Utility of Insulin Resistance in Estimating Cardiovascular Risk in Subjects with Type 1 Diabetes According to the Scores of the Steno Type 1 Risk Engine
Abstract
1. Introduction
2. Methods
2.1. Study Subjects
2.2. Study Design
2.2.1. Laboratory Analyses
2.2.2. Insulin Resistance
2.2.3. Assessment of Microvascular Complications
2.2.4. Steno Type 1 Risk Engine
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Livingstone, S.J.; Levin, D.; Looker, H.C.; Lindsay, R.S.; Wild, S.H.; Joss, N.; Leese, G.; Leslie, P.; McCrimmon, R.J.; Metcalfe, W.; et al. Scottish Diabetes Research Network epidemiology g., Scottish Renal R. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008–2010. JAMA 2015, 313, 37–44. [Google Scholar] [CrossRef]
- de Ferranti, S.D.; de Boer, I.H.; Fonseca, V.; Fox, C.S.; Golden, S.H.; Lavie, C.J.; Magge, S.N.; Marx, N.; McGuire, D.K.; Orchard, T.J.; et al. Type 1 diabetes mellitus and cardiovascular disease: A scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 2014, 37, 2843–2863. [Google Scholar] [CrossRef]
- Lind, M.; Svensson, A.M.; Kosiborod, M.; Gudbjornsdottir, S.; Pivodic, A.; Wedel, H.; Dahlqvist, S.; Clements, M.; Rosengren, A. Glycemic control and excess mortality in type 1 diabetes. N. Engl. J. Med. 2014, 371, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Rawshani, A.; Rawshani, A.; Franzen, S.; Eliasson, B.; Svensson, A.M.; Miftaraj, M.; McGuire, D.K.; Sattar, N.; Rosengren, A.; Gudbjornsdottir, S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N. Engl. J. Med. 2017, 376, 1407–1418. [Google Scholar] [CrossRef]
- Rawshani, A.; Sattar, N.; Franzen, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.M.; Eliasson, B.; Gudbjornsdottir, S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: A nationwide, register-based cohort study. Lancet 2018, 392, 477–486. [Google Scholar] [CrossRef]
- Livingstone, S.J.; Looker, H.C.; Hothersall, E.J.; Wild, S.H.; Lindsay, R.S.; Chalmers, J.; Cleland, S.; Leese, G.P.; McKnight, J.; Morris, A.D.; et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012, 9, e1001321. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular risk factors: The Framingham study. Circulation 1979, 59, 8–13. [Google Scholar] [CrossRef]
- Stevens, R.J.; Kothari, V.; Adler, A.I.; Stratton, I.M.; United Kingdom Prospective Diabetes Study Group. The UKPDS risk engine: A model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin. Sci. (Lond.) 2001, 101, 671–679. [Google Scholar] [CrossRef]
- Zgibor, J.C.; Piatt, G.A.; Ruppert, K.; Orchard, T.J.; Roberts, M.S. Deficiencies of cardiovascular risk prediction models for type 1 diabetes. Diabetes Care 2006, 29, 1860–1865. [Google Scholar] [CrossRef]
- Zgibor, J.C.; Ruppert, K.; Orchard, T.J.; Soedamah-Muthu, S.S.; Fuller, J.; Chaturvedi, N.; Roberts, M.S. Development of a coronary heart disease risk prediction model for type 1 diabetes: The Pittsburgh CHD in type 1 diabetes risk model. Diabetes Res. Clin. Pract. 2010, 88, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, J.; Eeg-Olofsson, K.; Eliasson, B.; Zethelius, B.; Gudbjornsdottir, S.; Swedish National Diabetes Register. A new model for 5-year risk of cardiovascular disease in type 1 diabetes; from the Swedish National Diabetes Register (NDR). Diabet. Med. 2011, 28, 1213–1220. [Google Scholar] [CrossRef]
- Vistisen, D.; Andersen, G.S.; Hansen, C.S.; Hulman, A.; Henriksen, J.E.; Bech-Nielsen, H.; Jorgensen, M.E. Prediction of first cardiovascular disease event in type 1 diabetes mellitus: The steno type 1 risk engine. Circulation 2016, 133, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Chillaron, J.J.; Goday, A.; Flores-Le-Roux, J.A.; Benaiges, D.; Carrera, M.J.; Puig, J.; Cano-Perez, J.F.; Pedro-Botet, J. Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 3530–3534. [Google Scholar] [CrossRef] [PubMed]
- Cleland, S.J. Cardiovascular risk in double diabetes mellitus—When two worlds collide. Nat. Rev. Endocrinol. 2012, 8, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Agin, A.; Jeandidier, N.; Gasser, F.; Grucker, D.; Sapin, R. Use of insulin immunoassays in clinical studies involving rapid-acting insulin analogues: Bi-insulin IRMA preliminary assessment. Clin. Chem. Lab. Med. 2006, 44, 1379–1382. [Google Scholar] [CrossRef]
- Janssen, J.; Llaurado, G.; Varewijck, A.J.; Groop, P.H.; Forsblom, C.; Fernandez-Veledo, S.; van den Dungen, E.S.R.; Vendrell, J.; Hofland, L.J.; Yki-Jarvinen, H. Serum insulin bioassay reflects insulin sensitivity and requirements in type 1 diabetes. J. Clin. Endocrinol. Metab. 2017, 102, 3814–3821. [Google Scholar] [CrossRef]
- Williams, K.V.; Erbey, J.R.; Becker, D.; Arslanian, S.; Orchard, T.J. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes 2000, 49, 626–632. [Google Scholar] [CrossRef]
- Dabelea, D.; D’Agostino, R.B.J.; Mason, C.C.; West, N.; Hamman, R.F.; Mayer-Davis, E.J.; Maahs, D.; Klingensmith, G.; Knowler, W.C.; Nadeau, K. Development, validation and use of an insulin sensitivity score in youths with diabetes: The SEARCH for Diabetes in Youth study. Diabetologia 2011, 54, 78–86. [Google Scholar] [CrossRef]
- Duca, L.M.; Maahs, D.M.; Schauer, I.E.; Bergman, B.C.; Nadeau, K.J.; Bjornstad, P.; Rewers, M.; Snell-Bergeon, J.K. Development and validation of a method to estimate insulin sensitivity in patients with and without type 1 diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Llaurado, G.; Cano, A.; Albert, L.; Ballesta, S.; Mazarico, I.; Luchtenberg, M.F.; Gonzalez-Sastre, M.; Megia, A.; Simo, R.; Vendrell, J.; et al. Arterial stiffness is highly correlated with the scores obtained from the Steno Type 1 Risk Engine in subjects with T1DM. PLoS ONE 2019, 14, e0220206. [Google Scholar] [CrossRef] [PubMed]
- Hallal, P.C.; Victora, C.G. Reliability and validity of the International Physical Activity Questionnaire (IPAQ). Med. Sci. Sports Exerc. 2004, 36, 556. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart. J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- National Cholesterol Education Program. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Kilpatrick, E.S.; Rigby, A.S.; Atkin, S.L. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care 2007, 30, 707–712. [Google Scholar] [CrossRef]
- Gonzalez-Clemente, J.M.; Mauricio, D.; Richart, C.; Broch, M.; Caixas, A.; Megia, A.; Gimenez-Palop, O.; Simon, I.; Martinez-Riquelme, A.; Gimenez-Perez, G.; et al. Diabetic neuropathy is associated with activation of the TNF-alpha system in subjects with type 1 diabetes mellitus. Clin. Endocrinol. (Oxf.) 2005, 63, 525–529. [Google Scholar] [CrossRef]
- American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. 1), S124–S138. [Google Scholar] [CrossRef]
- Bebu, I.; Braffett, B.H.; Pop-Busui, R.; Orchard, T.J.; Nathan, D.M.; Lachin, J.M.; Group, D.E.R. The relationship of blood glucose with cardiovascular disease is mediated over time by traditional risk factors in type 1 diabetes: The DCCT/EDIC study. Diabetologia 2017, 60, 2084–2091. [Google Scholar] [CrossRef] [PubMed]
- Purnell, J.Q.; Braffett, B.H.; Zinman, B.; Gubitosi-Klug, R.A.; Sivitz, W.; Bantle, J.P.; Ziegler, G.; Cleary, P.A.; Brunzell, J.D.; Group, D.E.R. Impact of excessive weight gain on cardiovascular outcomes in type 1 diabetes: Results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications (dcct/edic) study. Diabetes Care 2017, 40, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.J.; Olson, J.C.; Erbey, J.R.; Williams, K.; Forrest, K.Y.; Smithline Kinder, L.; Ellis, D.; Becker, D.J. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2003, 26, 1374–1379. [Google Scholar] [CrossRef]
- Pane, A.; Conget, I.; Boswell, L.; Ruiz, S.; Vinals, C.; Perea, V.; Gimenez, M.; Cofan, M.; Blanco, J.; Vinagre, I.; et al. Insulin resistance is associated with preclinical carotid atherosclerosis in patients with type 1 diabetes. Diabetes Metab. Res. Rev. 2020, e3323. [Google Scholar] [CrossRef] [PubMed]
- Llaurado, G.; Cano, A.; Hernandez, C.; Gonzalez-Sastre, M.; Rodriguez, A.A.; Punti, J.; Berlanga, E.; Albert, L.; Simo, R.; Vendrell, J.; et al. Type 1 diabetes: Developing the first risk-estimation model for predicting silent myocardial ischemia. The potential role of insulin resistance. PLoS ONE 2017, 12, e0174640. [Google Scholar] [CrossRef] [PubMed]
- Dabelea, D.; Kinney, G.; Snell-Bergeon, J.K.; Hokanson, J.E.; Eckel, R.H.; Ehrlich, J.; Garg, S.; Hamman, R.F.; Rewers, M. Effect of type 1 diabetes on the gender difference in coronary artery calcification: A role for insulin resistance? The coronary artery calcification in type 1 diabetes (CACTI) Study. Diabetes 2003, 52, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, P.; Maahs, D.M.; Duca, L.M.; Pyle, L.; Rewers, M.; Johnson, R.J.; Snell-Bergeon, J.K. Estimated insulin sensitivity predicts incident micro- and macrovascular complications in adults with type 1 diabetes over 6 years: The coronary artery calcification in type 1 diabetes study. J. Diabetes Complicat. 2016, 30, 586–590. [Google Scholar] [CrossRef]
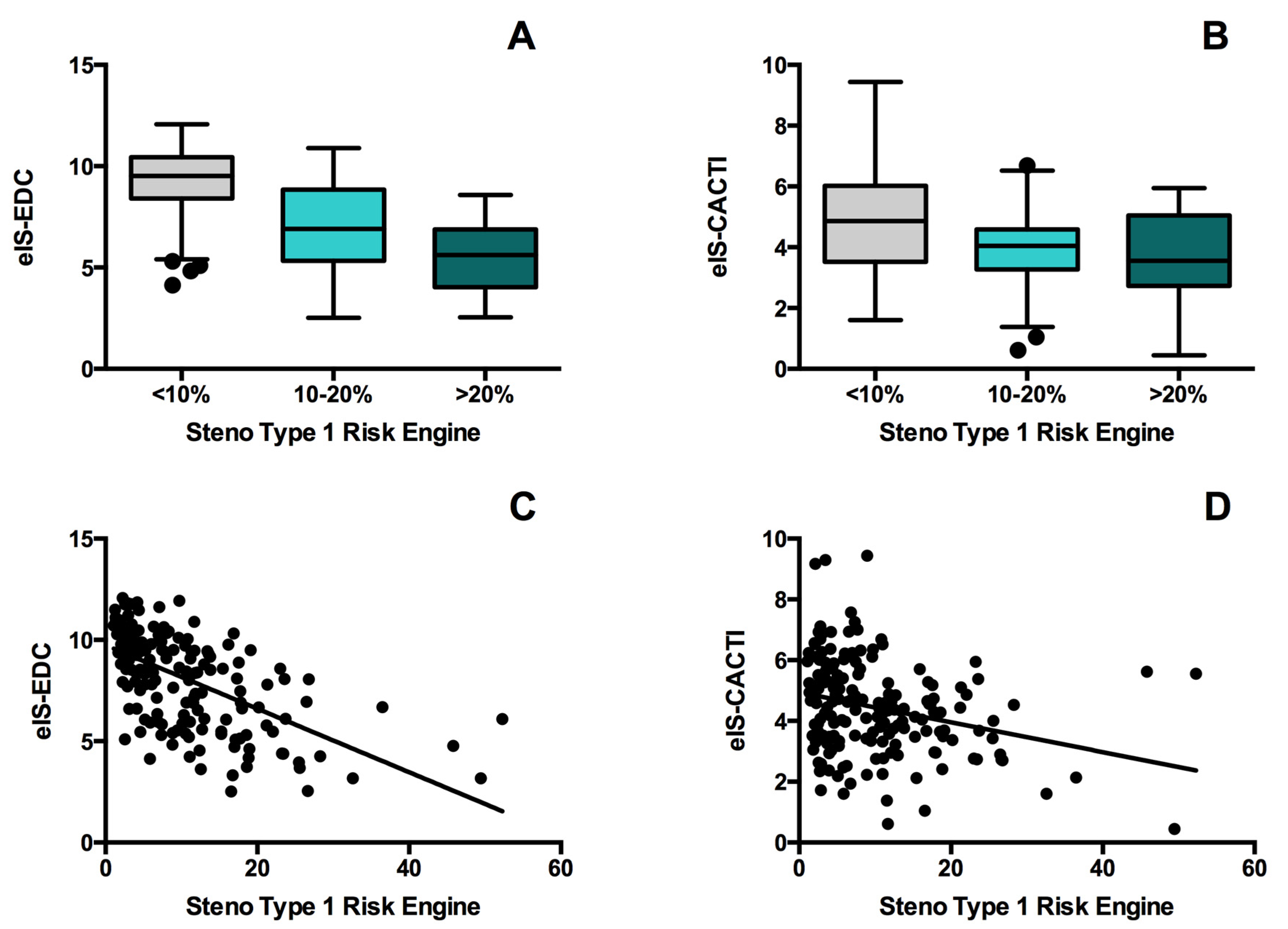
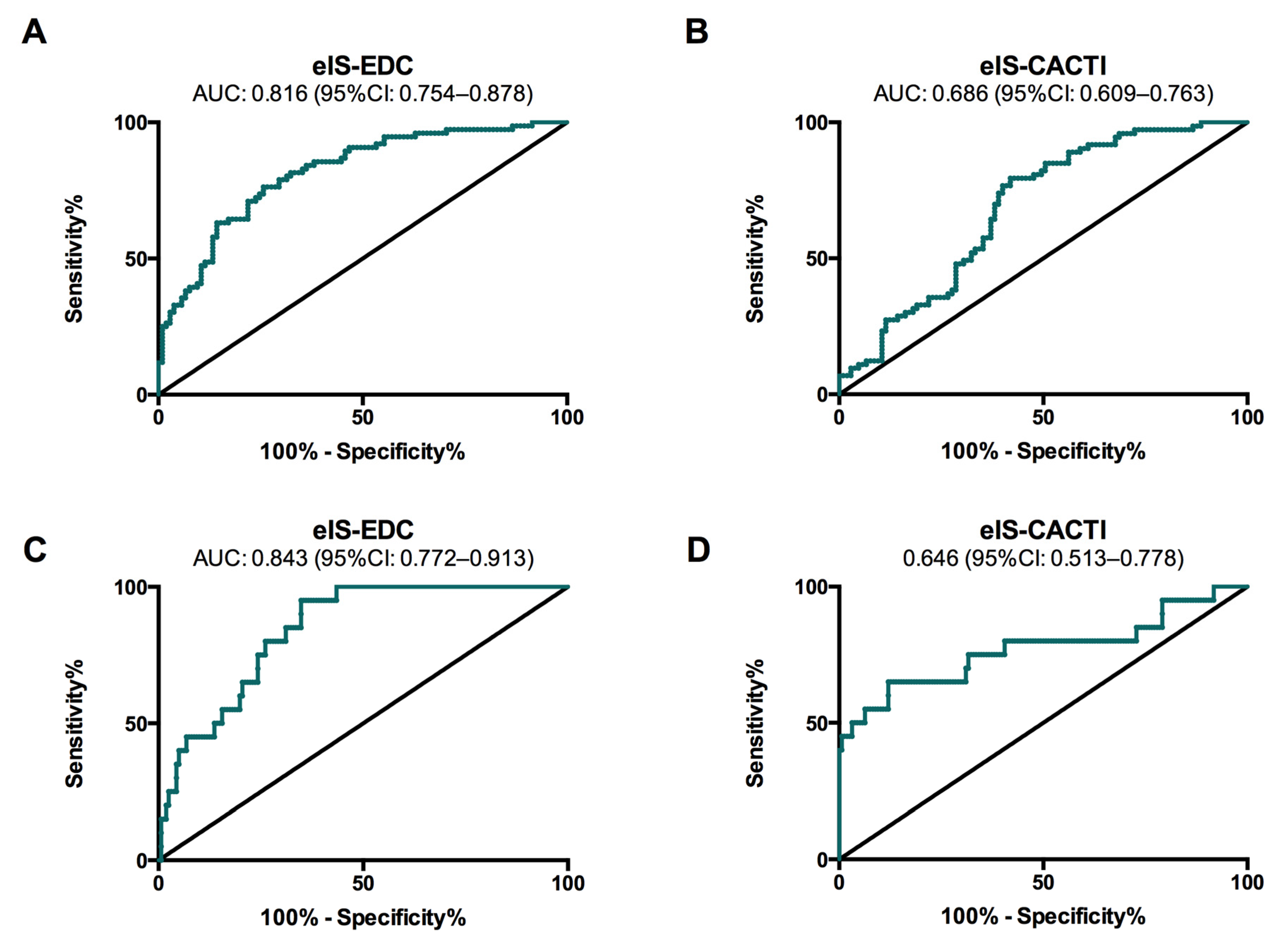
| Whole Population (n = 179) | Low-Risk (n = 105) | Moderate-Risk (n = 53) | High-Risk (n = 21) | p for Trend | |
|---|---|---|---|---|---|
| Clinical Characteristics | |||||
| Age (Years) | 41.2 (13.1) | 32.5 (8.3) | 50.8 (6.0) * | 60.7 (6.6) †,‡ | <0.001 |
| Sex (Male/Female), n | 91/88 | 52/53 | 29/24 | 10/11 | NS |
| Current Smokers, n (%) | 57 (31.8) | 31.0 (29.5) | 21 (39.6) * | 5 (23.8) | 0.012 |
| Regular Exercise, n (%) | 142 (79.3) | 86 (81.9) | 40 (75.5) | 16 (76.2) | 0.597 |
| Family History of Premature CVD, n (%) | 16 (8.9) | 7 (6.7) | 6 (11.3) | 3 (14.3) | NS |
| Family History of T2DM, n (%) | 37 (20.7) | 16 (15.2) | 15 (28.3) | 6 (28.6) | NS |
| Hypertension, n (%) | 49 (27.4) | 15 (14.3) | 20 (33.7) * | 14 (66.7) †,‡ | <0.001 |
| Dyslipidemia, n (%) | 98 (54.6) | 40 (38.1) | 40 (75.5) * | 18 (85.7) † | <0.001 |
| Diabetes | |||||
| Diabetes Duration (years) | 16 (12–23) | 14 (20–22) | 18 (15–27) * | 20 (15–29) † | <0.001 |
| Total Insulin Doses (UI/kg·day) | 0.6 (0.5–0.8) | 0.6 (0.5–0.8) | 0.7 (0.6–0.8) | 0.6 (0.5–0.7) | NS |
| Microvascular Complications, n (%) | 68 (38.4) | 28 (27.2) | 23 (43.4) | 18 (81.0) †,‡ | <0.001 |
| Retinopathy, n (%) | NS | ||||
| None, n (%) | 138 (77.1) | 86 (81.9) | 40 (75.5) | 12 (57.1) | |
| Non-Proliferative, n (%) | 20 (11.2) | 9 (8.6) | 6 (11.3) | 5 (23.8) | |
| Proliferative, n (%) | 21 (11.7) | 10 (9.5) | 7 (13.2) | 4 (19.1) | |
| Nephropathy, n (%) | 41 (23.2) | 14 (13.6) | 15 (28.3) | 12 (57.1) †,‡ | <0.001 |
| Neuropathy, n (%) | 7 (3.9) | 1 (1.0) | 3 (5.7) | 3 (14.3) † | 0.011 |
| Anthropometric Measurements | |||||
| Weight (kg) | 71.7 (13.0) | 69.8 (12.4) | 75.2 (14.3) * | 72.0 (10.7) | 0.045 |
| BMI (kg/m2) | 25.4 (3.7) | 24.3 (3.2) | 26.6 (3.8) * | 27.8 (4.4) † | <0.001 |
| Waist-to-hip ratio | 0.88 (0.81–0.94) | 0.84 (0.77–0.90) | 0.93 (0.86–0.99) * | 0.94 (0.90–0.98) † | <0.001 |
| Blood Pressure | |||||
| SBP (mmHg) | 125.6 (12.1) | 121.8 (11.0) | 128.8 (11.2) * | 136.9 (10.7) †,‡ | <0.001 |
| DBP (mmHg) | 72.0 (8.9) | 70.1 (8.2) | 74.4 (8.7) * | 75.7 (10.1) † | 0.002 |
| MAP (mmHg) | 89.9 (9.1) | 87.3 (8.4) | 92.5 (8.5) | 96.1 (9.6) | <0.001 |
| Laboratory Parameters | |||||
| Fasting Plasma Glucose (mmol/L) | 8.2 (3.8) | 7.8 (3.5) | 8.4 (3.8) | 9.5 (4.5) | NS |
| HbA1c (%) | 7.8 (1.0) | 7.6 (1.0) | 8.0 (1.0) | 8.5 (1.1) † | <0.001 |
| HbA1c (mmoL/moL) | 61.8 (11.4) | 59.2 (11.0) | 63.7 (10.5) | 69.9 (11.6) | |
| Urinary ACR (mg/g) | 4.7 (2.7–10.6) | 4.1 (2.4–7.7) | 6.1 (3.0–9.8) | 14.0 (5.3–54.0) †,‡ | <0.001 |
| eGFR (mL·min−1·1.73m−2) | 103.4 (91.2–113.6) | 108.7 (05.0–117.7) | 99.2 (91.8–104.0) * | 83.2 (73.3–93.6) †,‡ | <0.001 |
| Total Cholesterol (mg/dL) | 177.9 (158.5–201.1) | 174.0 (154.7–197.2) | 181.7 (166.3–201.1) | 197.2 (170.1–224.3) | NS |
| HDL-Cholesterol (mg/dL) | 65.7 (50.3–77.3) | 61.9 (50.3–73.5) | 65.7 (54.1–81.2) | 65.7 (58.0–85.1) † | 0.40 |
| LDL-Cholesterol (mg/dL) | 96.7 (81.2–112.1) | 96.7 (85.1–112.1) | 92.8 (81.2–108.3) | 100.5 (88.9–119.9) | NS |
| Triglycerides (mg/dL) | 64.6 (53.1–79.7) | 63.8 (47.8–77.9) | 64.7 (55.8–79.7) | 67.3 (60.2–97.4) | NS |
| Estimated Insulin Sensitivity | |||||
| eIS-EDC (mg·kg−1·min−1) | 8.6 (6.1–10.0) | 9.5 (8.4–10.4) | 6.9 (5.4–8.8) * | 5.6 (4.1–6.8) †,‡ | <0.001 |
| eIS-CACTI (mg·kg−1·min−1) | 4.4 (3.4–5.5) | 4.9 (3.5–6.0) | 4.0 (3.3–4.6) * | 3.6 (2.7–5.0) † | <0.001 |
| eIS-EDC | eIS-CACTI | |||
|---|---|---|---|---|
| rho | p | rho | p | |
| Clinical Characteristics | ||||
| Age (Years) | −0.545 | <0.001 | −0.189 | 0.012 |
| Female Sex | 0.382 | <0.001 | 0.338 | <0.001 |
| Family History of T2DM | −0.079 | 0.296 | −0.082 | 0.275 |
| Hypertension | −0.726 | <0.001 | −0.135 | 0.072 |
| Dyslipidemia | −0.382 | <0.001 | −0.308 | <0.001 |
| Diabetes | ||||
| Diabetes Duration (Years) | −0.255 | <0.001 | −0.010 | 0.891 |
| Total Insulin Doses (UI/kg·day) | −0.092 | 0.223 | −0.744 | <0.001 |
| Microvascular Complications | −0.490 | <0.001 | −0.045 | 0.551 |
| Retinopathy | −0.251 | <0.001 | −0.014 | 0.859 |
| Nephropathy | −0.580 | <0.001 | −0.113 | 0.137 |
| Peripheral Neuropathy | −0.058 | 0.444 | −0.057 | 0.453 |
| Anthropometric Measurements | ||||
| Weight (kg) | −0.333 | <0.001 | −0.543 | <0.001 |
| BMI (kg/m2) | −0.380 | <0.001 | −0.507 | <0.001 |
| WHR | −0.748 | <0.001 | −0.563 | <0.001 |
| Blood Pressure | ||||
| SBP (mmHg) | −0.435 | <0.001 | −0.359 | <0.001 |
| DBP (mmHg) | −0.422 | <0.001 | −0.382 | <0.001 |
| Laboratory Parameters | ||||
| HbA1c (%) | −0.354 | <0.001 | −0.280 | <0.001 |
| Urinary ACR (mg/g) | −0.125 | 0.095 | 0.048 | 0.523 |
| Total Cholesterol (mg/dL) | 0.002 | 0.980 | −0.020 | 0.824 |
| HDL-Cholesterol (mg/dL) | 0.156 | 0.078 | 0.365 | <0.001 |
| LDL-Cholesterol (mg/dL) | −0.049 | 0.583 | −0.100 | 0.259 |
| Triglycerides (mg/dL) | −0.207 | 0.019 | −0.550 | <0.001 |
| Steno Type 1 Risk Engine | ||||
| ST1RE Score | −0.635 | <0.001 | −0.291 | <0.001 |
| Estimated Insulin Sensitivity | ||||
| eIS-EDC (mg·kg−1·min−1) | - | - | 0.487 | <0.001 |
| eIS-CACTI (mg·kg−1·min−1) | 0.487 | <0.001 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano, A.; Llauradó, G.; Albert, L.; Mazarico, I.; Astiarraga, B.; González-Sastre, M.; Martínez, L.; Fernández-Veledo, S.; Simó, R.; Vendrell, J.; et al. Utility of Insulin Resistance in Estimating Cardiovascular Risk in Subjects with Type 1 Diabetes According to the Scores of the Steno Type 1 Risk Engine. J. Clin. Med. 2020, 9, 2192. https://doi.org/10.3390/jcm9072192
Cano A, Llauradó G, Albert L, Mazarico I, Astiarraga B, González-Sastre M, Martínez L, Fernández-Veledo S, Simó R, Vendrell J, et al. Utility of Insulin Resistance in Estimating Cardiovascular Risk in Subjects with Type 1 Diabetes According to the Scores of the Steno Type 1 Risk Engine. Journal of Clinical Medicine. 2020; 9(7):2192. https://doi.org/10.3390/jcm9072192
Chicago/Turabian StyleCano, Albert, Gemma Llauradó, Lara Albert, Isabel Mazarico, Brenno Astiarraga, Montserrat González-Sastre, Laia Martínez, Sonia Fernández-Veledo, Rafael Simó, Joan Vendrell, and et al. 2020. "Utility of Insulin Resistance in Estimating Cardiovascular Risk in Subjects with Type 1 Diabetes According to the Scores of the Steno Type 1 Risk Engine" Journal of Clinical Medicine 9, no. 7: 2192. https://doi.org/10.3390/jcm9072192
APA StyleCano, A., Llauradó, G., Albert, L., Mazarico, I., Astiarraga, B., González-Sastre, M., Martínez, L., Fernández-Veledo, S., Simó, R., Vendrell, J., & González-Clemente, J.-M. (2020). Utility of Insulin Resistance in Estimating Cardiovascular Risk in Subjects with Type 1 Diabetes According to the Scores of the Steno Type 1 Risk Engine. Journal of Clinical Medicine, 9(7), 2192. https://doi.org/10.3390/jcm9072192







