In Vitro Assessment of Fluoropyrimidine-Metabolizing Enzymes: Dihydropyrimidine Dehydrogenase, Dihydropyrimidinase, and β-Ureidopropionase
Abstract
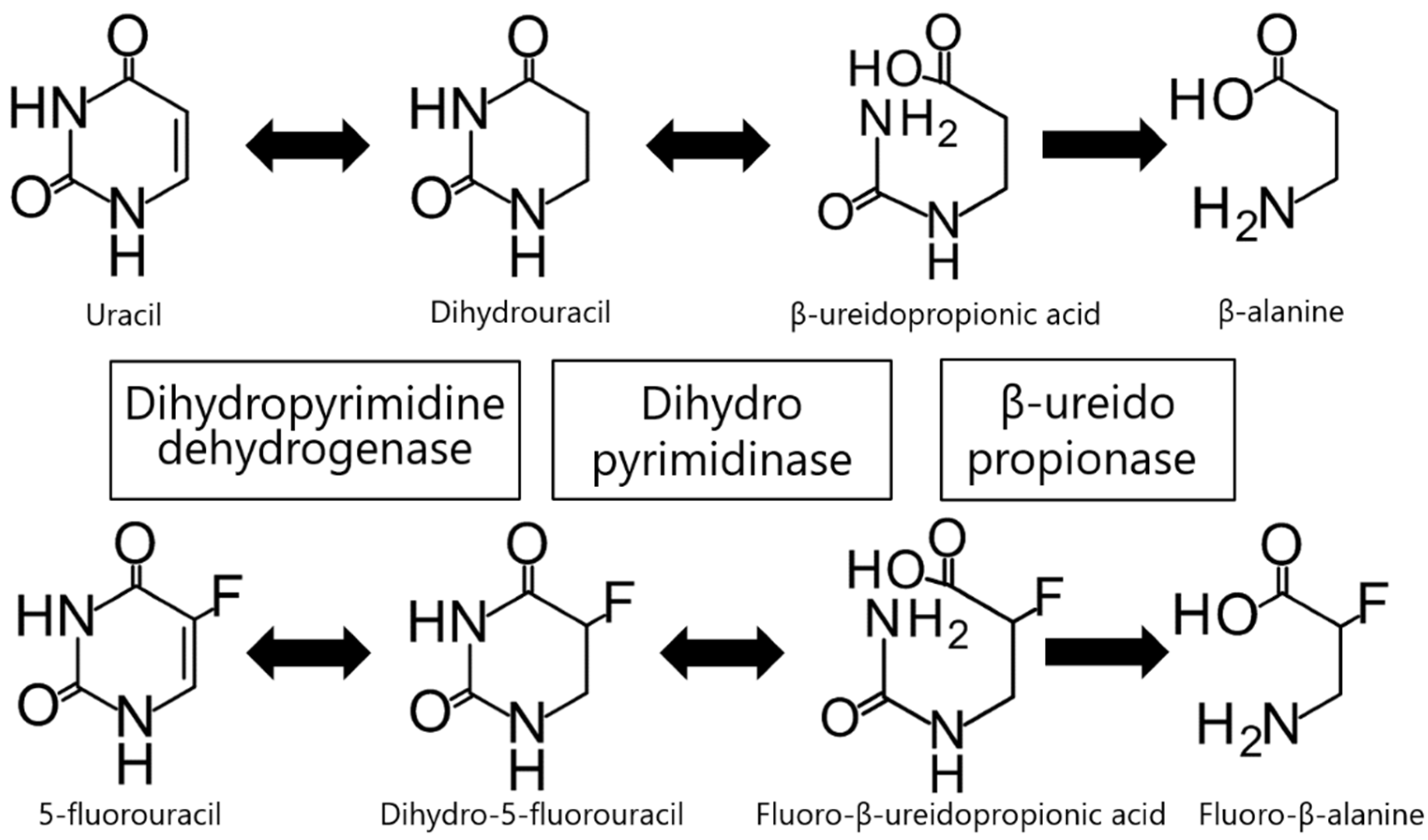
1. Introduction
2. Dihydropyrimidine Dehydrogenase (DPD)
3. Dihydropyrimidinase (DHP)
4. β-Ureidopropionase (β-UP)
5. Other Considerations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pan, X.; Wang, C.; Wang, F.; Li, P.; Hu, Z.; Shan, Y.; Zhang, J. Development of 5-Fluorouracil derivatives as anticancer agents. Curr. Med. Chem. 2011, 18, 4538–4556. [Google Scholar] [CrossRef] [PubMed]
- Kilic, L.; Ordu, C.; Yildiz, I.; Sen, F.; Keskin, S.; Ciftci, R.; Pilanci, K.N. Current adjuvant treatment modalities for gastric cancer: From history to the future. World J. Gastrointest. Oncol. 2016, 8, 439–449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lucas, A.S.; O’Neil, B.H.; Goldberg, R.M. A decade of advances in cytotoxic chemotherapy for metastatic colorectal cancer. Clin. Colorectal. Cancer 2011, 10, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.S.; Kwok, L.L.; Syn, N.; Chay, W.Y.; Chia, J.W.K.; Tham, C.K.; Wong, N.S.; Lo, S.K.; Dent, R.A.; Tan, S.; et al. Predictors of Hand-Foot Syndrome and Pyridoxine for Prevention of Capecitabine-Induced Hand-Foot Syndrome: A Randomized Clinical Trial. JAMA Oncol. 2017, 3, 1538–1545. [Google Scholar] [CrossRef]
- Lamberti, M.; Porto, S.; Zappavigna, S.; Addeo, E.; Marra, M.; Miraglia, N.; Sannolo, N.; Vanacore, D.; Stiuso, P.; Caraglia, M. A mechanistic study on the cardiotoxicity of 5-fluorouracil in vitro and clinical and occupational perspectives. Toxicol. Lett. 2014, 227, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Chung, H.C.; Choi, H.J.; Rha, S.Y.; Seong, J.S.; Jeung, H.C. Intermediate dose 5-fluorouracil-induced encephalopathy. Jpn. J. Clin. Oncol. 2006, 36, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Twelves, C.; Wong, A.; Nowacki, M.P.; Abt, M.; Burris, H., 3rd; Carrato, A.; Cassidy, J.; Cervantes, A.; Fagerberg, J.; Georgoulias, V.; et al. Capecitabine as adjuvant treatment for stage III colon cancer. N. Engl. J. Med. 2005, 352, 2696–2704. [Google Scholar] [CrossRef]
- Saltz, L.B.; Niedzwiecki, D.; Hollis, D.; Goldberg, R.M.; Hantel, A.; Thomas, J.P.; Fields, A.L.; Mayer, R.J. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 3456–3461. [Google Scholar] [CrossRef]
- Lam, S.W.; Guchelaar, H.J.; Boven, E. The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat. Rev. 2016, 50, 9–22. [Google Scholar] [CrossRef]
- Kunicka, T.; Prochazka, P.; Krus, I.; Bendova, P.; Protivova, M.; Susova, S.; Hlavac, V.; Liska, V.; Novak, P.; Schneiderova, M.; et al. Molecular profile of 5-fluorouracil pathway genes in colorectal carcinoma. BMC Cancer 2016, 16, 795. [Google Scholar] [CrossRef]
- Kim, J.Y.; Shin, E.; Kim, J.W.; Lee, H.S.; Lee, D.W.; Kim, S.H.; Lee, J.O.; Kim, Y.J.; Kim, J.H.; Bang, S.M.; et al. Impact of intratumoral expression levels of fluoropyrimidine-metabolizing enzymes on treatment outcomes of adjuvant S-1 therapy in gastric cancer. PLoS ONE 2015, 10, e0120324. [Google Scholar] [CrossRef] [PubMed]
- Daher, G.C.; Harris, B.E.; Diasio, R.B. Metabolism of pyrimidine analogues and their nucleosides. Pharmacol. Ther. 1990, 48, 189–222. [Google Scholar] [CrossRef]
- Heggie, G.D.; Sommadossi, J.P.; Cross, D.S.; Huster, W.J.; Diasio, R.B. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 1987, 47, 2203–2206. [Google Scholar] [PubMed]
- Porter, D.J.; Harrington, J.A.; Almond, M.R.; Lowen, G.T.; Spector, T. (R)-5-fluoro-5,6-dihydrouracil: Kinetics of oxidation by dihydropyrimidine dehydrogenase and hydrolysis by dihydropyrimidine aminohydrolase. Biochem. Pharmacol. 1994, 48, 775–779. [Google Scholar] [CrossRef]
- Kikugawa, M.; Kaneko, M.; Fujimoto-Sakata, S.; Maeda, M.; Kawasaki, K.; Takagi, T.; Tamaki, N. Purification, characterization and inhibition of dihydropyrimidinase from rat liver. Eur. J. Biochem. 1994, 219, 393–399. [Google Scholar] [CrossRef]
- Lu, Z.H.; Zhang, R.; Diasio, R.B. Purification and characterization of dihydropyrimidine dehydrogenase from human liver. J. Biol. Chem. 1992, 267, 17102–17109. [Google Scholar]
- Hamajima, N.; Kouwaki, M.; Vreken, P.; Matsuda, K.; Sumi, S.; Imaeda, M.; Ohba, S.; Kidouchi, K.; Nonaka, M.; Sasaki, M.; et al. Dihydropyrimidinase deficiency: Structural organization, chromosomal localization, and mutation analysis of the human dihydropyrimidinase gene. Am. J. Hum. Genet. 1998, 63, 717–726. [Google Scholar] [CrossRef]
- Vreken, P.; van Kuilenburg, A.B.; Hamajima, N.; Meinsma, R.; van Lenthe, H.; Gohlich-Ratmann, G.; Assmann, B.E.; Wevers, R.A.; van Gennip, A.H. cDNA cloning, genomic structure and chromosomal localization of the human BUP-1 gene encoding beta-ureidopropionase. Biochim. Biophys. Acta 1999, 1447, 251–257. [Google Scholar] [CrossRef]
- Amstutz, U.; Farese, S.; Aebi, S.; Largiader, C.R. Dihydropyrimidine dehydrogenase gene variation and severe 5-fluorouracil toxicity: A haplotype assessment. Pharmacogenomics 2009, 10, 931–944. [Google Scholar] [CrossRef]
- van Kuilenburg, A.B.P.; Haasjes, J.; Richel, D.J.; Zoetekouw, L.; Van Lenthe, H.; De Abreu, R.A.; Maring, J.G.; Vreken, P.; van Gennip, A.H. Clinical Implications of Dihydropyrimidine Dehydrogenase (DPD) Deficiency in Patients with Severe 5-Fluorouracil-associated Toxicity: Identification of New Mutations in the DPD Gene. Clin. Cancer Res. 2000, 6, 4705–4712. [Google Scholar]
- van Kuilenburg, A.B.; Meinsma, R.; Zonnenberg, B.A.; Zoetekouw, L.; Baas, F.; Matsuda, K.; Tamaki, N.; van Gennip, A.H. Dihydropyrimidinase deficiency and severe 5-fluorouracil toxicity. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 4363–4367. [Google Scholar]
- Amstutz, U.; Froehlich, T.K.; Largiader, C.R. Dihydropyrimidine dehydrogenase gene as a major predictor of severe 5-fluorouracil toxicity. Pharmacogenomics 2011, 12, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Meulendijks, D.; Cats, A.; Beijnen, J.H.; Schellens, J.H. Improving safety of fluoropyrimidine chemotherapy by individualizing treatment based on dihydropyrimidine dehydrogenase activity—Ready for clinical practice? Cancer Treat. Rev. 2016, 50, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Sistonen, J.; Buchel, B.; Froehlich, T.K.; Kummer, D.; Fontana, S.; Joerger, M.; van Kuilenburg, A.B.; Largiader, C.R. Predicting 5-fluorouracil toxicity: DPD genotype and 5,6-dihydrouracil:uracil ratio. Pharmacogenomics 2014, 15, 1653–1666. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, M. In vitro assessment of the allelic variants of cytochrome P450. Drug Metab. Pharmacokinet. 2012, 27, 68–84. [Google Scholar] [CrossRef] [PubMed]
- van Kuilenburg, A.B.; van Lenthe, H.; van Gennip, A.H. Activity of pyrimidine degradation enzymes in normal tissues. Nucleosides Nucleotides Nucleic Acids 2006, 25, 1211–1214. [Google Scholar] [CrossRef]
- Wei, X.; Elizondo, G.; Sapone, A.; McLeod, H.L.; Raunio, H.; Fernandez-Salguero, P.; Gonzalez, F.J. Characterization of the human dihydropyrimidine dehydrogenase gene. Genomics 1998, 51, 391–400. [Google Scholar] [CrossRef]
- Bakkeren, J.A.; De Abreu, R.A.; Sengers, R.C.; Gabreels, F.J.; Maas, J.M.; Renier, W.O. Elevated urine, blood and cerebrospinal fluid levels of uracil and thymine in a child with dihydrothymine dehydrogenase deficiency. Clin. Chim. Acta Int. J. Clin. Chem. 1984, 140, 247–256. [Google Scholar] [CrossRef]
- Berger, R.; Stoker-de Vries, S.A.; Wadman, S.K.; Duran, M.; Beemer, F.A.; de Bree, P.K.; Weits-Binnerts, J.J.; Penders, T.J.; van der Woude, J.K. Dihydropyrimidine dehydrogenase deficiency leading to thymine-uraciluria. An inborn error of pyrimidine metabolism. Clin. Chim. Acta Int. J. Clin. Chem. 1984, 141, 227–234. [Google Scholar] [CrossRef]
- van Kuilenburg, A.B. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur. J. Cancer 2004, 40, 939–950. [Google Scholar] [CrossRef]
- Al-Sanna’a, N.A.; Van Kuilenburg, A.B.; Atrak, T.M.; Abdul-Jabbar, M.A.; Van Gennip, A.H. Dihydropyrimidine dehydrogenase deficiency presenting at birth. J. Inherit. Metab. Dis. 2005, 28, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Boisdron-Celle, M.; Remaud, G.; Traore, S.; Poirier, A.L.; Gamelin, L.; Morel, A.; Gamelin, E. 5-Fluorouracil-related severe toxicity: A comparison of different methods for the pretherapeutic detection of dihydropyrimidine dehydrogenase deficiency. Cancer Lett. 2007, 249, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Kidouchi, K.; Sumi, S.; Mizokami, M.; Orito, E.; Kumada, K.; Ueda, R.; Wada, Y. Possible prediction of adverse reactions to pyrimidine chemotherapy from urinary pyrimidine levels and a case of asymptomatic adult dihydropyrimidinuria. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1996, 2, 1937–1941. [Google Scholar]
- Ezzeldin, H.; Diasio, R. Dihydropyrimidine dehydrogenase deficiency, a pharmacogenetic syndrome associated with potentially life-threatening toxicity following 5-fluorouracil administration. Clin. Colorectal. Cancer 2004, 4, 181–189. [Google Scholar] [CrossRef]
- Mounier-Boutoille, H.; Boisdron-Celle, M.; Cauchin, E.; Galmiche, J.P.; Morel, A.; Gamelin, E.; Matysiak-Budnik, T. Lethal outcome of 5-fluorouracil infusion in a patient with a total DPD deficiency and a double DPYD and UTG1A1 gene mutation. Br. J. Clin. Pharmacol. 2010, 70, 280–283. [Google Scholar] [CrossRef]
- Sahu, A.; Ramaswamy, A.; Ostwal, V. Dihydro pyrimidine dehydrogenase deficiency in patients treated with capecitabine based regimens: A tertiary care centre experience. J. Gastrointest. Oncol. 2016, 7, 380–386. [Google Scholar] [CrossRef]
- Sistonen, J.; Smith, C.; Fu, Y.K.; Largiader, C.R. A new DPYD genotyping assay for improving the safety of 5-fluorouracil therapy. Clin. Chim. Acta Int. J. Clin. Chem. 2012, 414, 109–111. [Google Scholar] [CrossRef]
- Iyer, S.N.; Singhal, R.S.; Hegde, M.R.; Ankala, A. Genetic variation in dihydropyrimidine dehydrogenase (DPYD) gene in a healthy adult Indian population. Ann. Hum. Biol. 2015, 42, 97–100. [Google Scholar] [CrossRef]
- Vaudo, C.E.; Gil, B.; Galuski, K.; Zarwan, C.; Nugent, F.W. Early-Onset 5-Fluorouracil Toxicity in a Patient Negative for Dihydropyrimidine Dehydrogenase Mutations: The Clinical Course of Reversal with Uridine Triacetate. Pharmacotherapy 2016, 36, e178–e182. [Google Scholar] [CrossRef]
- Thomas, F.; Hennebelle, I.; Delmas, C.; Lochon, I.; Dhelens, C.; Garnier Tixidre, C.; Bonadona, A.; Penel, N.; Goncalves, A.; Delord, J.P.; et al. Genotyping of a family with a novel deleterious DPYD mutation supports the pretherapeutic screening of DPD deficiency with dihydrouracil/uracil ratio. Clin. Pharmacol. Ther. 2016, 99, 235–242. [Google Scholar] [CrossRef]
- Henricks, L.M.; Siemerink, E.J.M.; Rosing, H.; Meijer, J.; Goorden, S.M.I.; Polstra, A.M.; Zoetekouw, L.; Cats, A.; Schellens, J.H.M.; van Kuilenburg, A.B.P. Capecitabine-based treatment of a patient with a novel DPYD genotype and complete dihydropyrimidine dehydrogenase deficiency. Int. J. Cancer 2018, 142, 424–430. [Google Scholar] [CrossRef]
- Gross, E.; Meul, C.; Raab, S.; Propping, C.; Avril, S.; Aubele, M.; Gkazepis, A.; Schuster, T.; Grebenchtchikov, N.; Schmitt, M.; et al. Somatic copy number changes in DPYD are associated with lower risk of recurrence in triple-negative breast cancers. Br. J. Cancer 2013, 109, 2347–2355. [Google Scholar] [CrossRef]
- van Kuilenburg, A.B.; Meijer, J.; Mul, A.N.; Meinsma, R.; Schmid, V.; Dobritzsch, D.; Hennekam, R.C.; Mannens, M.M.; Kiechle, M.; Etienne-Grimaldi, M.C.; et al. Intragenic deletions and a deep intronic mutation affecting pre-mRNA splicing in the dihydropyrimidine dehydrogenase gene as novel mechanisms causing 5-fluorouracil toxicity. Hum. Genet. 2010, 128, 529–538. [Google Scholar] [CrossRef]
- Qin, F.; Zhang, H.; Huang, Y.; Yang, L.; Yu, F.; Liu, X.; Fu, L.; Gu, F.; Ma, Y. Effect of dihydropyrimidine dehydrogenase single nucleotide polymorphisms on prognosis of breast cancer patients with chemotherapy. Oncotarget 2017, 8, 112060–112075. [Google Scholar] [CrossRef] [PubMed]
- Seck, K.; Riemer, S.; Kates, R.; Ullrich, T.; Lutz, V.; Harbeck, N.; Schmitt, M.; Kiechle, M.; Diasio, R.; Gross, E. Analysis of the DPYD gene implicated in 5-fluorouracil catabolism in a cohort of Caucasian individuals. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 5886–5892. [Google Scholar] [CrossRef]
- Amstutz, U.; Henricks, L.M.; Offer, S.M.; Barbarino, J.; Schellens, J.H.M.; Swen, J.J.; Klein, T.E.; McLeod, H.L.; Caudle, K.E.; Diasio, R.B.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin. Pharmacol. Ther. 2018, 103, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Lunenburg, C.; van der Wouden, C.H.; Nijenhuis, M.; Crommentuijn-van Rhenen, M.H.; de Boer-Veger, N.J.; Buunk, A.M.; Houwink, E.J.F.; Mulder, H.; Rongen, G.A.; van Schaik, R.H.N.; et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction of DPYD and fluoropyrimidines. Eur. J. Hum. Genet. 2020, 28, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.G.; Cheong, H.S.; Kim, J.Y.; Kim, L.H.; Han, C.S.; Kim, J.O.; Kim, H.D.; Kim, Y.H.; Chung, M.W.; Han, S.Y.; et al. Screening of dihydropyrimidine dehydrogenase genetic variants by direct sequencing in different ethnic groups. J. Korean Med. Sci. 2013, 28, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, K.; Saeki, M.; Saito, Y.; Ozawa, S.; Kurose, K.; Kaniwa, N.; Kawamoto, M.; Kamatani, N.; Kato, K.; Hamaguchi, T.; et al. Genetic variations and haplotype structures of the DPYD gene encoding dihydropyrimidine dehydrogenase in Japanese and their ethnic differences. J. Hum. Genet. 2007, 52, 804–819. [Google Scholar] [CrossRef]
- He, Y.F.; Wei, W.; Zhang, X.; Li, Y.H.; Li, S.; Wang, F.H.; Lin, X.B.; Li, Z.M.; Zhang, D.S.; Huang, H.Q.; et al. Analysis of the DPYD gene implicated in 5-fluorouracil catabolism in Chinese cancer patients. J. Clin. Pharm. Ther. 2008, 33, 307–314. [Google Scholar] [CrossRef]
- Lunenburg, C.; Henricks, L.M.; Guchelaar, H.J.; Swen, J.J.; Deenen, M.J.; Schellens, J.H.M.; Gelderblom, H. Prospective DPYD genotyping to reduce the risk of fluoropyrimidine-induced severe toxicity: Ready for prime time. Eur. J. Cancer 2016, 54, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, C.H.; Lu, Z.H.; Zhang, R.; Liang, M.D.; Larson, L.V.; Cantilena, L.R., Jr.; Grem, J.L.; Allegra, C.J.; Diasio, R.B.; Chu, E. Severe neurotoxicity following 5-fluorouracil-based chemotherapy in a patient with dihydropyrimidine dehydrogenase deficiency. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1996, 2, 477–481. [Google Scholar]
- Lu, Z.; Zhang, R.; Diasio, R.B. Dihydropyrimidine dehydrogenase activity in human peripheral blood mononuclear cells and liver: Population characteristics, newly identified deficient patients, and clinical implication in 5-fluorouracil chemotherapy. Cancer Res. 1993, 53, 5433–5438. [Google Scholar] [PubMed]
- Offer, S.M.; Wegner, N.J.; Fossum, C.; Wang, K.; Diasio, R.B. Phenotypic profiling of DPYD variations relevant to 5-fluorouracil sensitivity using real-time cellular analysis and in vitro measurement of enzyme activity. Cancer Res. 2013, 73, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Kuilenburg, A.; Meijer, J.; Tanck, M.W.T.; Dobritzsch, D.; Zoetekouw, L.; Dekkers, L.L.; Roelofsen, J.; Meinsma, R.; Wymenga, M.; Kulik, W.; et al. Phenotypic and clinical implications of variants in the dihydropyrimidine dehydrogenase gene. Biochim. Biophys. Acta 2016, 1862, 754–762. [Google Scholar] [CrossRef]
- van Kuilenburg, A.B.; Meijer, J.; Maurer, D.; Dobritzsch, D.; Meinsma, R.; Los, M.; Knegt, L.C.; Zoetekouw, L.; Jansen, R.L.; Dezentje, V.; et al. Severe fluoropyrimidine toxicity due to novel and rare DPYD missense mutations, deletion and genomic amplification affecting DPD activity and mRNA splicing. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 721–730. [Google Scholar] [CrossRef]
- Ogura, K.; Ohnuma, T.; Minamide, Y.; Mizuno, A.; Nishiyama, T.; Nagashima, S.; Kanamaru, M.; Hiratsuka, A.; Watabe, T.; Uematsu, T. Dihydropyrimidine dehydrogenase activity in 150 healthy Japanese volunteers and identification of novel mutations. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 5104–5111. [Google Scholar] [CrossRef]
- Offer, S.M.; Fossum, C.C.; Wegner, N.J.; Stuflesser, A.J.; Butterfield, G.L.; Diasio, R.B. Comparative functional analysis of DPYD variants of potential clinical relevance to dihydropyrimidine dehydrogenase activity. Cancer Res. 2014, 74, 2545–2554. [Google Scholar] [CrossRef]
- Elraiyah, T.; Jerde, C.R.; Shrestha, S.; Wu, R.; Nie, Q.; Giama, N.H.; Sarangi, V.; Roberts, L.R.; Offer, S.M.; Diasio, R.B. Novel Deleterious Dihydropyrimidine Dehydrogenase Variants May Contribute to 5-Fluorouracil Sensitivity in an East African Population. Clin. Pharmacol. Ther. 2017, 101, 382–390. [Google Scholar] [CrossRef]
- Hishinuma, E.; Narita, Y.; Saito, S.; Maekawa, M.; Akai, F.; Nakanishi, Y.; Yasuda, J.; Nagasaki, M.; Yamamoto, M.; Yamaguchi, H.; et al. Functional Characterization of 21 Allelic Variants of Dihydropyrimidine Dehydrogenase Identified in 1070 Japanese Individuals. Drug Metab. Dispos. Biol. Fate Chem. 2018, 46, 1083–1090. [Google Scholar] [CrossRef]
- Schnackerz, K.D.; Dobritzsch, D.; Lindqvist, Y.; Cook, P.F. Dihydropyrimidine dehydrogenase: A flavoprotein with four iron-sulfur clusters. Biochim. Biophys. Acta 2004, 1701, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Dobritzsch, D.; Ricagno, S.; Schneider, G.; Schnackerz, K.D.; Lindqvist, Y. Crystal structure of the productive ternary complex of dihydropyrimidine dehydrogenase with NADPH and 5-iodouracil. Implications for mechanism of inhibition and electron transfer. J. Biol. Chem. 2002, 277, 13155–13166. [Google Scholar] [CrossRef] [PubMed]
- Dobritzsch, D.; Schneider, G.; Schnackerz, K.D.; Lindqvist, Y. Crystal structure of dihydropyrimidine dehydrogenase, a major determinant of the pharmacokinetics of the anti-cancer drug 5-fluorouracil. EMBO J. 2001, 20, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Mattison, L.K.; Johnson, M.R.; Diasio, R.B. A comparative analysis of translated dihydropyrimidine dehydrogenase cDNA; conservation of functional domains and relevance to genetic polymorphisms. Pharmacogenetics 2002, 12, 133–144. [Google Scholar] [CrossRef]
- Lohkamp, B.; Voevodskaya, N.; Lindqvist, Y.; Dobritzsch, D. Insights into the mechanism of dihydropyrimidine dehydrogenase from site-directed mutagenesis targeting the active site loop and redox cofactor coordination. Biochim. Biophys. Acta 2010, 1804, 2198–2206. [Google Scholar] [CrossRef]
- Henricks, L.M.; Lunenburg, C.A.; Meulendijks, D.; Gelderblom, H.; Cats, A.; Swen, J.J.; Schellens, J.H.; Guchelaar, H.J. Translating DPYD genotype into DPD phenotype: Using the DPYD gene activity score. Pharmacogenomics 2015, 16, 1277–1286. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, C.; Jerde, C.R.; Nie, Q.; Li, H.; Offer, S.M.; Diasio, R.B. Gene-Specific Variant Classifier (DPYD-Varifier) to Identify Deleterious Alleles of Dihydropyrimidine Dehydrogenase. Clin. Pharmacol. Ther. 2018, 104, 709–718. [Google Scholar] [CrossRef]
- Hamajima, N.; Matsuda, K.; Sakata, S.; Tamaki, N.; Sasaki, M.; Nonaka, M. A novel gene family defined by human dihydropyrimidinase and three related proteins with differential tissue distribution. Gene 1996, 180, 157–163. [Google Scholar] [CrossRef]
- Duran, M.; Rovers, P.; de Bree, P.K.; Schreuder, C.H.; Beukenhorst, H.; Dorland, L.; Berger, R. Dihydropyrimidinuria: A new inborn error of pyrimidine metabolism. J. Inherit. Metab. Dis. 1991, 14, 367–370. [Google Scholar] [CrossRef]
- Ohba, S.; Kidouchi, K.; Sumi, S.; Imaeda, M.; Takeda, N.; Yoshizumi, H.; Tatematsu, A.; Kodama, K.; Yamanaka, K.; Kobayashi, M.; et al. Dihydropyrimidinuria: The first case in Japan. Adv. Exp. Med. Biol. 1994, 370, 383–386. [Google Scholar] [CrossRef]
- van Kuilenburg, A.B.; Meijer, J.; Dobritzsch, D.; Meinsma, R.; Duran, M.; Lohkamp, B.; Zoetekouw, L.; Abeling, N.G.; van Tinteren, H.L.; Bosch, A.M. Clinical, biochemical and genetic findings in two siblings with a dihydropyrimidinase deficiency. Mol. Genet. Metab. 2007, 91, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.W.; Yau, M.M.; Ma, C.K.; Siu, T.S.; Tam, S.; Lam, C.W. Diagnosis of dihydropyrimidinase deficiency in a Chinese boy with dihydropyrimidinuria. Hong Kong Med. J./Xianggang Yi Xue Za Zhi 2013, 19, 272–275. [Google Scholar] [CrossRef]
- Sumi, S.; Imaeda, M.; Kidouchi, K.; Ohba, S.; Hamajima, N.; Kodama, K.; Togari, H.; Wada, Y. Population and family studies of dihydropyrimidinuria: Prevalence, inheritance mode, and risk of fluorouracil toxicity. Am. J. Med Genet. 1998, 78, 336–340. [Google Scholar] [CrossRef]
- van Gennip, A.H.; Abeling, N.G.; Stroomer, A.E.; van Lenthe, H.; Bakker, H.D. Clinical and biochemical findings in six patients with pyrimidine degradation defects. J. Inherit. Metab. Dis. 1994, 17, 130–132. [Google Scholar] [CrossRef] [PubMed]
- van Gennip, A.H.; de Abreu, R.A.; van Lenthe, H.; Bakkeren, J.; Rotteveel, J.; Vreken, P.; van Kuilenburg, A.B. Dihydropyrimidinase deficiency: Confirmation of the enzyme defect in dihydropyrimidinuria. J. Inherit. Metab. Dis. 1997, 20, 339–342. [Google Scholar] [CrossRef] [PubMed]
- van Kuilenburg, A.B.; Dobritzsch, D.; Meijer, J.; Meinsma, R.; Benoist, J.F.; Assmann, B.; Schubert, S.; Hoffmann, G.F.; Duran, M.; de Vries, M.C.; et al. Dihydropyrimidinase deficiency: Phenotype, genotype and structural consequences in 17 patients. Biochim. Biophys. Acta 2010, 1802, 639–648. [Google Scholar] [CrossRef]
- Nakajima, Y.; Meijer, J.; Dobritzsch, D.; Ito, T.; Zhang, C.; Wang, X.; Watanabe, Y.; Tashiro, K.; Meinsma, R.; Roelofsen, J.; et al. Dihydropyrimidinase deficiency in four East Asian patients due to novel and rare DPYS mutations affecting protein structural integrity and catalytic activity. Mol. Genet. Metab. 2017, 122, 216–222. [Google Scholar] [CrossRef]
- Akai, F.; Hosono, H.; Hirasawa, N.; Hiratsuka, M. Novel single nucleotide polymorphisms of the dihydropyrimidinase gene (DPYS) in Japanese individuals. Drug Metab. Pharmacokinet. 2015, 30, 127–129. [Google Scholar] [CrossRef]
- Hiratsuka, M.; Yamashita, H.; Akai, F.; Hosono, H.; Hishinuma, E.; Hirasawa, N.; Mori, T. Genetic polymorphisms of dihydropyrimidinase in a Japanese patient with capecitabine-induced toxicity. PLoS ONE 2015, 10, e0124818. [Google Scholar] [CrossRef]
- Fidlerova, J.; Kleiblova, P.; Bilek, M.; Kormunda, S.; Formankova, Z.; Novotny, J.; Kleibl, Z. Contribution of dihydropyrimidinase gene alterations to the development of serious toxicity in fluoropyrimidine-treated cancer patients. Cancer Chemother. Pharmacol. 2010, 65, 661–669. [Google Scholar] [CrossRef]
- Nakajima, Y.; Meijer, J.; Zhang, C.; Wang, X.; Kondo, T.; Ito, T.; Dobritzsch, D.; Van Kuilenburg, A.B. Altered Pre-mRNA Splicing Caused by a Novel Intronic Mutation c.1443+5G>A in the Dihydropyrimidinase (DPYS) Gene. Int. J. Mol. Sci. 2016, 17, 86. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.R.; Ezzeldin, H.H.; Guarcello, V.; Mattison, L.K.; Fridley, B.L.; Diasio, R.B. Genetic regulation of dihydropyrimidinase and its possible implication in altered uracil catabolism. Pharm. Genom. 2007, 17, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Hishinuma, E.; Akai, F.; Narita, Y.; Maekawa, M.; Yamaguchi, H.; Mano, N.; Oda, A.; Hirasawa, N.; Hiratsuka, M. Functional characterization of 21 allelic variants of dihydropyrimidinase. Biochem. Pharmacol. 2017, 143, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.C.; Chen, M.C.; Hsu, C.C.; Chan, S.I.; Yang, Y.S.; Chen, C.J. Crystal structures of vertebrate dihydropyrimidinase and complexes from Tetraodon nigroviridis with lysine carbamylation: Metal and structural requirements for post-translational modification and function. J. Biol. Chem. 2013, 288, 30645–30658. [Google Scholar] [CrossRef]
- Brooks, K.P.; Jones, E.A.; Kim, B.D.; Sander, E.G. Bovine liver dihydropyrimidine amidohydrolase: Purification, properties, and characterization as a zinc metalloenzyme. Arch. Biochem. Biophys. 1983, 226, 469–483. [Google Scholar] [CrossRef]
- Lohkamp, B.; Andersen, B.; Piskur, J.; Dobritzsch, D. The crystal structures of dihydropyrimidinases reaffirm the close relationship between cyclic amidohydrolases and explain their substrate specificity. J. Biol. Chem. 2006, 281, 13762–13776. [Google Scholar] [CrossRef]
- Tzeng, C.T.; Huang, Y.H.; Huang, C.Y. Crystal structure of dihydropyrimidinase from Pseudomonas aeruginosa PAO1: Insights into the molecular basis of formation of a dimer. Biochem. Biophys. Res. Commun. 2016, 478, 1449–1455. [Google Scholar] [CrossRef]
- Kim, G.J.; Kim, H.S. C-terminal regions of D-hydantoinases are nonessential for catalysis, but affect the oligomeric structure. Biochem. Biophys. Res. Commun. 1998, 243, 96–100. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, X.; Shi, Y.; Yuan, J. Subunit dissociation and stability alteration of D hydantoinase deleted at the terminal amino acid residue. Biotechnol. Lett. 2007, 29, 303–308. [Google Scholar] [CrossRef][Green Version]
- Abendroth, J.; Niefind, K.; Schomburg, D. X-ray structure of a dihydropyrimidinase from Thermus sp. at 1.3 A resolution. J. Mol. Biol. 2002, 320, 143–156. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Rislund, L.; Andersen, B.; Sandrini, M.P.; Cook, P.F.; Schnackerz, K.D.; Piskur, J. Dihydropyrimidine amidohydrolases and dihydroorotases share the same origin and several enzymatic properties. Nucleic Acids Res. 2003, 31, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Sakata, S.F.; Matsuda, K.; Horikawa, Y.; Tamaki, N. Expression and properties of human liver beta-ureidopropionase. J. Nutr. Sci. Vitaminol. 2001, 47, 132–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Kuilenburg, A.B.; Meinsma, R.; Beke, E.; Assmann, B.; Ribes, A.; Lorente, I.; Busch, R.; Mayatepek, E.; Abeling, N.G.; van Cruchten, A.; et al. beta-Ureidopropionase deficiency: An inborn error of pyrimidine degradation associated with neurological abnormalities. Hum. Mol. Genet. 2004, 13, 2793–2801. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Shibata, T.; Yoshinaga, H.; Kuhara, T.; Nakajima, Y.; Kato, T.; Maeda, Y.; Ohse, M.; Oka, M.; Kageyama, M.; et al. A Japanese case of β-ureidopropionase deficiency with dysmorphic features. Brain Dev. 2017, 39, 58–61. [Google Scholar] [CrossRef]
- van Kuilenburg, A.B.; Dobritzsch, D.; Meijer, J.; Krumpel, M.; Selim, L.A.; Rashed, M.S.; Assmann, B.; Meinsma, R.; Lohkamp, B.; Ito, T.; et al. ß-ureidopropionase deficiency: Phenotype, genotype and protein structural consequences in 16 patients. Biochim. Biophys. Acta 2012, 1822, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; van Kuilenburg, A.B.; Abeling, N.G.; Vasta, V.; Hahn, S.H. A Korean Case of β-Ureidopropionase Deficiency Presenting with Intractable Seizure, Global Developmental Delay, and Microcephaly. JIMD Rep. 2015, 19, 117–121. [Google Scholar] [CrossRef]
- Nakajima, Y.; Meijer, J.; Dobritzsch, D.; Ito, T.; Meinsma, R.; Abeling, N.G.; Roelofsen, J.; Zoetekouw, L.; Watanabe, Y.; Tashiro, K.; et al. Clinical, biochemical and molecular analysis of 13 Japanese patients with beta-ureidopropionase deficiency demonstrates high prevalence of the c.977G > A (p.R326Q) mutation [corrected]. J. Inherit. Metab. Dis. 2014, 37, 801–812. [Google Scholar] [CrossRef]
- Yaplito-Lee, J.; Pitt, J.; Meijer, J.; Zoetekouw, L.; Meinsma, R.; van Kuilenburg, A.B. Beta-ureidopropionase deficiency presenting with congenital anomalies of the urogenital and colorectal systems. Mol. Genet. Metab. 2008, 93, 190–194. [Google Scholar] [CrossRef]
- Fang, Y.; Cai, C.; Wang, C.; Sun, B.; Zhang, X.; Fan, W.; Hu, W.; Meng, Y.; Lin, S.; Zhang, C.; et al. Clinical and genetic analysis of 7 Chinese patients with beta-ureidopropionase deficiency. Medicine 2019, 98, e14021. [Google Scholar] [CrossRef]
- Shu, J.; Lv, X.; Jiang, S.; Zhang, Y.; Zhang, C.; Meng, Y.; Situ, A.; Xu, H.; Song, L. Genetic analysis of the UPB1 gene in two new Chinese families with beta-ureidopropionase deficiency and the carrier frequency of the mutation c.977G>A in Northern China. Child’s Nerv. Syst. Off. J. Int. Soc. Pediatric Neurosurg. 2014, 30, 2109–2114. [Google Scholar] [CrossRef]
- Thomas, H.R.; Ezzeldin, H.H.; Guarcello, V.; Mattison, L.K.; Fridley, B.L.; Diasio, R.B. Genetic regulation of beta-ureidopropionase and its possible implication in altered uracil catabolism. Pharm. Genom. 2008, 18, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Fidlerova, J.; Kleiblova, P.; Kormunda, S.; Novotny, J.; Kleibl, Z. Contribution of the beta-ureidopropionase (UPB1) gene alterations to the development of fluoropyrimidine-related toxicity. Pharmacol. Rep. 2012, 64, 1234–1242. [Google Scholar] [CrossRef]
- Matsusaka, S.; Lenz, H.J. Pharmacogenomics of fluorouracil -based chemotherapy toxicity. Expert Opin. Drug Metab. Toxicol. 2015, 11, 811–821. [Google Scholar] [CrossRef] [PubMed]
- de Bock, C.E.; Garg, M.B.; Scott, N.; Sakoff, J.A.; Scorgie, F.E.; Ackland, S.P.; Lincz, L.F. Association of thymidylate synthase enhancer region polymorphisms with thymidylate synthase activity in vivo. Pharm. J. 2011, 11, 307–314. [Google Scholar] [CrossRef]
- Amirfallah, A.; Kocal, G.C.; Unal, O.U.; Ellidokuz, H.; Oztop, I.; Basbinar, Y. DPYD, TYMS and MTHFR Genes Polymorphism Frequencies in a Series of Turkish Colorectal Cancer Patients. J. Pers. Med. 2018, 8, 45. [Google Scholar] [CrossRef]
- Lima, A.; Azevedo, R.; Sousa, H.; Seabra, V.; Medeiros, R. Current approaches for TYMS polymorphisms and their importance in molecular epidemiology and pharmacogenetics. Pharmacogenomics 2013, 14, 1337–1351. [Google Scholar] [CrossRef]
- Loganayagam, A.; Arenas Hernandez, M.; Corrigan, A.; Fairbanks, L.; Lewis, C.M.; Harper, P.; Maisey, N.; Ross, P.; Sanderson, J.D.; Marinaki, A.M. Pharmacogenetic variants in the DPYD, TYMS, CDA and MTHFR genes are clinically significant predictors of fluoropyrimidine toxicity. Br. J. Cancer 2013, 108, 2505–2515. [Google Scholar] [CrossRef]
- Amstutz, U.; Offer, S.M.; Sistonen, J.; Joerger, M.; Diasio, R.B.; Largiader, C.R. Polymorphisms in MIR27A Associated with Early-Onset Toxicity in Fluoropyrimidine-Based Chemotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 2038–2044. [Google Scholar] [CrossRef]
- Meulendijks, D.; Henricks, L.M.; Amstutz, U.; Froehlich, T.K.; Largiader, C.R.; Beijnen, J.H.; de Boer, A.; Deenen, M.J.; Cats, A.; Schellens, J.H. Rs895819 in MIR27A improves the predictive value of DPYD variants to identify patients at risk of severe fluoropyrimidine-associated toxicity. Int. J. Cancer 2016, 138, 2752–2761. [Google Scholar] [CrossRef] [PubMed]
- Offer, S.M.; Butterfield, G.L.; Jerde, C.R.; Fossum, C.C.; Wegner, N.J.; Diasio, R.B. microRNAs miR-27a and miR-27b directly regulate liver dihydropyrimidine dehydrogenase expression through two conserved binding sites. Mol. Cancer Ther. 2014, 13, 742–751. [Google Scholar] [CrossRef] [PubMed]
| dbSNP rsID | PharmVar ID | Location | Nucleotide Change | Amino Acid Substitution | Domain | Expression System | Substrates | Effect | References |
|---|---|---|---|---|---|---|---|---|---|
| rs150036960 | PV00901 | Exon 2 | 46C > G | L16V | V | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs72549310 | PV01042 | Exon 2 | 61C > T | R21X | I | HEK293T/c17 | 5-FU | No function | [58] |
| rs80081766 | PV01307 | Exon 2 | 62G > A | R21Q | I | HEK293T/c17 | 5-FU | Normal function | [58] |
| – | – | Exon 2 | 74A > G | H25R | I | 293FT | 5-FU | 156% of CLint ratio | [60] |
| rs1801265 | PV00910 | Exon 2 | 85T > C (DPYD*9A) | C29R | I | HEK293T/c17 HEK293 Flp-In | 5-FU Thymine | Increased function Decreased function | [54] [55] |
| rs371587702 | PV00962 | Exon 3 | 194C > T | T65M | I | HEK293T/c17 | 5-FU | Normal function | [58] |
| – | – | Exon 4 | 257C > T | P86L | I | HEK293T/c17 | 5-FU | No function | [59] |
| rs143986398 | PV00887 | Exon 4 | 274C > G | P92A | I | HEK293T/c17 | 5-FU | Decreased function | [58] |
| rs72549309 | PV01041 | Exon 4 | 295delTCAT (DPYD*7) | F100fs | I | HEK293T/c17 | 5-FU | No function | [58] |
| rs150385342 | PV00902 | Exon 4 | 313G > A | A105T | I | HEK293T/c17 | 5-FU | Normal function | [58] |
| – | – | Exon 5 | 325T > A | Y109N | I | 293FT | 5-FU | 79% of CLint ratio | [60] |
| rs141462178 | PV00878 | Exon 5 | 343A > G | M115V | I | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs200562975 | PV00927 | Exon 5 | 451A > G | N151D | I | 293FT HEK293T/c17 | 5-FU 5-FU | 107% of CLint ratio Normal function | [60] [58] |
| rs2297595 | PV0943 | Exon 6 | 496A > G | M166V | I | 293FT HEK293T/c17 HEK293 Flp-In | 5-FU 5-FU Thymine | 77% of CLint ratio Increased function Decreased function | [60] [58] [55] |
| rs139834141 | PV00871 | Exon 6 | 498G > A | M166I | I | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs371792178 | – | Exon 6 | 524C > T | S175L | II | 293FT | 5-FU | 131% of CLint ratio | [60] |
| rs115232898 | PV00862 | Exon 6 | 557A > G | Y186C | II | HEK293T/c17 | 5-FU | Decreased function | [58] |
| rs72549308 | PV01040 | Exon 6 | 601A > C | S201R | II | HEK293T/c17 | 5-FU | No function | [58] |
| rs72549307 | PV01039 | Exon 6 | 632A > G | Y211C | II | HEK293T/c17 | 5-FU | Decreased function | [58] |
| rs1801266 | PV00911 | Exon 7 | 703C > T (DPYD*8) | R235W | II | HEK293T/c17 | 5-FU | Decreased function | [58] |
| rs780025995 | PV01299 | Exon 7 | 710C > T | P237L | II | HEK293T/c17 | 5-FU | Decreased function | [59] |
| rs45589337 | PV00984 | Exon 8 | 775A > G | K259E | II | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs777220476 | PV01275 | Exon 9 | 851G > T | G284V | II | HEK293 Flp-In | Thymine | No function | [56] |
| rs146356975 | PV00895 | Exon 9 | 868A > G | K290E | III | HEK293T/c17 | 5-FU | Decreased function | [58] |
| rs143878757 | PV00886 | Exon 9 | 893C > T | T298M | III | 293FT | 5-FU | 50% of CLint ratio | [60] |
| rs183105782 | PV00914 | Exon 9 | 910T > C | Y304H | III | HEK293T/c17 | 5-FU | Decreased function | [58] |
| rs150437414 | PV00904 | Exon 9 | 929T > C | L310S | III | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs145112791 | PV00891 | Exon 9 | 934C > T | L312F | III | HEK293T/c17 | 5-FU | Normal function | [58] |
| – | – | Exon 9 | 937G > T | V313L | III | 293FT | 5-FU | 30% of CLint ratio | [60] |
| rs201018345 | PV00933 | Exon 10 | 967G > A | A323T | III | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs72549306 | PV01038 | Exon 10 | 1003G > A | V335M | III | 293FT | 5-FU | 47% of CLint ratio | [60] |
| rs72549306 | PV01037 | Exon 10 | 1003G > T (DPYD*11) | V335L | III | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs183385770 | PV00915 | Exon 10 | 1024G > A | D342N | III | HEK293T/c17 | 5-FU | Decreased function | [58] |
| rs190577302 | PV00919 | Exon 10 | 1054C > G | L352V | III | HEK293T/c17 | 5-FU | Decreased function | [58] |
| rs143154602 | PV00882 | Exon 10 | 1057C > T | R353C | III | HEK293T/c17 | 5-FU | No function | [58] |
| – | – | Exon 10 | 1097G > C | G366A | III | 293FT Escherichia coli | 5-FU 5-FU | 71% of CLint ratio 47% of CLint ratio | [60] [57] |
| rs72549305 | PV01036 | Exon 10 | 1108A > G | I370V | III | HEK293T/c17 | 5-FU | Normal function | [58] |
| – | – | Exon 11 | 1139C > T | A380V | III | 293FT | 5-FU | 33% of CLint ratio | [60] |
| – | – | Exon 11 | 1150A > G | K384E | III | 293FT | 5-FU | 68% of CLint ratio | [60] |
| rs78060119 | PV01302 | Exon 11 | 1156G > T(DPYD*12) | E386X | III | HEK293T/c17 | 5-FU | No function | [58] |
| rs140602333 | PV00874 | Exon 11 | 1180C > T | R394W | III | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs143815742 | PV00883 | Exon 11 | 1181G > T | R394L | III | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs143815742 | PV00884 | Exon 11 | 1181G > A | R394Q | III | HEK293T/c17 | 5-FU | Normal function | [59] |
| – | – | Exon 11 | 1201G > A | G401R | III | HEK293 Flp-In | Thymine | Decreased function | [55] |
| rs61622928 | PV01018 | Exon 11 | 1218G > A | M406I | III | HEK293T/c17 HEK293 Flp-In | 5-FU Thymine | Normal function Normal function | [58] [55] |
| rs200064537 | PV00925 | Exon 11 | 1260T > A | N420K | III | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs764666241 | PV0183 | Exon 11 | 1278G > T | M426I | III | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs200693895 | PV00931 | Exon 11 | 1280T > C | V427A | III | HEK293 Flp-In | Thymine | Normal function | [56] |
| rs142512579 | PV00880 | Exon 11 | 1294G > A | D432N | III | HEK293T/c17 | 5-FU | Normal function | [58] |
| – | – | Exon 11 | 1300G > C | V434L | III | 293FT | 5-FU | 44% of CLint ratio | [60] |
| rs186169810 | PV00916 | Exon 11 | 1314T > G | F438L | III | HEK293T/c17 | 5-FU | Decreased function | [58] |
| rs72975710 | PV01043 | Exon 12 | 1349C > T | A450V | II | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs144395748 | PV00888 | Exon 12 | 1358C > G | P453R | II | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs199549923 | PV00921 | Exon 12 | 1403C > A | T468N | II | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs72549304 | PV01035 | Exon 12 | 1475C > T | S492L | II | HEK293T/c17 | 5-FU | Decreased function | [58] |
| rs111858276 | PV00857 | Exon 12 | 1484A > G | D495G | II | HEK293T/c17 | 5-FU | Decreased function | [58] |
| rs138391898 | PV00867 | Exon 12 | 1519G > A | V507I | II | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs760663364 | PV01150 | Exon13 | 1538C > T | A513V | II | HEK293T/c17 | 5-FU | Decreased function | [59] |
| rs148994843 | PV00900 | Exon 13 | 1543G > A | V515I | II | 293FT HEK293T/c17 | 5-FU 5-FU | 36% of CLint ratio Normal function | [60] [58] |
| – | – | Exon 13 | 1567C > T | L523F | II | HEK293T/c17 | 5-FU | Normal function | [59] |
| rs190951787 | PV00920 | Exon 13 | 1577C > G | T526S | IV | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs1180771326 | PV00864 | Exon 13 | 1582A > G | I528V | IV | HEK293T/c17 | 5-FU | Normal function | [59] |
| rs1801158 | PV00907 | Exon 13 | 1601G > A (DPYD*4) | S534N | IV | HEK293T/c17 HEK293 Flp-In | 5-FU Thymine | Increased function Decreased function | [54] [55] |
| rs142619737 | – | Exon 13 | 1615G > C | G539R | IV | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs1801159 | PV00908 | Exon 13 | 1627A > G (DPYD*5) | I543V | IV | 293FT HEK293T/c17 HEK293 Flp-In | 5-FU 5-FU Thymine | 102% of CLint ratio Normal function Normal function | [60] [54] [55] |
| rs55886062 | PV01000 | Exon 13 | 1679T > G (DPYD*13) | I560S | IV | HEK293T/c17 | 5-FU | Decreased function | [54] |
| rs201615754 | PV00937 | Exon 13 | 1682G > T | R561L | IV | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs59086055 | PV01015 | Exon 14 | 1774C > T | R592W | IV | 293FT HEK293T/c17 | 5-FU 5-FU | 2% of CLint ratio No function | [60] [58] |
| rs138616379 | PV00869 | Exon 14 | 1775G > A | R592Q | IV | HEK293T/c17 | 5-FU | Decreased function | [58] |
| rs145773863 | PV00894 | Exon 14 | 1777G > A | G593R | IV | HEK293T/c17 | 5-FU | No function | [58] |
| rs147601618 | PV00898 | Exon 14 | 1796T > C | M599T | IV | HEK293T/c17 | 5-FU | Normal function | [58] |
| Rs72549304 | PV01034 | Exon 4 | 1898delC (DPYD*3) | P633fs | IV | HEK293T/c17 | 5-FU | No function | [58] |
| rs3918289 | PV00982 | Exon 14 | 1905C > T/G | N635K | IV | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs3918290 | PV00983 | Intron 14 | 1905 + 1G > A (DPYD*2A) | Exon 14 skipping | IV | HEK293T/c17 | 5-FU | No function | [54] |
| rs55971861 | PV01003 | Exon 15 | 1906A > C | I636L | IV | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs138545885 | PV00868 | Exon 16 | 1990G > T | A664S | IV | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs137999090 | PV00866 | Exon 16 | 2021G > A | G674D | IV | HEK293T/c17 | 5-FU | No function | [58] |
| – | – | Exon 17 | 2096G > C | R699T | IV | HEK293T/c17 | 5-FU | Normal function | [59] |
| rs145548112 | PV00893 | Exon 17 | 2161G > A | A721T | IV | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs146529561 | PV00896 | Exon 18 | 2186C > T | A729V | IV | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs1801160 | PV00909 | Exon 18 | 2194G > A (DPYD*6) | V732I | IV | 293FT HEK293T/c17 HEK293 Flp-In | 5-FU 5-FU Thymine | 114% of CLint ratio Normal function Decreased function | [60] [54] [55] |
| rs60511679 | PV01017 | Exon 18 | 2195T > G | V732G | IV | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs112766203 | PV00858 | Exon 18 | 2279C > T | T760I | IV | HEK293T/c17 | 5-FU | Decreased function | [58] |
| rs56005131 | PV01004 | Exon 19 | 2303C > A | T768K | IV | 293FT HEK293T/c17 E. coli | 5-FU 5-FU 5-FU | 48% of CLint ratio Normal function 83% of CLint ratio | [60] [58] [57] |
| rs199634007 | PV00922 | Exon 19 | 2336C > A | T779N | IV | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs547099198 | PV00994 | Exon 19 | 2378C > T | T793I | IV | HEK293T/c17 | 5-FU | Decreased function | [59] |
| – | – | Exon 19 | 2420A > G | H807R | IV | 293FT | 5-FU | 50% of CLint ratio | [60] |
| – | – | Exon 20 | 2476G > A | V826M | IV | 293FT | 5-FU | 35% of CLint ratio | [60] |
| rs200687447 | PV00930 | Exon 20 | 2482G > A | E828K | IV | HEK293T/c17 | 5-FU | Increased function | [58] |
| rs60139309 | PV01016 | Exon 20 | 2582A > G | K861R | V | HEK293T/c17 | 5-FU | Increased function | [58] |
| rs201035051 | PV00934 | Exon 21 | 2623A > C | K875Q | V | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs55674432 | PV00996 | Exon 21 | 2639G > T | G880V | V | HEK293T/c17 | 5-FU | No function | [58] |
| rs147545709 | PV00897 | Exon 21 | 2656C > T | R886C | V | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs1801267 | PV00912 | Exon 21 | 2657G > A (DPYD*9B) | R886H | V | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs188052243 | PV00918 | Exon 21 | 2678A > G | N893S | V | 293FT HEK293T/c17 | 5-FU 5-FU | 61% of CLint ratio Decreased function | [60] [58] |
| – | – | Exon 22 | 2777G > T | G926V | V | 293FT | 5-FU | No function | [58] |
| – | – | Exon 22 | 2822T > C | V941A | V | HEK293T/c17 | 5-FU | Decreased function | [59] |
| – | – | Exon 22 | 2843T > C | I948T | V | HEK293 Flp-In | Thymine | Decreased function | [56] |
| rs67376798 | PV01031 | Exon 22 | 2846A > T | D949V | V | HEK293T/c17 HEK293 Flp-In | 5-FU Thymine | Decreased function Decreased function | [58] [55] |
| rs141044036 | PV00876 | Exon 22 | 2872A > G | K958E | V | HEK293T/c17 | 5-FU | No function | [58] |
| rs145529148 | PV00892 | Exon 23 | 2915A > G | Q972R | V | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs72547602 | PV01033 | Exon 23 | 2921A > T | D974V | V | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs72547601 | PV01032 | Exon 23 | 2933A > G | H978R | V | HEK293T/c17 | 5-FU | No function | [58] |
| rs61757362 | PV01019 | Exon 23 | 2948C > T | T983I | V | HEK293T/c17 | 5-FU | Decreased function | [58] |
| rs202144771 | PV00941 | Exon 23 | 2977C > T | L993F | V | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs139459586 | PV00870 | Exon 23 | 2978T > G | L993R | V | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs1801268 | PV00913 | Exon 23 | 2983G > T (DPYD*10) | V995F | V | HEK293T/c17 | 5-FU | No function | [58] |
| rs140114515 | PV00873 | Exon 23 | 3049G > A | V1017I | V | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs148799944 | PV00899 | Exon 23 | 3061G > C | V1021L | V | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs114096998 | PV00860 | Exon 23 | 3067C > A | P1023T | V | HEK293T/c17 | 5-FU | Normal function | [58] |
| rs114096998 | PV00861 | Exon 23 | 3067C > T | P1023S | V | HEK293T/c17 | 5-FU | Decreased function | [59] |
| dbSNP rsID | Location | Nucleotide Change | Amino Acid Substitution | Expression System | Substrates | Effect | References |
|---|---|---|---|---|---|---|---|
| rs199618701 | Exon 1 | 17G > A | R6Q | 293FT | FUH2 | 120% of CLint ratio | [83] |
| rs57732538 | Exon 1 | 19C > G | L7V | 293FT RKO E. coli | FUH2UH2UH2 | 116% of CLint ratio 65% of wild-type DHP No function | [83] [82] [76] |
| rs572241599 | Exon 1 | 48C > G | N16K | 293FT | FUH2 | No function | [83] |
| – | Exon 1 | 203C > G | T68R | 293FT COS-7 | FUH25-bromo-UH2 | No function 1.5% of wild-type DHP | [83] [17] |
| rs370718225 | Exon 1 | 209T > C | M70T | 293FT E. coli | FUH2UH2 | No function No function | [83] [76] |
| – | Exon 1 | 242A > G | D81G | 293FT E. coli | FUH2UH2 | No function No function | [83] [76] |
| – | Exon 2 | 349T > C | W117R | 293FT | FUH2 | 44% of CLint ratio | [83] |
| rs36027551 | Exon 3 | 541C > T | R181W | 293FT RKO | FUH2UH2 | 110% of CLint ratio 99% of wild-type DHP | [83] [82] |
| rs751371011 | Exon 4 | 750G > A | M250I | HEK293 | UH2 | 2% of wild-type DHP | [77] |
| – | Exon 5 | 833G > A | G278D | 293FT E. coli | FUH2UH2 | No function No function | [83] [21] |
| – | Exon 5 | 884A > G | H295R | HEK293 | UH2 | 9.8% of wild-type DHP | [77] |
| rs200913682 | Exon 5 | 905G > A | R302Q | 293FT E. coli | FUH2UH2 | No function 3.9% of wild-type DHP | [83] [76] |
| rs121964923 | Exon 6 | 1001A > G | Q334R | 293FT HEK293 COS-7 | FUH2UH25-bromo-UH2 | 20% of CLint ratio 9.7% of wild-type DHP 2.5% of wild-type DHP | [83] [77] [17] |
| rs530911437 | Exon 6 | 1010T > C | L337P | 293FT E. coli | FUH2UH2 | No function No function | [83] [76] |
| rs201457190 | Exon 6 | 1027A > G | T343A | 293FT E. coli | FUH2UH2 | 43% of CLint ratio 49% of wild-type DHP | [83] [76] |
| rs121964924 | Exon 6 | 1078T > C | W360R | 293FT E. coli E. coli COS-7 | FUH2UH2UH25-bromo-UH2 | No function No function No function 1.2% of wild-type DHP | [83] [71] [76] [17] |
| rs138282507 | Exon 6 | 1090G > A | V364M | 293FT E. coli | FUH2UH2 | 8% of CLint ratio No function | [83] [76] |
| rs201258823 | Exon 7 | 1137C > A | S379R | 293FT E. coli | FUH2UH2 | No function 0.20–.9% of wild-type DHP | [83] [76] |
| rs267606774 | Exon 7 | 1235G > T | R412M | 293FT E. coli | FUH2UH2 | 36% of CLint ratio No function | [83] [71] |
| – | Exon 8 | 1253C > T | T418I | HEK293 | UH2 | 64% of wild-type DHP | [77] |
| rs267606773 | Exon 8 | 1303G > A | G435R | 293FT COS-7 | FUH25-bromo-UH2 | No function 5.1% of wild-type DHP | [83] [17] |
| rs201280871 | Exon 8 | 1393C > T | R465X | 293FT E. coli | FUH2UH2 | No function No function | [83] [76] |
| rs61758444 | Exon 8 | 1423C > T | R475X | 293FT E. coli | FUH2UH2 | No function 0.2–0.9% of wild-type DHP | [83] [76] |
| rs142574766 | Exon 9 | 1468C > T | R490C | 293FT E. coli COS-7 | FUH2UH25-bromo-UH2 | No function 0.2–0.9% of wild-type DHP 1.7% of wild-type DHP | [83] [76] [17] |
| Rs189448963 | Exon 9 | 1469G > A | R490H | HEK293 | UH2 | 0.3% of wild-type DHP | [77] |
| db SNP rsID | Location | Nucleotide Change | Amino Acid Substitution | Expression System | Substrates | Effect | References |
|---|---|---|---|---|---|---|---|
| – | Exon 1 | c.38T > C | p.L13S | E. coli | bUPA | 6% of wild-type β-UP | [95] |
| rs200145797 | Exon 1 | c.91G > A | p.G31S | HEK293 | bUPA | 52% of wild-type β-UP | [97] |
| rs121908066 | Exon 2 | c.209G > C | p.R70P | No reports of in vitro study | [98] | ||
| rs34035085 | Exon 2 | c.254C > A | p.A85E | E. coli RKO | bUPA bUPA | No function 2.7% of wild-type β-UP | [93] [101] |
| – | Exon 6 | c.703G > A | p.G235R | E. coli | bUPA | No function | [95] |
| rs144135211 | Exon 6 | c.706C > T | p.R236W | E. coli | bUPA | No function | [95] |
| rs145766755 | Exon 7 | c.792C > A | p.S264R | E. coli | bUPA | 20% of wild-type β-UP | [95] |
| – | Exon 7 | c.811G > A | p.E271K | HEK293 | bUPA | 0.7% of wild-type β-UP | [97] |
| – | Exon 7 | c.851G > T | p.C284F | No reports of in vitro study | [99] | ||
| rs1375840064 | Exon 7 | c.853G > A | p.A285T | No reports of in vitro study | [99] | ||
| – | Exon 7 | c.857T > C | p.I286T | HEK293 | bUPA | 70% of wild-type β-UP | [97] |
| rs118163237 | Exon 9 | c.977G > A | p.R326Q | E. coli HEK293 | bUPA bUPA | No function 1.3% of wild-type β-UP | [95] [97] |
| rs369879221 | Exon 10 | c.1076C > T | p.T359M | E. coli | bUPA | No function | [95] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hishinuma, E.; Gutiérrez Rico, E.; Hiratsuka, M. In Vitro Assessment of Fluoropyrimidine-Metabolizing Enzymes: Dihydropyrimidine Dehydrogenase, Dihydropyrimidinase, and β-Ureidopropionase. J. Clin. Med. 2020, 9, 2342. https://doi.org/10.3390/jcm9082342
Hishinuma E, Gutiérrez Rico E, Hiratsuka M. In Vitro Assessment of Fluoropyrimidine-Metabolizing Enzymes: Dihydropyrimidine Dehydrogenase, Dihydropyrimidinase, and β-Ureidopropionase. Journal of Clinical Medicine. 2020; 9(8):2342. https://doi.org/10.3390/jcm9082342
Chicago/Turabian StyleHishinuma, Eiji, Evelyn Gutiérrez Rico, and Masahiro Hiratsuka. 2020. "In Vitro Assessment of Fluoropyrimidine-Metabolizing Enzymes: Dihydropyrimidine Dehydrogenase, Dihydropyrimidinase, and β-Ureidopropionase" Journal of Clinical Medicine 9, no. 8: 2342. https://doi.org/10.3390/jcm9082342
APA StyleHishinuma, E., Gutiérrez Rico, E., & Hiratsuka, M. (2020). In Vitro Assessment of Fluoropyrimidine-Metabolizing Enzymes: Dihydropyrimidine Dehydrogenase, Dihydropyrimidinase, and β-Ureidopropionase. Journal of Clinical Medicine, 9(8), 2342. https://doi.org/10.3390/jcm9082342






