Natriuretic Peptides, Cognitive Impairment and Dementia: An Intriguing Pathogenic Link with Implications in Hypertension
Abstract
1. Introduction
2. Populations-Based Evidence
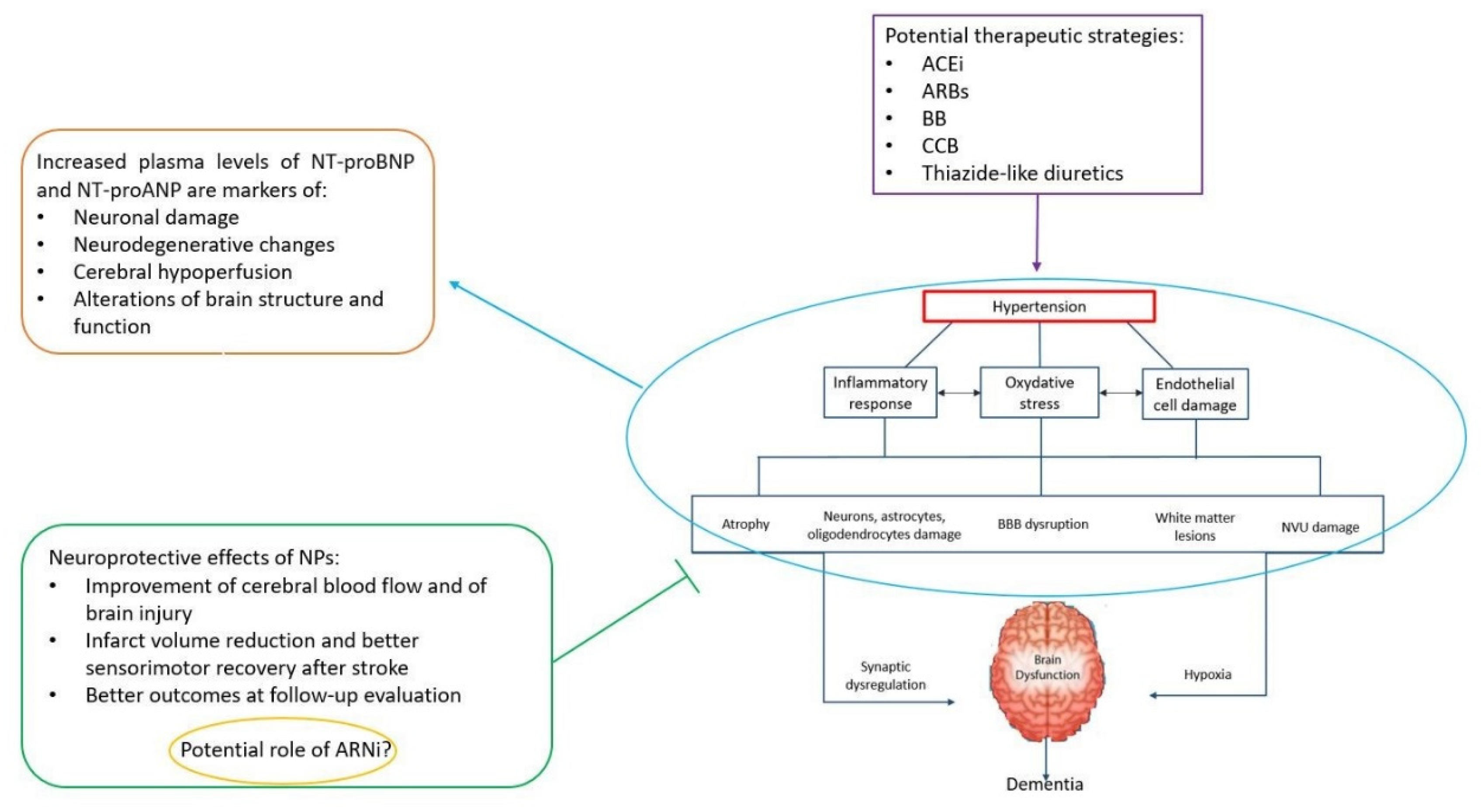
3. Mechanistic Insights on the Link between NPs and Cognitive Decline/Dementia
3.1. CVD-Dependent Pathogenic Mechanisms
3.2. CVD-Independent Pathogenic Mechanisms
4. NPs, Cognitive Impairment and Dementia: Implications in Hypertension
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Levin, E.R.; Gardner, D.G.; Samson, W.K. Natriuretic peptides. N. Engl. J. Med. 1998, 339, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Sciarretta, S.; Valenti, V.; Stanzione, R.; Volpe, M. Natriuretic peptides: An update on bioactivity, potential therapeutic use, and implication in cardiovascular diseases. Am. J. Hypertens. 2008, 21, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Nishikimi, T.; Kuwahara, K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides 2019, 111, 18–25. [Google Scholar] [CrossRef]
- Volpe, M.; Rubattu, S.; Burnett, J., Jr. Natriuretic peptides in cardiovascular diseases: Current use and perspectives. Eur. Heart J. 2014, 35, 419–425. [Google Scholar] [CrossRef]
- van Peet, P.G.; de Craen, A.J.; Gussekloo, J.; de Ruijter, W. Plasma NT-proBNP as predictor of change in functional status, cardiovascular morbidity and mortality in the oldest old: The Leiden 85-plus study. Age (Dordr) 2014, 36, 9660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagata, T.; Ohara, T.; Hata, J.; Sakata, S.; Furuta, Y.; Yoshida, D.; Honda, T.; Hirakawa, Y.; Ide, T.; Kanba, S.; et al. NT-proBNP and Risk of Dementia in a General Japanese Elderly Population: The Hisayama Study. J. Am. Heart Assoc. 2019, 8, e011652. [Google Scholar] [CrossRef]
- Zonneveld, H.I.; Arfan Ikram, M.; Hofman, A.; Niessen, W.J.; van der Lugt, A.; Krestin, G.P.; Franco, O.H.; Vernooij, M.W. N-Terminal Pro-B-Type Natriuretic Peptide and Subclinical Brain Damage in the General Population. Radiology 2017, 283, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, I.T.; Elbejjani, M.; Sabayan, B.; Jacobs, D.R., Jr.; Meirelles, O.; Sanchez, O.A.; Tracy, R.; Bryan, N.; Launer, L.J. N-Terminal pro-Brain Natriuretic Peptide and Associations With Brain Magnetic Resonance Imaging (MRI) Features in Middle Age: The CARDIA Brain MRI Study. Front. Neurol. 2018, 9, 307. [Google Scholar] [CrossRef]
- Riching, A.S.; Major, J.L.; Londono, P.; Bagchi, R.A. The Brain-Heart Axis: Alzheimer’s, Diabetes, and Hypertension. ACS Pharmacol. Transl. Sci. 2019, 3, 21–28. [Google Scholar] [CrossRef]
- Ostovaneh, M.R.; Moazzami, K.; Yoneyama, K.; Venkatesh, B.A.; Heckbert, S.R.; Wu, C.O.; Shea, S.; Post, W.S.; Fitzpatrick, A.L.; Burke, G.L.; et al. Change in NT-proBNP (N-Terminal Pro-B-Type Natriuretic Peptide) Level and Risk of Dementia in Multi-Ethnic Study of Atherosclerosis (MESA). Hypertension 2020, 75, 316–323. [Google Scholar] [CrossRef]
- Di Daniele, N.; Celotto, R.; Alunni Fegatelli, D.; Gabriele, M.; Rovella, V.; Scuteri, A. Common Carotid Artery Calcification Impacts on Cognitive Function in Older Patients. High Blood Press. Cardiovasc. Prev. 2019, 26, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Rizzoni, M.; Nardin, M.; Chiarini, G.; Agabiti-Rosei, C.; Aggiusti, C.; Paini, A.; Salvetti, M.; Muiesan, M.L. Vascular Aging and Disease of the Small Vessels. High Blood Press. Cardiovasc. Prev. 2019, 26, 183–189. [Google Scholar] [CrossRef]
- Hilal, S.; Chai, Y.L.; Ikram, M.K.; Elangovan, S.; Yeow, T.B.; Xin, X.; Chong, J.Y.; Venketasubramanian, N.; Richards, A.M.; Chong, J.P.C.; et al. Markers of Cardiac Dysfunction in Cognitive Impairment and Dementia. Medicine (Baltimore) 2015, 94, e297. [Google Scholar] [CrossRef] [PubMed]
- Hilal, S.; Chai, Y.L.; van Veluw, S.; Shaik, M.A.; Ikram, M.K.; Venketasubramanian, N.; Richards, A.M.; Biessels, G.J.; Chen, C. Association Between Subclinical Cardiac Biomarkers and Clinically Manifest Cardiac Diseases With Cortical Cerebral Microinfarcts. JAMA Neurol. 2017, 74, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Tynkkynen, J.; Hernesniemi, J.A.; Laatikainen, T.; Havulinna, A.S.; Salo, P.; Blankenberg, S.; Zeller, T.; Salomaa, V. High-sensitivity cardiac troponin I and NT-proBNP as predictors of incident dementia and Alzheimer’s disease: The FINRISK Study. J. Neurol. 2017, 264, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.T.; Holtzman, D.M.; Fagan, A.M.; Shaw, L.M.; Perrin, R.; Arnold, S.E.; Grossman, M.; Xiong, C.; Craig-Schapiro, R.; Clark, C.M.; et al. Alzheimer’s Disease Neuroimaging Initiative. Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease [published correction appears in Neurology. 2012 Oct 30;79(18):1935]. Neurology 2012, 79, 897–905. [Google Scholar] [CrossRef]
- Sabayan, B.; van Buchem, M.A.; Sigurdsson, S.; Zhang, Q.; Harris, T.B.; Gudnason, V.; Arai, A.E.; Launer, L.J. Cardiac hemodynamics are linked with structural and functional features of brain aging: The age, gene/environment susceptibility (AGES)-Reykjavik Study. J. Am. Heart Assoc. 2015, 4, e001294. [Google Scholar] [CrossRef]
- Mirza, S.S.; de Bruijn, R.F.; Koudstaal, P.J.; van den Meiracker, A.H.; Franco, O.H.; Hofman, A.; Tiemeier, H.; Ikram, M.A. The N-terminal pro B-type natriuretic peptide, and risk of dementia and cognitive decline: A 10-year follow-up study in the general population. J. Neurol. Neurosurg. Psychiatry 2016, 87, 356–362. [Google Scholar] [CrossRef]
- Hiltunen, M.; Kerola, T.; Kettunen, R.; Hartikainen, S.; Sulkava, R.; Vuolteenaho, O.; Nieminen, T. The prognostic capacity of B-type natriuretic peptide on cognitive disorder varies by age. Ann. Med. 2013, 45, 74–78. [Google Scholar] [CrossRef]
- Mazure, C.M.; Swendsen, J. Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. 2016, 15, 451–452. [Google Scholar] [CrossRef]
- Rocca, W.A.; Mielke, M.M.; Vemuri, P.; Miller, V.M. Sex and gender differences in the causes of dementia: A narrative review. Maturitas 2014, 79, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Cheng, S.; Choong, K.; Larson, M.G.; Murabito, J.M.; Newton-Cheh, C.; Bhasin, S.; McCabe, E.L.; Miller, K.K.; Redfield, M.M.; et al. Influence of sex and hormone status on circulating natriuretic peptides. J. Am. Coll. Cardiol. 2011, 58, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Buerger, K.; Uspenskaya, O.; Hartmann, O.; Hansson, O.; Minthon, L.; Blennow, K.; Moeller, H.J.; Teipel, S.J.; Ernst, A.; Bergmann, A.; et al. Prediction of Alzheimer’s disease using midregional proadrenomedullin and midregional proatrial natriuretic peptide: A retrospective analysis of 134 patients with mild cognitive impairment. J. Clin. Psychiatry 2011, 72, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Holm, H.; Nägga, K.; Nilsson, E.D.; Ricci, F.; Melander, O.; Hansson, O.; Bachus, E.; Magnusson, M.; Fedorowski, A. Biomarkers of microvascular endothelial dysfunction predict incident dementia: A population-based prospective study. J. Intern. Med. 2017, 282, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Abete, P.; Della-Morte, D.; Gargiulo, G.; Basile, C.; Langellotto, A.; Galizia, G.; Testa, G.; Canonico, V.; Bonaduce, D.; Cacciatore, F. Cognitive impairment and cardiovascular diseases in the elderly. A heart-brain continuum hypothesis. Ageing Res. Rev. 2014, 18, 41–52. [Google Scholar] [CrossRef]
- Mueller, K.; Thiel, F.; Beutner, F.; Teren, A.; Frisch, S.; Ballarini, T.; Möller, H.E.; Ihle, K.; Thiery, J.; Schuler, G.; et al. Brain Damage With Heart Failure: Cardiac Biomarker Alterations and Gray Matter Decline. Circ. Res. 2020, 126, 750–764. [Google Scholar] [CrossRef]
- Mahinrad, S.; de Craen, A.J.M.; Yasar, S.; van Heemst, D.; Sabayan, B. Natriuretic peptides in the central nervous system: Novel targets for cognitive impairment. Neurosci. Biobehav. Rev. 2016, 68, 148–156. [Google Scholar] [CrossRef]
- Cao, L.H.; Yang, X.L. Natriuretic peptides and their receptors in the central nervous system. Prog. Neurobiol. 2008, 84, 234–248. [Google Scholar] [CrossRef]
- Prado, J.; Baltrons, M.A.; Pifarré, P.; García, A. Glial cells as sources and targets of natriuretic peptides. Neurochem. Int. 2010, 57, 367–374. [Google Scholar] [CrossRef]
- Quirion, R. Receptor sites for atrial natriuretic factors in brain and associated structures: An overview. Cell. Mol. Neurobiol. 1989, 9, 45–55. [Google Scholar] [CrossRef]
- Colini Baldeschi, A.; Pittaluga, E.; Andreola, F.; Rossi, S.; Cozzolino, M.; Nicotera, G.; Sferrazza, G.; Pierimarchi, P.; Serafino, A. Atrial Natriuretic Peptide Acts as a Neuroprotective Agent in in Vitro Models of Parkinson’s Disease via Up-regulation of the Wnt/β-Catenin Pathway. Front. Aging Neurosci. 2018, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Cotugno, M.; Forte, M.; Stanzione, R.; Bianchi, F.; Madonna, M.; Marchitti, S.; Volpe, M. Effects of dual angiotensin type 1 receptor/neprilysin inhibition vs. angiotensin type 1 receptor inhibition on target organ injury in the stroke-prone spontaneously hypertensive rat. J. Hypertens. 2018, 36, 1902–1914. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Omland, T.; Wolf, P.A.; Vasan, R.S. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N. Engl. J. Med. 2004, 350, 655–663. [Google Scholar] [CrossRef]
- James, M.L.; Wang, H.; Venkatraman, T.; Song, P.; Lascola, C.D.; Laskowitz, D.T. Brain natriuretic peptide improves long-term functional recovery after acute CNS injury in mice. J. Neurotrauma. 2010, 27, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Susavila, H.; Rodríguez-Yáñez, M.; Dopico-López, A.; Arias, S.; Santamaría, M.; Ávila-Gómez, P.; Doval-García, J.M.; Sobrino, T.; Iglesias-Rey, R.; Castillo, J.; et al. Heads and Tails of Natriuretic Peptides: Neuroprotective Role of Brain Natriuretic Peptide. J. Am. Heart Assoc. 2017, 6, e007329. [Google Scholar] [CrossRef]
- Schaare, H.L.; Kharabian Masouleh, S.; Beyer, F.; Kumral, D.; Uhlig, M.; Reinelt, J.D.; Reiter, A.M.F.; Lampe, L.; Babayan, A.; Erbey, M.; et al. Association of peripheral blood pressure with gray matter volume in 19-to 40-year-old adults [published correction appears in Neurology. 2019 Mar 5;92(10):495]. Neurology 2019, 92, e758–e773. [Google Scholar] [CrossRef] [PubMed]
- Launer, L.J.; Ross, G.W.; Petrovitch, H.; Masaki, K.; Foley, D.; White, L.R.; Havlik, R.J. Midlife blood pressure and dementia: The Honolulu-Asia aging study. Neurobiol. Aging 2000, 21, 49–55. [Google Scholar] [CrossRef]
- Iadecola, C.; Yaffe, K.; Biller, J.; Bratzke, L.C.; Faraci, F.M.; Gorelick, P.B.; Gulati, M.; Kamel, H.; Knopman, D.S.; Launer, L.J.; et al. Impact of Hypertension on Cognitive Function: A Scientific Statement From the American Heart Association. Hypertension 2016, 68, e67–e94. [Google Scholar] [CrossRef] [PubMed]
- Pandav, R.; Dodge, H.H.; DeKosky, S.T.; Ganguli, M. Blood pressure and cognitive impairment in India and the United States: A cross-national epidemiological study. Arch. Neurol. 2003, 60, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Gilsanz, P.; Mayeda, E.R.; Glymour, M.M.; Quesenberry, C.P.; Mungas, D.M.; DeCarli, C.; Dean, A.; Whitmer, R.A. Female sex, early-onset hypertension, and risk of dementia. Neurology 2017, 89, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A.; Galecki, A.T.; Langa, K.M.; Unverzagt, F.W.; Kabeto, M.U.; Giordani, B.; Cushman, M.; McClure, L.A.; Safford, M.M.; Wadley, V.G. Blood Pressure and Cognitive Decline Over 8 Years in Middle-Aged and Older Black and White Americans. Hypertension 2019, 73, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Gottesman, R.F. Neurovascular and Cognitive Dysfunction in Hypertension. Circ. Res. 2019, 124, 1025–1044. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, A.; Brancati, A.M.; Gianni, W.; Assisi, A.; Volpe, M. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: A pilot study. J. Hypertens. 2005, 23, 1211–1216. [Google Scholar] [CrossRef]
- Rouch, L.; Cestac, P.; Sallerin, B.; Andrieu, S.; Bailly, H.; Beunardeau, M.; Cohen, A.; Dubail, D.; Hernandorena, I.; Seux, M.L.; et al. Pulse Wave Velocity Is Associated With Greater Risk of Dementia in Mild Cognitive Impairment Patients. Hypertension 2018, 72, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.F.; Cunha, A.R.; Cunha, M.R.; Gismondi, R.A.; Oigman, W. The Role of Renin-Angiotensin-Aldosterone System and Its New Components in Arterial Stiffness and Vascular Aging. High Blood Press. Cardiovasc. Prev. 2018, 25, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, Y.; Hoshide, S.; Kanegae, H.; Kario, K. Increased Arterial Stiffness Amplifies the Association Between Home Blood Pressure Variability and Cardiac Overload: The J-HOP Study. Hypertension 2020, 75, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Robb, S.D.; Murdoch, D.R.; Morton, J.J.; Ford, I.; Morrison, C.E.; Tunstall-Pedoe, H.; McMurray, J.J.; Dargie, H.J. Biochemical detection of left-ventricular systolic dysfunction. Lancet 1998, 351, 9–13. [Google Scholar] [CrossRef]
- Vilar-Bergua, A.; Riba-Llena, I.; Penalba, A.; Cruz, L.M.; Jiménez-Balado, J.; Montaner, J.; Delgado, P. N-terminal pro-brain natriuretic peptide and subclinical brain small vessel disease. Neurology 2016, 87, 2533–2539. [Google Scholar] [CrossRef]
- Rubattu, S.; Stanzione, R.; Cotugno, M.; Bianchi, F.; Marchitti, S.; Forte, M. Epigenetic control of natriuretic peptides: Implications for health and disease. Cell. Mol. Life Sci. 2020, in press. [Google Scholar] [CrossRef]
- Kerola, T.; Nieminen, T.; Hartikainen, S.; Sulkava, R.; Vuolteenaho, O.; Kettunen, R. B-type natriuretic peptide as a predictor of declining cognitive function and dementia—A cohort study of an elderly general population with a 5-year follow-up. Ann. Med. 2010, 42, 207–215. [Google Scholar] [CrossRef]
- Staessen, J.A.; Fagard, R.; Thijs, L.; Celis, H.; Arabidze, G.G.; Birkenhäger, W.H.; Bulpitt, C.J.; de Leeuw, P.W.; Dollery, C.T.; Fletcher, A.E.; et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997, 350, 757–764. [Google Scholar] [CrossRef]
- Hanon, O.; Forette, F. Prevention of dementia: Lessons from SYST-EUR and PROGRESS. J. Neurol. Sci. 2004, 226, 71–74. [Google Scholar] [CrossRef]
- Ikram, M.A.; Brusselle, G.G.O.; Murad, S.D.; van Duijn, C.M.; Franco, O.H.; Goedegebure, A.; Klaver, C.C.W.; Nijsten, T.E.C.; Peeters, R.P.; Stricker, B.H.; et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur. J. Epidemiol. 2017, 32, 807–850. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Buerger, K.; Teipel, S.; Uspenskaya, O.; Hartmann, O.; Hansson, O.; Minthon, L.; Rujescu, D.; Moeller, H.J.; Zetterberg, H.; et al. Antihypertensive therapy is associated with reduced rate of conversion to Alzheimer’s disease in midregional proatrial natriuretic peptide stratified subjects with mild cognitive impairment. Biol. Psychiatry 2011, 70, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Hernandorena, I.; Duron, E.; Vidal, J.S.; Hanon, O. Treatment options and considerations for hypertensive patients to prevent dementia. Expert Opin. Pharmacother. 2017, 18, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Sink, K.M.; Leng, X.; Williamson, J.; Kritchevsky, S.B.; Yaffe, K.; Kuller, L.; Yasar, S.; Atkinson, H.; Robbins, M.; Psaty, B.; et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: Results from the Cardiovascular Health Study. Arch. Intern. Med. 2009, 169, 1195–1202. [Google Scholar] [CrossRef]
- Csikai, E.; Andrejkovics, M.; Balajthy-Hidegh, B.; Hofgárt, G.; Kardos, L.; Diószegi, Á.; Rostás, R.; Czuriga-Kovács, K.R.; Csongrádi, É.; Csiba, L. Influence of angiotensin-converting enzyme inhibition on reversibility of alterations in arterial wall and cognitive performance associated with early hypertension: A follow-up study. Medicine (Baltimore) 2019, 98, e16966. [Google Scholar] [CrossRef]
- Tzourio, C.; Anderson, C.; Chapman, N.; Woodward, M.; Neal, B.; MacMahon, S.; Chalmers, J.; PROGRESS Collaborative Group. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch. Intern. Med. 2003, 163, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cui, Y.; Zhao, Y.; Dong, Y.; Duan, D.; Wang, J.; Sheng, L.; Ji, T.; Zhou, T.; Hu, W.; et al. Effects of sartans and low-dose statins on cerebral white matter hyperintensities and cognitive function in older patients with hypertension: A randomized, double-blind and placebo-controlled clinical trial. Hypertens. Res. 2019, 42, 717–729. [Google Scholar] [CrossRef]
- Bavishi, C.; Messerli, F.H.; Kadosh, B.; Ruilope, L.M.; Kario, K. Role of neprilysin inhibitor combinations in hypertension: Insights from hypertension and heart failure trials. Eur. Heart J. 2015, 36, 1967–1973. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Cannon, J.A.; Shen, L.; Jhund, P.S.; Kristensen, S.L.; Køber, L.; Chen, F.; Gong, J.; Lefkowitz, M.P.; Rouleau, J.L.; Shi, V.C.; et al. Dementia-related adverse events in PARADIGM-HF and other trials in heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Kicking the tyres of a heart failure trial: Physician response to the approval of sacubitril/valsartan in the USA. Eur. J. Heart Fail. 2016, 18, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Langenickel, T.H.; Tsubouchi, C.; Ayalasomayajula, S.; Pal, P.; Valentin, M.A.; Hinder, M.; Jhee, S.; Gevorkyan, H.; Rajman, I. The effect of LCZ696 (sacubitril/valsartan) on amyloid-β concentrations in cerebrospinal fluid in healthy subjects. Br. J. Clin. Pharmacol. 2016, 81, 878–890. [Google Scholar] [CrossRef] [PubMed]
- Lowy, A.; Munk, V.C.; Ong, S.H.; Burnier, M.; Vrijens, B.; Tousset, E.P.; Urquhart, J. Effects on blood pressure and cardiovascular risk of variations in patients’ adherence to prescribed antihypertensive drugs: Role of duration of drug action. Int. J. Clin. Pract. 2011, 65, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Yasar, S.; Anderson, C.S.; Andrews, S.; Antikainen, R.; Arima, H.; Beckett, N.; Beer, J.C.; Bertens, A.S.; Booth, A. Investigation of antihypertensive class, dementia, and cognitive decline: A meta-analysis. Neurology 2020, 94, e267–e281. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, G.; Bianchi, F.; Cotugno, M.; Volpe, M.; Rubattu, S. Natriuretic Peptides, Cognitive Impairment and Dementia: An Intriguing Pathogenic Link with Implications in Hypertension. J. Clin. Med. 2020, 9, 2265. https://doi.org/10.3390/jcm9072265
Gallo G, Bianchi F, Cotugno M, Volpe M, Rubattu S. Natriuretic Peptides, Cognitive Impairment and Dementia: An Intriguing Pathogenic Link with Implications in Hypertension. Journal of Clinical Medicine. 2020; 9(7):2265. https://doi.org/10.3390/jcm9072265
Chicago/Turabian StyleGallo, Giovanna, Franca Bianchi, Maria Cotugno, Massimo Volpe, and Speranza Rubattu. 2020. "Natriuretic Peptides, Cognitive Impairment and Dementia: An Intriguing Pathogenic Link with Implications in Hypertension" Journal of Clinical Medicine 9, no. 7: 2265. https://doi.org/10.3390/jcm9072265
APA StyleGallo, G., Bianchi, F., Cotugno, M., Volpe, M., & Rubattu, S. (2020). Natriuretic Peptides, Cognitive Impairment and Dementia: An Intriguing Pathogenic Link with Implications in Hypertension. Journal of Clinical Medicine, 9(7), 2265. https://doi.org/10.3390/jcm9072265




