammopathy in Gaucher disease; a systematic review of the literature. Br. J. Haematol. 2013, 161, 832–842. [Google Scholar] [CrossRef]
- Rosenbloom, B.; Balwani, M.; Bronstein, J.M.; Kolodny, E.; Sathe, S.; Gwosdow, A.R.; Taylor, J.S.; Cole, J.A.; Zimran, A.; Weinreb, N.J. The incidence of Parkinsonism in patients with type 1 Gaucher disease: Data from the ICGG Gaucher Registry. Blood Cells Mol. Dis. 2011, 46, 95–102. [Google Scholar] [CrossRef]
- Revel-Vilk, S.; Szer, J.; Mehta, A.; Zimran, A. How we manage Gaucher Disease in the era of choices. Br. J. Haematol. 2018, 182, 467–480. [Google Scholar] [CrossRef]
- Dinur, T.; Zimran, A.; Becker-Cohen, M.; Arkadir, D.; Cozma, C.; Hovakimyan, M.; Oppermann, S.; DeMuth, L.; Rolfs, A.; Revel-Vilk, S.; et al. Long Term Follow-Up of 103 Untreated Adult Patients with Type 1 Gaucher Disease. J. Clin. Med. 2019, 8, 1662. [Google Scholar] [CrossRef]
- National Diagnosis and Treatment Protocol-Protocole National de Diagnostic et de Soins. Available online: http://wwwhas-santefr/portail/jcms/c_2580600/fr/maladie-de-gaucher (accessed on 11 December 2015).
- Zimran, A.; Kay, A.; Gelbart, T.; Garver, P.; Thurston, D.; Saven, A.; Beutler, E. Gaucher disease. Clinical, laboratory, radiologic, and genetic features of 53 patients. Medicine (Baltimore) 1992, 71, 337–353. [Google Scholar] [CrossRef]
- Beutler, E.; Demina, A.; Laubscher, K.; Garver, P.; Gelbart, T.; Balicki, D.; Vaughan, L. The Clinical Course of Treated and Untreated Gaucher Disease. A Study of 45 Patients. Blood Cells Mol. Dis. 1995, 21, 86–108. [Google Scholar] [CrossRef] [PubMed]
- Ida, H.; Rennert, O.M.; Ito, T.; Maekawa, K.; Eto, Y. Type 1 Gaucher Disease: Phenotypic Expression and Natural History in Japanese Patients. Blood Cells Mol. Dis. 1998, 24, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Maaswinkel-Mooij, P.; Hollak, C.; Van Eysden-Plaisier, M.; Prins, M.; Aerts, J.M.G.; Pöll, R. The natural course of Gaucher disease in The Netherlands: Implications for monitoring of disease manifestations. J. Inherit. Metab. Dis. 2000, 23, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Piran, S.; Roberts, A.; Patterson, M.A.; Amato, D. The clinical course of untreated Gaucher disease in 22 patients over 10 years: Hematological and skeletal manifestations. Blood Cells Mol. Dis. 2009, 43, 289–293. [Google Scholar] [CrossRef]
- Boomsma, J.M.; Van Dussen, L.; Wiersma, M.G.; Groener, J.E.; Aerts, J.M.G.; Maas, M.; Hollak, C.E.M. Spontaneous regression of disease manifestations can occur in type 1 Gaucher disease; results of a retrospective cohort study. Blood Cells Mol. Dis. 2010, 44, 181–187. [Google Scholar] [CrossRef]
- Caubel, I.; De Villemeur, T.B.; Belmatoug, N. Gaucher’s disease in children: First clinical signs, natural course and benefits of enzyme replacement therapy. Arch. Pédiatr. 2003, 10, 681–688. [Google Scholar] [CrossRef]
- Amato, D. Gaucher Disease in Ontario, Canada: Clinical Manifestations, Natural Progression, and Treatment Response. J. Rare Dis. Res. Treat. 2018, 3, 7–16. [Google Scholar] [CrossRef]
- Weinreb, N.J.; Barbouth, D.S.; Lee, R.E. Causes of death in 184 patients with type 1 Gaucher disease from the United States who were never treated with enzyme replacement therapy. Blood Cells Mol. Dis. 2018, 68, 211–217. [Google Scholar] [CrossRef]
- WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. 2011. Available online: https://wwwwhoint/vmnis/indicators/haemoglobinpdf (accessed on 11 February 2020).
- Kishnani, P.; DiRocco, M.; Kaplan, P.; Mehta, A.; Pastores, G.; Smith, S.; Puga, A.; Lemay, R.; Weinreb, N. A randomized trial comparing the efficacy and safety of imiglucerase (Cerezyme) infusions every 4 weeks versus every 2 weeks in the maintenance therapy of adult patients with Gaucher disease type 1. Mol. Genet. Metab. 2009, 96, 164–170. [Google Scholar] [CrossRef]
- Alzheimer, A.F. 2020. Available online: https://www.francealzheimer.org/maladie-dalzheimer-maladies-apparentees-similitudes-differences/maladie-a-corps-de-lewy/ (accessed on 15 February 2020).
- Weinreb, N.J.; Deegan, P.; Kacena, K.A.; Mistry, P.; Pastores, G.M.; Velentgas, P.; Dahl, S.V. Life expectancy in Gaucher disease type 1. Am. J. Hematol. 2008, 83, 896–900. [Google Scholar] [CrossRef]
- Van Dussen, L.; Biegstraaten, M.; Dijkgraaf, M.G.; Hollak, C.E. Modelling Gaucher disease progression: Long-term enzyme replacement therapy reduces the incidence of splenectomy and bone complications. Orphanet J. Rare Dis. 2014, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Balwani, M.; Fuerstman, L.; Kornreich, R.; Edelmann, L.; Desnick, R.J. Type 1 Gaucher Disease. Arch. Intern. Med. 2010, 170, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Zion, Y.C.; Pappadopulos, E.; Wajnrajch, M.; Rosenbaum, H. Rethinking fatigue in Gaucher disease. Orphanet J. Rare Dis. 2016, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Roca-Espiau, M.; Andrade-Campos, M.; Cebolla, J.J.; De Frutos, L.L.; Medrano-Engay, B.; López-Royo, M.-P.; Giraldo, P. Muscle-tendon weakness contributes to chronic fatigue syndrome in Gaucher’s disease. J. Orthop. Surg. Res. 2019, 14, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, P.; Baris, H.; De Meirleir, L.; Di Rocco, M.; El-Beshlawy, A.; Huemer, M.; Martins, A.M.; Naşcu, I.; Rohrbach, M.; Steinbach, L.; et al. Revised recommendations for the management of Gaucher disease in children. Eur. J. Nucl. Med. Mol. Imaging 2012, 172, 447–458. [Google Scholar] [CrossRef]
- Sisodiya, S.M.; Weil, R.S.; Bresner, C.; Lawton, M.A.; Grosset, K.; Tan, M.; Bajaj, N.; Barker, R.A.; Burn, D.J.; Foltynie, T.; et al. Features of GBA-associated Parkinson’s disease at presentation in the UK Tracking Parkinson’s study. J. Neurol. Neurosurg. Psychiatry 2018, 89, 702–709. [Google Scholar] [CrossRef]
- Lopez, G.; Steward, A.; Ryan, E.; Groden, C.; Wiggs, E.; Segalà, L.; Monestime, G.M.; Tayebi, N.; Sidransky, E. Clinical Evaluation of Sibling Pairs With Gaucher Disease Discordant for Parkinsonism. Mov. Disord. 2019, 35, 359–365. [Google Scholar] [CrossRef]
| Never-Treated and Non-Splenectomized Patients with GD1, n = 36 | |
|---|---|
| Median age at first symptoms | 17.5 (0–59) |
| Median age at diagnosis | 24.8 (0.1–72.1) |
| Median age at the last evaluation | 36.6 (2.4–75.1) |
| Median time between diagnosis and last evaluation (years) | 7.8 (0.4–32.4) |
| Sex ratio F/M | 19/17 |
| GBA1 genotype N370S/N370S (%) N370S/other (non L444P) (%) L444P/N370S (%) L444P/other (%) Other/Other (%) | n = 3 17/31 (22.6) 12/31 (38.7) 5/31 (16) 2/31 (6) 5/31 (16.1) |
| Conditions leading to diagnosis (several possibilities) Splenomegaly (%) Thrombocytopenia (%) Family screening (%) Unknown (%) | 47 42 16 14 |
| Clinical Parameters | Diagnosis | Last Evaluation | Biological Parameters | Slope | p-Value |
|---|---|---|---|---|---|
| Asthenia | 6/34 (17.6%) | 2/34 (6%) | Hemoglobin, g/dL/year | +0.05 | <0.001 |
| Abdominal pain | 0/34 (0%) | 2/34 (6%) | Platelets, giga/L/year | −1456.57 | 0.075 |
| Chronic bone pain | 1/34 (3%) | 5/34 (14.7%) | Leucocytes, giga/L/year | −24.71 | 0.487 |
| Acute bone crisis | 0/34 (0%) | 1/34 (3%) | Chitotriosidase, nmol/h/mL/year | −66.76 | 0.456 |
| Splenomegaly | 19/28 (67.8%) | 20/28 (71.4%) | Ferritin, ng/mL/year | +1.66 | 0.807 |
| Hepatomegaly | 10/16 (62.5%) | 12/16 (75%) | CCL18, ng/mL/year | −6.81 | 0.182 |
| GBA1 Genotype Amino Acid Change (Nucleotide Change) | Age at Diagnosis | Age at Last Evaluation | Age at Treatment Criteria Fulfillment | Criteria for Treatment at Diagnosis | Criteria for Treatmentat Last Evaluation | Reason for Absence of Treatment | |
|---|---|---|---|---|---|---|---|
| M | N370S/V121A (c.1226A > G/c.479T > C) | 72 | 74 | Not known | Bilateral aseptic osteonecrosis ** | Bilateral aseptic osteonecrosis | Patient’s refusal |
| M | N370S/L444P (c.1226A > G/c.1448T > C) | 53 | 60 | 43 | Humeral fracture Bilateral femoral infarcts | Humeral fracture Bilateral femoral infarcts | Patient’s refusal |
| M | N370S/S125N (c.1226A > G/c.491G > A) | 4 | 9 | 9 | - | Thrombocytopenia 50 × 10⁹/L | Not known |
| M | ND/ND | 17 | 47 | 40 | - | Thrombocytopenia (86 × 10⁹/L) + hemorrhagic syndrome | Patient’s refusal |
| F | N370S/L444P (c.1226A > G/c.1448T > C) | 4 | 9 | 9 | - | Bone crisis | Treated 1 year after inclusion |
| F | N370S/L444P (c.1226A > G/c.1448T > C) | 61 | 63 | 63 | - | Vertebral fracture L5 | Severe Lewy body dementia |
| F | N370S/ND (c.1226A > G/ND) | 32 | 55 | 45 | - | Femoral infarction | Patient’s refusal |
| M | N370S/N370S (c.1226A > G/c.1226A > G) | 51 | 69 | 69 | - | Vertebral fracture | Patient’s refusal |
| n = 15 | |
| Median age at diagnosis (years) | 4.2 (0.1–17) |
| Median age at last follow-up (years) | 8.6 (2.4–17.6) |
| Median follow-up duration (years) | 4.4 (2.1–15.5) |
| Sex ratio F/M | 7/8 |
| GBA1 genotype N370S/N370S (%) N370S/other (non L444P) (%) N370S/L444P (%) Other | n = 10 0/10 (0) 6/10 (60) 2/10 (20) 2/10 (20) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serratrice, C.; Stirnemann, J.; Berrahal, A.; Belmatoug, N.; Camou, F.; Caillaud, C.; Billette de Villemeur, T.; Dalbies, F.; Cador, B.; Froissart, R.; et al. A Cross-Sectional Retrospective Study of Non-Splenectomized and Never-Treated Patients with Type 1 Gaucher Disease. J. Clin. Med. 2020, 9, 2343. https://doi.org/10.3390/jcm9082343
Serratrice C, Stirnemann J, Berrahal A, Belmatoug N, Camou F, Caillaud C, Billette de Villemeur T, Dalbies F, Cador B, Froissart R, et al. A Cross-Sectional Retrospective Study of Non-Splenectomized and Never-Treated Patients with Type 1 Gaucher Disease. Journal of Clinical Medicine. 2020; 9(8):2343. https://doi.org/10.3390/jcm9082343
Chicago/Turabian StyleSerratrice, Christine, Jérôme Stirnemann, Amina Berrahal, Nadia Belmatoug, Fabrice Camou, Catherine Caillaud, Thierry Billette de Villemeur, Florence Dalbies, Bérengère Cador, Roseline Froissart, and et al. 2020. "A Cross-Sectional Retrospective Study of Non-Splenectomized and Never-Treated Patients with Type 1 Gaucher Disease" Journal of Clinical Medicine 9, no. 8: 2343. https://doi.org/10.3390/jcm9082343
APA StyleSerratrice, C., Stirnemann, J., Berrahal, A., Belmatoug, N., Camou, F., Caillaud, C., Billette de Villemeur, T., Dalbies, F., Cador, B., Froissart, R., Masseau, A., Brassier, A., Hivert, B., Swiader, L., Bertchansky, I., de Moreuil, C., Chabrol, B., Durieu, I., Leguy Seguin, V., ... Berger, M. G. (2020). A Cross-Sectional Retrospective Study of Non-Splenectomized and Never-Treated Patients with Type 1 Gaucher Disease. Journal of Clinical Medicine, 9(8), 2343. https://doi.org/10.3390/jcm9082343





