Prevention of Prosthetic Joint Infection: From Traditional Approaches towards Quality Improvement and Data Mining
Abstract
1. Introduction
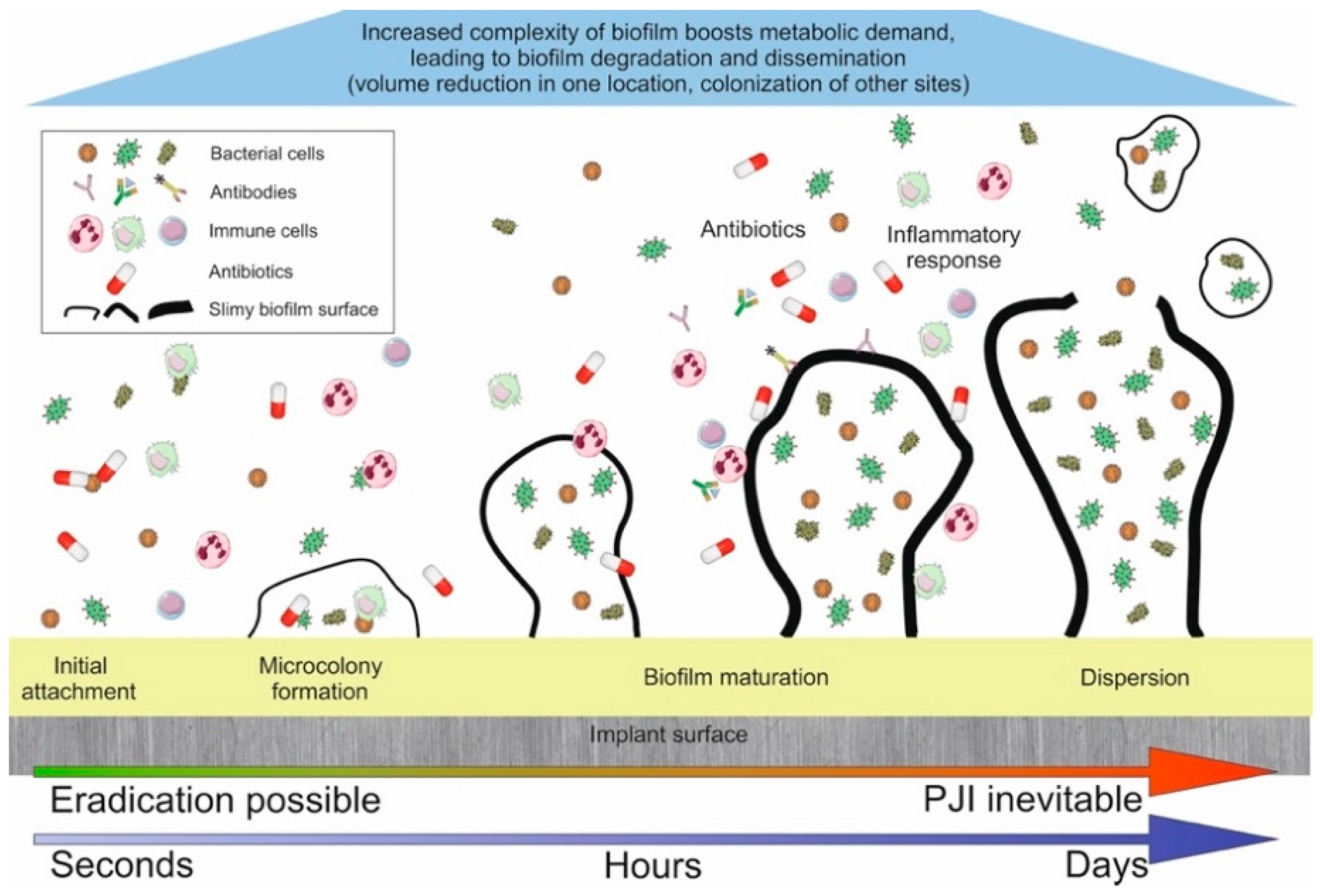
1.1. Key Steps Towards PJI
1.2. Why Has PJI Not Been Eradicated Despite Modern Preventative Measures?
2. Preoperative Strategies
2.1. At-Risk Patients
2.2. Preventative Strategies for At-Risk Patients
3. Perioperative Strategies
3.1. Preparation of Operative Field
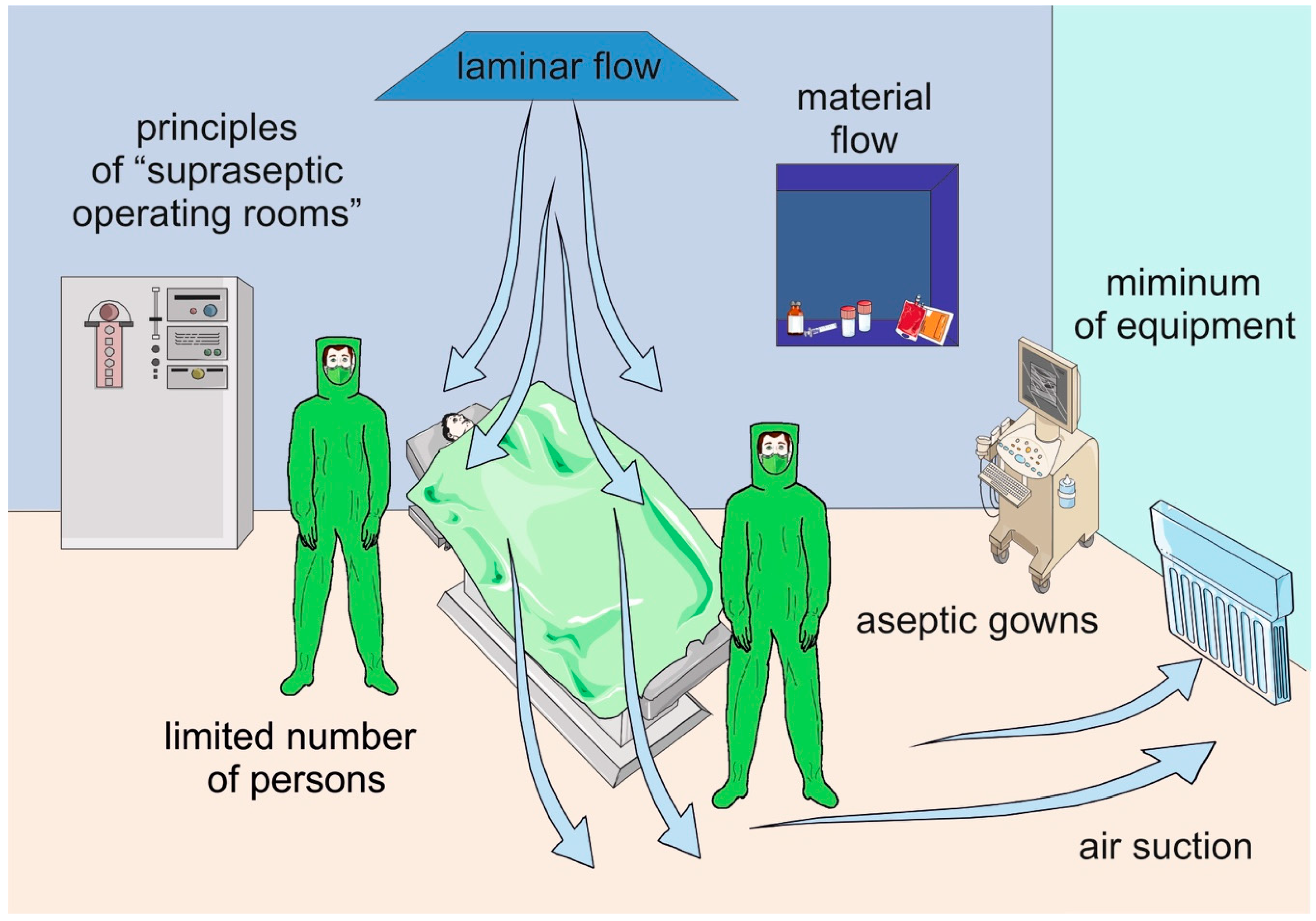
3.2. Operating Room—Technical Parameters, Traffic
3.3. Team, Personnel
3.4. Systemic/Local Antibiotics
3.5. Intraoperative Care for TJA Patients
3.6. Surgeon Performance
3.7. Anti-Infective Implant
4. Postoperative Strategies
4.1. Wound Care
4.2. Measures Against Hematogenous and Directly Spreading PJI
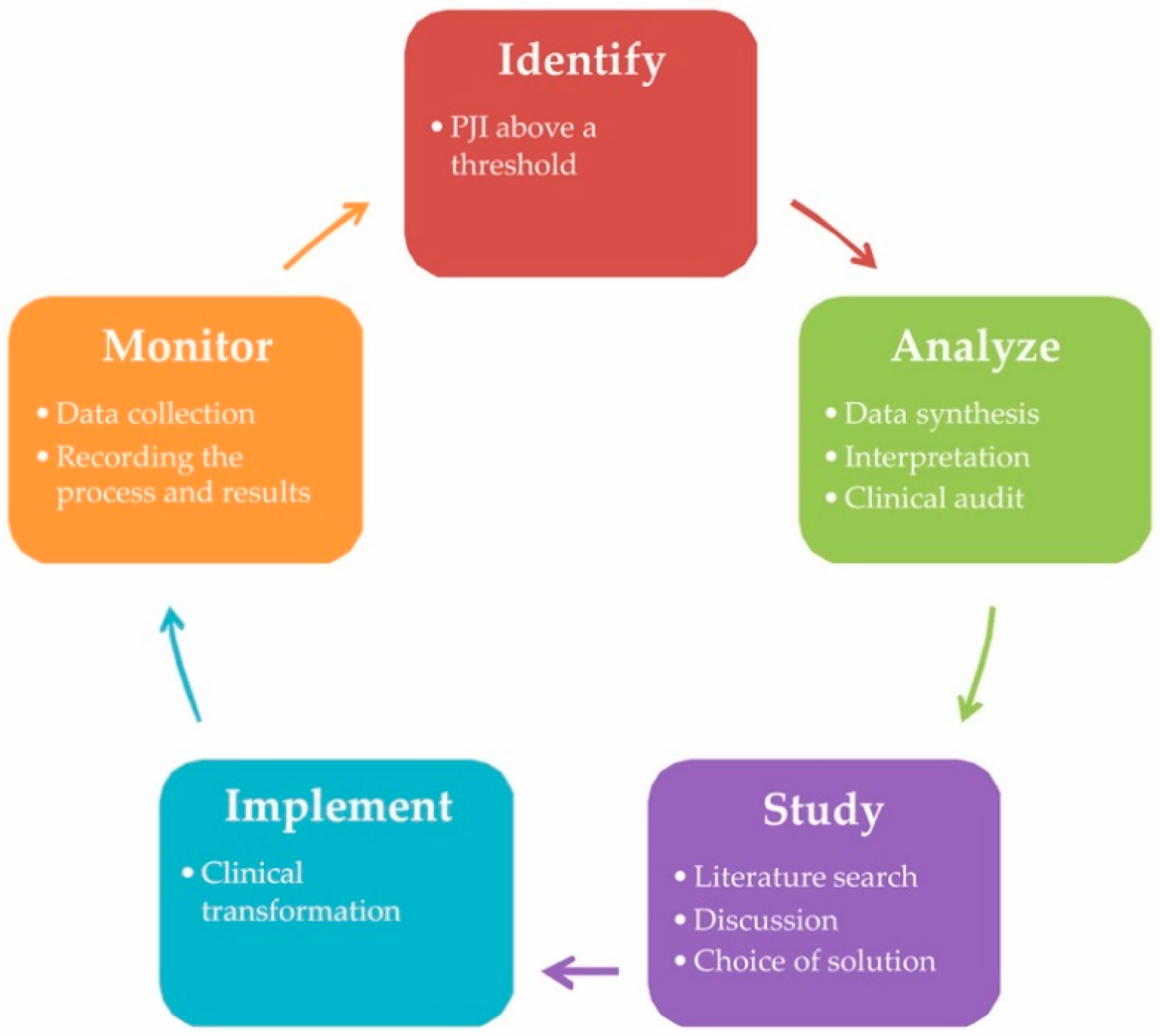
5. A Case for Quality Improvement into Practice
6. Who Is the Best Provider?
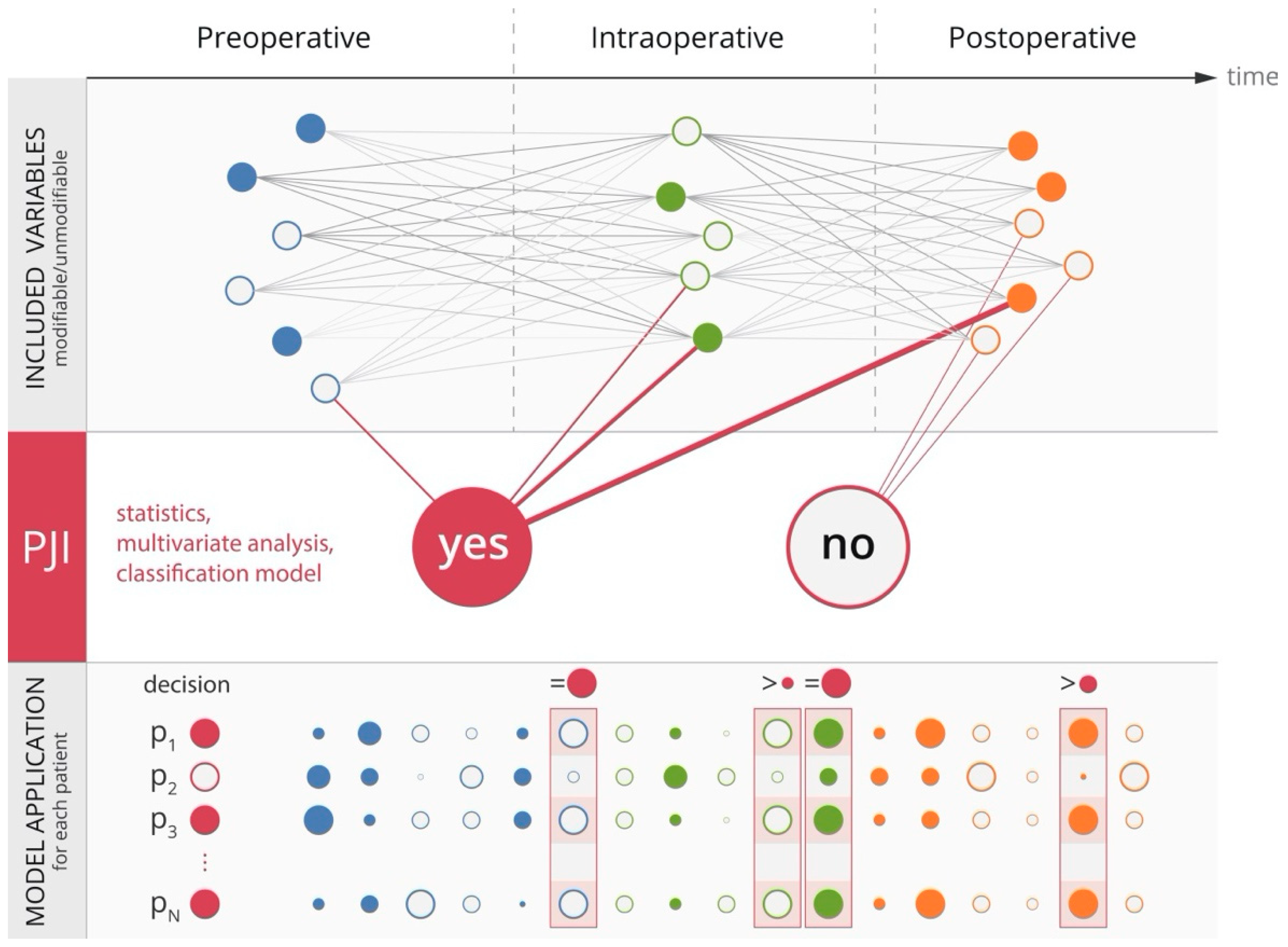
7. Contribution of Machine Learning to Prevention of PJI
8. A Step towards Precision Prevention of PJI?
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fischbacher, A.; Borens, O. Prosthetic-joint infections: Mortality over the last 10 years. J. Bone Jt. Infect. 2019, 4, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Natsuhara, K.M.; Shelton, T.J.; Meehan, J.P.; Lum, Z.C. Mortality during total hip periprosthetic joint infection. J. Arthroplast. 2019, 34, S337–S342. [Google Scholar] [CrossRef] [PubMed]
- Gundtoft, P.H.; Pedersen, A.B.; Varnum, C.; Overgaard, S. Increased mortality after prosthetic joint infection in primary tha. Clin. Orthop. Relat. Res. 2017, 475, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Puhto, T.; Puhto, A.P.; Vielma, M.; Syrjala, H. Infection triples the cost of a primary joint arthroplasty. Infect. Dis. 2019, 51, 348–355. [Google Scholar] [CrossRef]
- Musil, D.; Snorek, M.; Gallo, J.; Jahoda, D.; Stehlik, J. Economic analysis of the costs of hospital stay of patients with infection as a complication of total replacements-part 2: Total hip arthroplasty. Acta Chir. Orthop. Traumatol. Cechoslov. 2019, 86, 241–248. (In Czech) [Google Scholar]
- Musil, D.; Snorek, M.; Gallo, J.; Jahoda, D.; Stehlik, J. Economic analysis of the costs of hospital stay of patients with infection as a complication of total replacements-part 1: Total knee arthroplasty. Acta Chir. Orthop. Traumatol. Cechoslov. 2019, 86, 173–180. (In Czech) [Google Scholar]
- Marculescu, C.E.; Mabry, T.; Berbari, E.F. Prevention of surgical site infections in joint replacement surgery. Surg. Infect. 2016, 17, 152–157. [Google Scholar] [CrossRef]
- Wang, F.D.; Wang, Y.P.; Chen, C.F.; Chen, H.P. The incidence rate, trend and microbiological aetiology of prosthetic joint infection after total knee arthroplasty: A 13 years’ experience from a tertiary medical center in taiwan. J. Microbiol. Immunol. Infect. 2018, 51, 717–722. [Google Scholar] [CrossRef]
- Boddapati, V.; Fu, M.C.; Mayman, D.J.; Su, E.P.; Sculco, P.K.; McLawhorn, A.S. Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. J. Arthroplast. 2018, 33, 521–526. [Google Scholar] [CrossRef]
- Dale, H.; Hallan, G.; Hallan, G.; Espehaug, B.; Havelin, L.I.; Engesaeter, L.B. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop. 2009, 80, 639–645. [Google Scholar] [CrossRef]
- Matthews, J.; Bamal, R.; McLean, A.; Bindra, R. Bacteriological profile of community-acquired musculoskeletal infections: A study from queensland. ANZ J. Surg. 2018, 88, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Lourtet-Hascoet, J.; Felice, M.P.; Bicart-See, A.; Bouige, A.; Giordano, G.; Bonnet, E. Species and antimicrobial susceptibility testing of coagulase-negative staphylococci in periprosthetic joint infections. Epidemiol. Infect. 2018, 146, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Triffault-Fillit, C.; Ferry, T.; Laurent, F.; Pradat, P.; Dupieux, C.; Conrad, A.; Becker, A.; Lustig, S.; Fessy, M.H.; Chidiac, C.; et al. Microbiologic epidemiology depending on time to occurrence of prosthetic joint infection: A prospective cohort study. Clin. Microbiol. Infect. 2019, 25, 353–358. [Google Scholar] [CrossRef]
- Rosteius, T.; Jansen, O.; Fehmer, T.; Baecker, H.; Citak, M.; Schildhauer, T.A.; Gessmann, J. Evaluating the microbial pattern of periprosthetic joint infections of the hip and knee. J. Med Microbiol. 2018, 67, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.; Chang, C.H.; Lin, Y.C.; Lee, S.H.; Hsieh, P.H.; Chang, Y. Different microbiological profiles between hip and knee prosthetic joint infections. J. Orthop. Surg. 2019, 27, 2309499019847768. [Google Scholar] [CrossRef] [PubMed]
- Armit, D.; Vickers, M.; Parr, A.; Van Rosendal, S.; Trott, N.; Gunasena, R.; Parkinson, B. Humidity a potential risk factor for prosthetic joint infection in a tropical australian hospital. ANZ J. Surg. 2018, 88, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.J.; Spratt, D.; Liddle, A.D. Implant materials and prosthetic joint infection: The battle with the biofilm. EFORT Open Rev. 2019, 4, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G.; Naylor, P.; Myrvik, Q. Infections from biomaterials and implants: A race for the surface. Med Prog. Technol. 1988, 14, 205–224. [Google Scholar]
- Moriarty, T.F.; Harris, L.G.; Mooney, R.A.; Wenke, J.C.; Riool, M.; Zaat, S.A.J.; Moter, A.; Schaer, T.P.; Khanna, N.; Kuehl, R.; et al. Recommendations for design and conduct of preclinical in vivo studies of orthopedic device-related infection. J. Orthop. Res. 2019, 37, 271–287. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Kimkes, T.E.P.; Heinemann, M. How bacteria recognise and respond to surface contact. FEMS Microbiol. Rev. 2019, 44, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The nexus between periodontal inflammation and dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Herman-Bausier, P.; Labate, C.; Towell, A.M.; Derclaye, S.; Geoghegan, J.A.; Dufrene, Y.F. Staphylococcus aureus clumping factor a is a force-sensitive molecular switch that activates bacterial adhesion. Proc. Natl. Acad. Sci. USA 2018, 115, 5564–5569. [Google Scholar] [CrossRef]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Orazi, G.; O’Toole, G.A. “It takes a village”: Mechanisms underlying antimicrobial recalcitrance of polymicrobial biofilms. J. Bacteriol. 2019, 202, e00530-19. [Google Scholar] [CrossRef]
- Josse, J.; Laurent, F.; Diot, A. Staphylococcal adhesion and host cell invasion: Fibronectin-binding and other mechanisms. Front. Microbiol. 2017, 8, 2433. [Google Scholar] [CrossRef]
- Schulz, F.; Horn, M. Intranuclear bacteria: Inside the cellular control center of eukaryotes. Trends Cell Biol. 2015, 25, 339–346. [Google Scholar] [CrossRef]
- Broz, P. Recognition of intracellular bacteria by inflammasomes. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef] [PubMed]
- de Mesy Bentley, K.L.; Trombetta, R.; Nishitani, K.; Bello-Irizarry, S.N.; Ninomiya, M.; Zhang, L.; Chung, H.L.; McGrath, J.L.; Daiss, J.L.; Awad, H.A.; et al. Evidence of staphylococcus aureus deformation, proliferation, and migration in canaliculi of live cortical bone in murine models of osteomyelitis. J. Bone Miner. Res. 2017, 32, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Escoll, P.; Buchrieser, C. Metabolic reprogramming: An innate cellular defence mechanism against intracellular bacteria? Curr. Opin. Immunol. 2019, 60, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.D.; Benedict, C.; Schioth, H.B. Experimenter gender and replicability in science. Sci. Adv. 2018, 4, e1701427. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. Why replication has more scientific value than original discovery. Behav. Brain Sci. 2018, 41, e137. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.C.; Son, M.S.; Chang, E.T.; Zimmerli, W.; Parvizi, J. Are we winning or losing the battle with periprosthetic joint infection: Trends in periprosthetic joint infection and mortality risk for the medicare population. J. Arthroplast. 2018, 33, 3238–3245. [Google Scholar] [CrossRef]
- Berbari, E.F.; Hanssen, A.D.; Duffy, M.C.; Steckelberg, J.M.; Ilstrup, D.M.; Harmsen, W.S.; Osmon, D.R. Risk factors for prosthetic joint infection: Case-control study. Clin. Infect. Dis. 1998, 27, 1247–1254. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Beswick, A.D.; Team, I. Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0150866. [Google Scholar] [CrossRef]
- Deegan, B.F.; Richard, R.D.; Bowen, T.R.; Perkins, R.M.; Graham, J.H.; Foltzer, M.A. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics 2014, 37, e613–e618. [Google Scholar] [CrossRef]
- Miric, A.; Inacio, M.C.; Namba, R.S. Can total knee arthroplasty be safely performed in patients with chronic renal disease? Acta Orthop. 2014, 85, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Kuo, F.C.; Wang, J.W. Total knee arthroplasty in patients with dialysis: Early complications and mortality. Biomed. J. 2014, 37, 84–89. [Google Scholar]
- Li, W.C.; Shih, C.H.; Ueng, S.W.; Shih, H.N.; Lee, M.S.; Hsieh, P.H. Uncemented total hip arthroplasty in chronic hemodialysis patients. Acta Orthop. 2010, 81, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Lieu, D.; Harris, I.A.; Naylor, J.M.; Mittal, R. Review article: Total hip replacement in haemodialysis or renal transplant patients. J. Orthop. Surg. 2014, 22, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Onochie, E.; Kayani, B.; Dawson-Bowling, S.; Millington, S.; Achan, P.; Hanna, S. Total hip arthroplasty in patients with chronic liver disease: A systematic review. SICOT J. 2019, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Kim, H.J.; Cho, I.Y.; Lee, D.H. Infection and revision rates following primary total knee arthroplasty in patients with rheumatoid arthritis versus osteoarthritis: A meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3800–3807. [Google Scholar] [CrossRef]
- George, M.D.; Baker, J.F.; Winthrop, K.; Alemao, E.; Chen, L.; Connolly, S.; Hsu, J.Y.; Simon, T.A.; Wu, Q.; Xie, F.; et al. Risk of biologics and glucocorticoids in patients with rheumatoid arthritis undergoing arthroplasty: A cohort study. Ann. Intern. Med. 2019, 170, 825–836. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, F.; Qi, J.; Xiang, W.; Zhang, J. Meta-analysis shows that obesity may be a significant risk factor for prosthetic joint infections. Int. Orthop. 2016, 40, 659–667. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Papadopoulos, D.V.; Lytras, T.; Tsantes, A.E.; Mavrogenis, A.F.; Korompilias, A.V.; Gelalis, I.D.; Tsantes, C.G.; Bonovas, S. Association of malnutrition with periprosthetic joint and surgical site infections after total joint arthroplasty: A systematic review and meta-analysis. J. Hosp. Infect. 2019, 103, 69–77. [Google Scholar] [CrossRef]
- Bedard, N.A.; DeMik, D.E.; Owens, J.M.; Glass, N.A.; DeBerg, J.; Callaghan, J.J. Tobacco use and risk of wound complications and periprosthetic joint infection: A systematic review and meta-analysis of primary total joint arthroplasty procedures. J. Arthroplast. 2019, 34, 385–396.E4. [Google Scholar] [CrossRef]
- Kolz, J.M.; Rainer, W.G.; Wyles, C.C.; Houdek, M.T.; Perry, K.I.; Lewallen, D.G. Lymphedema: A significant risk factor for infection and implant failure after total knee arthroplasty. J. Am. Acad. Orthop. Surg. 2020. [Google Scholar] [CrossRef]
- O’Neill, S.C.; Queally, J.M.; Hickey, A.; Mulhall, K.J. Outcome of total hip and knee arthroplasty in hiv-infected patients: A systematic review. Orthop. Rev. 2019, 11, 8020. [Google Scholar] [CrossRef] [PubMed]
- Somerson, J.S.; Boylan, M.R.; Hug, K.T.; Naziri, Q.; Paulino, C.B.; Huang, J.I. Risk factors associated with periprosthetic joint infection after total elbow arthroplasty. Shoulder Elb. 2019, 11, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Althoff, A.; Cancienne, J.M.; Cooper, M.T.; Werner, B.C. Patient-related risk factors for periprosthetic ankle joint infection: An analysis of 6977 total ankle arthroplasties. J. Foot Ankle Surg. 2018, 57, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, B.P.; Weston, J.T.; Osmon, D.R.; Hanssen, A.D.; Berry, D.J.; Abdel, M.P. Prior hip or knee prosthetic joint infection in another joint increases risk three-fold of prosthetic joint infection after primary total knee arthroplasty: A matched control study. Bone Jt. J. 2019, 101-B, 91–97. [Google Scholar] [CrossRef]
- Malcolm, T.L.; Robinson le, D.; Klika, A.K.; Ramanathan, D.; Higuera, C.A.; Murray, T.G. Predictors of staphylococcus aureus colonization and results after decolonization. Interdiscip. Perspect. Infect. Dis. 2016, 2016, 4367156. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, X.; Zeng, Y.; Feng, W.; Li, J.; Zeng, J.; Zeng, Y. Can nasal staphylococcus aureus screening and decolonization prior to elective total joint arthroplasty reduce surgical site and prosthesis-related infections? A systematic review and meta-analysis. J. Orthop. Surg. Res. 2020, 15, 60. [Google Scholar] [CrossRef]
- Ma, N.; Cameron, A.; Tivey, D.; Grae, N.; Roberts, S.; Morris, A. Systematic review of a patient care bundle in reducing staphylococcal infections in cardiac and orthopaedic surgery. ANZ J. Surg. 2017, 87, 239–246. [Google Scholar] [CrossRef]
- Kim, K.Y.; Anoushiravani, A.A.; Chen, K.K.; Li, R.; Bosco, J.A.; Slover, J.D.; Iorio, R. Perioperative orthopedic surgical home: Optimizing total joint arthroplasty candidates and preventing readmission. J. Arthroplast. 2019, 34, S91–S96. [Google Scholar] [CrossRef]
- Maradit Kremers, H.; Lewallen, L.W.; Mabry, T.M.; Berry, D.J.; Berbari, E.F.; Osmon, D.R. Diabetes mellitus, hyperglycemia, hemoglobin a1c and the risk of prosthetic joint infections in total hip and knee arthroplasty. J. Arthroplast. 2015, 30, 439–443. [Google Scholar] [CrossRef]
- Shohat, N.; Tarabichi, M.; Tan, T.L.; Goswami, K.; Kheir, M.; Malkani, A.L.; Shah, R.P.; Schwarzkopf, R.; Parvizi, J. 2019 john insall award: Fructosamine is a better glycaemic marker compared with glycated haemoglobin (hba1c) in predicting adverse outcomes following total knee arthroplasty: A prospective multicentre study. Bone Jt. J. 2019, 101-B, 3–9. [Google Scholar] [CrossRef]
- Colunga-Lozano, L.E.; Gonzalez Torres, F.J.; Delgado-Figueroa, N.; Gonzalez-Padilla, D.A.; Hernandez, A.V.; Roman, Y.; Cuello-Garcia, C.A. Sliding scale insulin for non-critically ill hospitalised adults with diabetes mellitus. Cochrane Database Syst. Rev. 2018, 11, CD011296. [Google Scholar] [CrossRef] [PubMed]
- Uppal, C.; Blanshard, A.; Ahluwalia, R.; Dhatariya, K. Achieving a preoperative target hba1c of <69 mmol/mol in elective vascular and orthopedic surgery: A retrospective single center observational study. Diabetes Ther. 2019, 10, 1959–1967. [Google Scholar] [PubMed]
- Zhong, J.; Wang, B.; Chen, Y.; Li, H.; Lin, N.; Xu, X.; Lu, H. Relationship between body mass index and the risk of periprosthetic joint infection after primary total hip arthroplasty and total knee arthroplasty. Ann. Transl. Med. 2020, 8, 464. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.; Agius, L.; Burke, N.; Rahardja, R.; Young, S.W. Bmi is a better predictor of periprosthetic joint infection risk than local measures of adipose tissue after tka. J. Arthroplast. 2020, 35, S313–S318. [Google Scholar] [CrossRef]
- Kwasny, M.J.; Edelstein, A.I.; Manning, D.W. Statistical methods dictate the estimated impact of body mass index on major and minor complications after total joint arthroplasty. Clin. Orthop. Relat. Res. 2018, 476, 2418–2429. [Google Scholar] [CrossRef]
- Baratz, M.D.; Hallmark, R.; Odum, S.M.; Springer, B.D. Twenty percent of patients may remain colonized with methicillin-resistant staphylococcus aureus despite a decolonization protocol in patients undergoing elective total joint arthroplasty. Clin. Orthop. Relat. Res. 2015, 473, 2283–2290. [Google Scholar] [CrossRef]
- Tandon, T.; Tadros, B.J.; Akehurst, H.; Avasthi, A.; Hill, R.; Rao, M. Risk of surgical site infection in elective hip and knee replacements after confirmed eradication of mrsa in chronic carriers. J. Arthroplast. 2017, 32, 3711–3717. [Google Scholar] [CrossRef]
- Yeganeh, M.H.; Kheir, M.M.; Shahi, A.; Parvizi, J. Rheumatoid arthritis, disease modifying agents, and periprosthetic joint infection: What does a joint surgeon need to know? J. Arthroplast. 2018, 33, 1258–1264. [Google Scholar] [CrossRef]
- Eiselt, D. Presurgical skin preparation with a novel 2% chlorhexidine gluconate cloth reduces rates of surgical site infection in orthopaedic surgical patients. Orthop. Nurs. 2009, 28, 141–145. [Google Scholar] [CrossRef]
- Kapadia, B.H.; Elmallah, R.K.; Mont, M.A. A randomized, clinical trial of preadmission chlorhexidine skin preparation for lower extremity total joint arthroplasty. J. Arthroplast. 2016, 31, 2856–2861. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xu, K.; Hou, W.; Yang, Z.; Xu, P. Preoperative chlorhexidine reduces the incidence of surgical site infections in total knee and hip arthroplasty: A systematic review and meta-analysis. Int. J. Surg. 2017, 39, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.C.; Tan, T.L.; Wang, J.W.; Wang, C.J.; Ko, J.Y.; Lee, M.S. Use of antimicrobial-impregnated incise drapes to prevent periprosthetic joint infection in primary total joint arthroplasty: A retrospective analysis of 9774 cases. J. Arthroplast. 2020, 35, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Hesselvig, A.B.; Arpi, M.; Madsen, F.; Bjarnsholt, T.; Odgaard, A.; Group, I.S. Does an antimicrobial incision drape prevent intraoperative contamination? A randomized controlled trial of 1187 patients. Clin. Orthop. Relat. Res. 2020, 478, 1007–1015. [Google Scholar] [CrossRef]
- Rezapoor, M.; Tan, T.L.; Maltenfort, M.G.; Parvizi, J. Incise draping reduces the rate of contamination of the surgical site during hip surgery: A prospective, randomized trial. J. Arthroplast. 2018, 33, 1891–1895. [Google Scholar] [CrossRef]
- Villa, J.M.; Pannu, T.S.; Riesgo, A.M.; Patel, P.D.; Mont, M.A.; Higuera-Rueda, C.A. Dual antibiotic prophylaxis in total knee arthroplasty: Where do we stand? J. Knee Surg. 2020, 33, 100–105. [Google Scholar] [CrossRef]
- Burger, J.R.; Hansen, B.J.; Leary, E.V.; Aggarwal, A.; Keeney, J.A. Dual-agent antibiotic prophylaxis using a single preoperative vancomycin dose effectively reduces prosthetic joint infection rates with minimal renal toxicity risk. J. Arthroplast. 2018, 33, S213–S218. [Google Scholar] [CrossRef]
- DeFrancesco, C.J.; Fu, M.C.; Kahlenberg, C.A.; Miller, A.O.; Bostrom, M.P. Extended antibiotic prophylaxis may be linked to lower peri-prosthetic joint infection rates in high-risk patients: An evidence-based review. HSS J. 2019, 15, 297–301. [Google Scholar] [CrossRef]
- Inabathula, A.; Dilley, J.E.; Ziemba-Davis, M.; Warth, L.C.; Azzam, K.A.; Ireland, P.H.; Meneghini, R.M. Extended oral antibiotic prophylaxis in high-risk patients substantially reduces primary total hip and knee arthroplasty 90-day infection rate. J. Bone Jt. Surg. 2018, 100, 2103–2109. [Google Scholar] [CrossRef]
- Chen, A.F.; Fleischman, A.; Austin, M.S. Use of intrawound antibiotics in orthopaedic surgery. J. Am. Acad. Orthop. Surg. 2018, 26, e371–e378. [Google Scholar] [CrossRef]
- Wang, Q.; Goswami, K.; Shohat, N.; Aalirezaie, A.; Manrique, J.; Parvizi, J. Longer operative time results in a higher rate of subsequent periprosthetic joint infection in patients undergoing primary joint arthroplasty. J. Arthroplast. 2019, 34, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chen, B.P.; Soleas, I.M.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged operative duration increases risk of surgical site infections: A systematic review. Surg. Infect. 2017, 18, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Barnes, S.; Shohat, N.; Edmiston, C.E., Jr. Environment of care: Is it time to reassess microbial contamination of the operating room air as a risk factor for surgical site infection in total joint arthroplasty? Am. J. Infect. Control 2017, 45, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Driesman, A.; Shen, M.; Feng, J.E.; Waren, D.; Slover, J.; Bosco, J.; Schwarzkopf, R. Perioperative chlorhexidine gluconate wash during joint arthroplasty has equivalent periprosthetic joint infection rates in comparison to betadine wash. J. Arthroplast. 2020, 35, 845–848. [Google Scholar] [CrossRef]
- Slullitel, P.A.; Dobransky, J.S.; Bali, K.; Poitras, S.; Bhullar, R.S.; Ottawa Arthroplasty, G.; Kim, P.R. Is there a role for preclosure dilute betadine irrigation in the prevention of postoperative infection following total joint arthroplasty? J. Arthroplast. 2020, 35, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.L.; Malizos, K.; Capuano, N.; Mezzoprete, R.; D’Arienzo, M.; Van Der Straeten, C.; Scarponi, S.; Drago, L. Does an antibiotic-loaded hydrogel coating reduce early post-surgical infection after joint arthroplasty? J. Bone Jt. Infect. 2016, 1, 34–41. [Google Scholar] [CrossRef]
- Sebastian, S.; Liu, Y.; Christensen, R.; Raina, D.B.; Tagil, M.; Lidgren, L. Antibiotic containing bone cement in prevention of hip and knee prosthetic joint infections: A systematic review and meta-analysis. J. Orthop. Translat. 2020, 23, 53–60. [Google Scholar] [CrossRef]
- van Vugt, T.A.G.; Walraven, J.M.B.; Geurts, J.A.P.; Arts, J.J.C. Antibiotic-loaded collagen sponges in clinical treatment of chronic osteomyelitis: A systematic review. J. Bone Jt. Surg. 2018, 100, 2153–2161. [Google Scholar] [CrossRef]
- Parry, M.C.; Laitinen, M.K.; Albergo, J.I.; Gaston, C.L.; Stevenson, J.D.; Grimer, R.J.; Jeys, L.M. Silver-coated (agluna(r)) tumour prostheses can be a protective factor against infection in high risk failure patients. Eur. J. Surg. Oncol. 2019, 45, 704–710. [Google Scholar] [CrossRef]
- Gustin, M.P.; Ohannessian, R.; Giard, M.; Caillat-Vallet, E.; Savey, A.; Vanhems, P.; CCLIN Sud-Est study group. Use of surveillance data to calculate the sample size and the statistical power of randomized clinical trials testing staphylococcus aureus vaccine efficacy in orthopedic surgery. Vaccine 2017, 35, 6934–6937. [Google Scholar] [CrossRef]
- Fowler, V.G.; Allen, K.B.; Moreira, E.D.; Moustafa, M.; Isgro, F.; Boucher, H.W.; Corey, G.R.; Carmeli, Y.; Betts, R.; Hartzel, J.S.; et al. Effect of an investigational vaccine for preventing staphylococcus aureus infections after cardiothoracic surgery: A randomized trial. JAMA 2013, 309, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Rezapoor, M.; Alvand, A.; Jacek, E.; Paziuk, T.; Maltenfort, M.G.; Parvizi, J. Operating room traffic increases aerosolized particles and compromises the air quality: A simulated study. J. Arthroplast. 2018, 33, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.G.; Balkam, C.B.; Purcell, R.L.; Parks, N.L.; Holdsworth, J.E. Operating room traffic in total joint arthroplasty: Identifying patterns and training the team to keep the door shut. Am. J. Infect. Control 2018, 46, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Baldini, A.; Blevins, K.; Del Gaizo, D.; Enke, O.; Goswami, K.; Griffin, W.; Indelli, P.F.; Jennison, T.; Kenanidis, E.; Manner, P.; et al. General assembly, prevention, operating room-personnel: Proceedings of international consensus on orthopedic infections. J. Arthroplast. 2019, 34, S97–S104. [Google Scholar] [CrossRef]
- Curtis, G.L.; Faour, M.; Jawad, M.; Klika, A.K.; Barsoum, W.K.; Higuera, C.A. Reduction of particles in the operating room using ultraviolet air disinfection and recirculation units. J. Arthroplast. 2018, 33, S196–S200. [Google Scholar] [CrossRef]
- Illingworth, K.D.; Mihalko, W.M.; Parvizi, J.; Sculco, T.; McArthur, B.; el Bitar, Y.; Saleh, K.J. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: A multicenter approach: Aaos exhibit selection. J. Bone Jt. Surg. 2013, 95, e50. [Google Scholar] [CrossRef]
- Andersson, A.E.; Bergh, I.; Karlsson, J.; Eriksson, B.I.; Nilsson, K. Traffic flow in the operating room: An explorative and descriptive study on air quality during orthopedic trauma implant surgery. Am. J. Infect. Control 2012, 40, 750–755. [Google Scholar] [CrossRef]
- Clyburn, T.A.; Evans, R.P.; Moucha, C.S.; Prokuski, L. Surgical site infection prevention: The operating room environment. Instr. Course Lect. 2011, 60, 565–574. [Google Scholar]
- Hester, R.A.; Nelson, C.L.; Harrison, S. Control of contamination of the operative team in total joint arthroplasty. J. Arthroplast. 1992, 7, 267–269. [Google Scholar] [CrossRef]
- Jolivet, S.; Lucet, J.C. Surgical field and skin preparation. Orthop. Traumatol. Surg. Res. 2019, 105, S1–S6. [Google Scholar] [CrossRef]
- Hijas-Gomez, A.I.; Lucas, W.C.; Checa-Garcia, A.; Martinez-Martin, J.; Fahandezh-Saddi, H.; Gil-de-Miguel, A.; Duran-Poveda, M.; Rodriguez-Caravaca, G. Surgical site infection incidence and risk factors in knee arthroplasty: A 9-year prospective cohort study at a university teaching hospital in spain. Am. J. Infect. Control 2018, 46, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.L.; Brown, J.A.; Salathiel, J.; Gollner, S. An intervention to improve patient understanding and use of preoperative chlorhexidine washes. Infect Dis. Health 2019, 24, 194–200. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Klika, A.K.; Higuera, C.A. Use of chlorhexidine preparations in total joint arthroplasty. J. Bone Jt. Infect. 2017, 2, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Matsen, F.A.; Whitson, A.J.; Hsu, J.E. While home chlorhexidine washes prior to shoulder surgery lower skin loads of most bacteria, they are not effective against cutibacterium (propionibacterium). Int. Orthop. 2020, 44, 531–534. [Google Scholar] [CrossRef]
- Privitera, G.P.; Costa, A.L.; Brusaferro, S.; Chirletti, P.; Crosasso, P.; Massimetti, G.; Nespoli, A.; Petrosillo, N.; Pittiruti, M.; Scoppettuolo, G.; et al. Skin antisepsis with chlorhexidine versus iodine for the prevention of surgical site infection: A systematic review and meta-analysis. Am. J. Infect. Control 2017, 45, 180–189. [Google Scholar] [CrossRef]
- Letzelter, J.; Hill, J.B.; Hacquebord, J. An overview of skin antiseptics used in orthopaedic surgery procedures. J. Am. Acad. Orthop. Surg. 2019, 27, 599–606. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.W.; Guo, B.; Xu, C.C. Preoperative antisepsis with chlorhexidine versus povidone-iodine for the prevention of surgical site infection: A systematic review and meta-analysis. World J. Surg. 2020, 44, 1412–1424. [Google Scholar] [CrossRef]
- Cooke, C.L.; Greene, R.S.; van Eck, C.F.; Uquilas, C.; Limpisvasti, O. Bioelectric silver-zinc dressing equally effective to chlorhexidine in reducing skin bacterial load in healthy volunteers. Arthroscopy 2018, 34, 2886–2891. [Google Scholar] [CrossRef]
- Peel, T.N.; Dowsey, M.M.; Buising, K.L.; Cheng, A.C.; Choong, P.F.M. Chlorhexidine-alcohol versus iodine-alcohol for surgical site skin preparation in an elective arthroplasty (acaisa) study: A cluster randomized controlled trial. Clin. Microbiol. Infect. 2019, 25, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.; Phillips, C. Cyanoacrylate microbial sealants for skin preparation prior to surgery. Cochrane Database Syst. Rev. 2016, 18, CD008062. [Google Scholar] [CrossRef]
- Webster, J.; Alghamdi, A. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst. Rev. 2015, 17, CD006353. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.N.; Chen, A.F.; Taneja, M.; Kucukdurmaz, F.; Rothman, R.H.; Parvizi, J. Single vs repeat surgical skin preparations for reducing surgical site infection after total joint arthroplasty: A prospective, randomized, double-blinded study. J. Arthroplast. 2016, 31, 1289–1294. [Google Scholar] [CrossRef]
- Langvatn, H.; Schrama, J.C.; Cao, G.; Hallan, G.; Furnes, O.; Lingaas, E.; Walenkamp, G.; Engesaeter, L.B.; Dale, H. Operating room ventilation and the risk of revision due to infection after total hip arthroplasty: Assessment of validated data in the norwegian arthroplasty register. J. Hosp. Infect. 2020. [Google Scholar] [CrossRef]
- Teo, B.J.X.; Woo, Y.L.; Phua, J.K.S.; Chong, H.C.; Yeo, W.; Tan, A.H.C. Laminar flow does not affect risk of prosthetic joint infection after primary total knee replacement in asian patients. J. Hosp. Infect. 2020, 104, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, P.; Kubilay, N.Z.; Allegranzi, B.; Egger, M.; Gastmeier, P. Effect of laminar airflow ventilation on surgical site infections: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 553–561. [Google Scholar] [CrossRef]
- McHugh, S.M.; Hill, A.D.; Humphreys, H. Laminar airflow and the prevention of surgical site infection. More harm than good? Surgeon 2015, 13, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Knobben, B.A.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Evaluation of measures to decrease intra-operative bacterial contamination in orthopaedic implant surgery. J. Hosp. Infect. 2006, 62, 174–180. [Google Scholar] [CrossRef]
- Ban, K.A.; Minei, J.P.; Laronga, C.; Harbrecht, B.G.; Jensen, E.H.; Fry, D.E.; Itani, K.M.; Dellinger, E.P.; Ko, C.Y.; Duane, T.M. American college of surgeons and surgical infection society: Surgical site infection guidelines, 2016 update. J. Am. Coll. Surg. 2017, 224, 59–74. [Google Scholar] [CrossRef]
- Allegranzi, B.; Zayed, B.; Bischoff, P.; Kubilay, N.Z.; de Jonge, S.; de Vries, F.; Gomes, S.M.; Gans, S.; Wallert, E.D.; Wu, X.; et al. New who recommendations on intraoperative and postoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e288–e303. [Google Scholar] [CrossRef]
- Agodi, A.; Auxilia, F.; Barchitta, M.; Cristina, M.L.; D’Alessandro, D.; Mura, I.; Nobile, M.; Pasquarella, C.; Italian Study Group of Hospital Hygiene. Operating theatre ventilation systems and microbial air contamination in total joint replacement surgery: Results of the gisio-ischia study. J. Hosp. Infect. 2015, 90, 213–219. [Google Scholar] [CrossRef]
- National Institute for Health Research Global Research Health Unit on Global Surgery. Delphi prioritization and development of global surgery guidelines for the prevention of surgical-site infection. Br. J. Surg. 2020, 107, 970–977. [Google Scholar] [CrossRef]
- Mackain-Bremner, A.A.; Owens, K.; Wylde, V.; Bannister, G.C.; Blom, A.W. Adherence to recommendations designed to decrease intra-operative wound contamination. Ann. R Coll. Surg. Engl. 2008, 90, 412–416. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lo Giudice, D.; Trimarchi, G.; La Fauci, V.; Squeri, R.; Calimeri, S. Hospital infection control and behaviour of operating room staff. Cent. Eur. J. Public Health 2019, 27, 292–295. [Google Scholar] [CrossRef]
- Zucco, R.; Lavano, F.; Nobile, C.G.A.; Papadopoli, R.; Bianco, A. Adherence to evidence-based recommendations for surgical site infection prevention: Results among italian surgical ward nurses. PLoS ONE 2019, 14, e0222825. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.H.; Wang, Y.C.; Loh, E.W.; Tam, K.W. Antiseptic efficacies of waterless hand rub, chlorhexidine scrub, and povidone-iodine scrub in surgical settings: A meta-analysis of randomized controlled trials. J. Hosp. Infect. 2019, 101, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.; Dumville, J.C.; Norman, G.; Fortnam, M. Surgical hand antisepsis to reduce surgical site infection. Cochrane Database Syst. Rev. 2016, 22, CD004288. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.E. Operating room hand preparation: To scrub or to rub? Surg Infect. (Larchmt) 2019, 20, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Croke, L. Recommended practices for surgical hand antisepsis. AORN J. 2019, 109, P8–P10. [Google Scholar] [CrossRef]
- Young, S.W.; Zhu, M.; Shirley, O.C.; Wu, Q.; Spangehl, M.J. Do ‘surgical helmet systems’ or ‘body exhaust suits’ affect contamination and deep infection rates in arthroplasty? A systematic review. J. Arthroplast. 2016, 31, 225–233. [Google Scholar] [CrossRef]
- Ling, F.; Halabi, S.; Jones, C. Comparison of air exhausts for surgical body suits (space suits) and the potential for periprosthetic joint infection. J. Hosp. Infect. 2018, 99, 279–283. [Google Scholar] [CrossRef]
- Moores, T.S.; Khan, S.A.; Chatterton, B.D.; Harvey, G.; Lewthwaite, S.C. A microbiological assessment of sterile surgical helmet systems using particle counts and culture plates: Recommendations for safe use whilst scrubbing. J. Hosp. Infect. 2019, 101, 354–360. [Google Scholar] [CrossRef]
- Vermeiren, A.; Verheyden, M.; Verheyden, F. Do double-fan surgical helmet systems result in less gown-particle contamination than single-fan designs? Clin. Orthop. Relat. Res. 2020, 478, 1359–1365. [Google Scholar] [CrossRef]
- Klaber, I.; Ruiz, P.; Schweitzer, D.; Lira, M.J.; Botello, E.; Wozniak, A. Contamination rate of the surgical gowns during total hip arthroplasty. Arch. Orthop. Trauma Surg. 2019, 139, 1015–1019. [Google Scholar] [CrossRef]
- Fraser, J.F.; Young, S.W.; Valentine, K.A.; Probst, N.E.; Spangehl, M.J. The gown-glove interface is a source of contamination: A comparative study. Clin. Orthop. Relat. Res. 2015, 473, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, P.; Mundis, G.M., Jr.; Eastlack, R.; Nourian, A.; Pawelek, J.; Nguyen, S.; Akbarnia, B.A. Do longer surgical procedures result in greater contamination of surgeons’ hands? Clin. Orthop. Relat. Res. 2016, 474, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Beldame, J.; Lagrave, B.; Lievain, L.; Lefebvre, B.; Frebourg, N.; Dujardin, F. Surgical glove bacterial contamination and perforation during total hip arthroplasty implantation: When gloves should be changed. Orthop. Traumatol. Surg. Res. 2012, 98, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Aboltins, C.A.; Berdal, J.E.; Casas, F.; Corona, P.S.; Cuellar, D.; Ferrari, M.C.; Hendershot, E.; Huang, W.; Kuo, F.C.; Malkani, A.; et al. Hip and knee section, prevention, antimicrobials (systemic): Proceedings of international consensus on orthopedic infections. J. Arthroplast. 2019, 34, S279–S288. [Google Scholar] [CrossRef]
- Stefansdottir, A.; Robertsson, O.; Annette, W.D.; Kiernan, S.; Gustafson, P.; Lidgren, L. Inadequate timing of prophylactic antibiotics in orthopedic surgery. We can do better. Acta Orthop. 2009, 80, 633–638. [Google Scholar] [CrossRef]
- Siddiqi, A.; Forte, S.A.; Docter, S.; Bryant, D.; Sheth, N.P.; Chen, A.F. Perioperative antibiotic prophylaxis in total joint arthroplasty: A systematic review and meta-analysis. J. Bone Jt. Surg. 2019, 101, 828–842. [Google Scholar] [CrossRef]
- Tan, T.L.; Gomez, M.M.; Kheir, M.M.; Maltenfort, M.G.; Chen, A.F. Should preoperative antibiotics be tailored according to patient’s comorbidities and susceptibility to organisms? J. Arthroplast. 2017, 32, 1089–1094.E3. [Google Scholar] [CrossRef]
- Al-Mayahi, M.; Cian, A.; Lipsky, B.A.; Suva, D.; Muller, C.; Landelle, C.; Miozzari, H.H.; Uckay, I. Administration of antibiotic agents before intraoperative sampling in orthopedic infections alters culture results. J. Infect. 2015, 71, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Bedencic, K.; Kavcic, M.; Faganeli, N.; Mihalic, R.; Mavcic, B.; Dolenc, J.; Bajc, Z.; Trebse, R. Does preoperative antimicrobial prophylaxis influence the diagnostic potential of periprosthetic tissues in hip or knee infections? Clin. Orthop. Relat. Res. 2016, 474, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.L.; Shohat, N.; Rondon, A.J.; Foltz, C.; Goswami, K.; Ryan, S.P.; Seyler, T.M.; Parvizi, J. Perioperative antibiotic prophylaxis in total joint arthroplasty: A single dose is as effective as multiple doses. J. Bone Jt. Surg. 2019, 101, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.S.; Black, C.S.; Ryan, S.P.; Seyler, T.M. Extended oral antibiotics and infection prophylaxis after a primary or revision total knee arthroplasty. J. Knee Surg. 2020, 33, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, M.; Jamsen, E.; Karppelin, M.; Huttunen, R.; Syrjanen, J. The effect of preoperative oral antibiotic use on the risk of periprosthetic joint infection after primary knee or hip replacement: A retrospective study with a 1-year follow-up. Clin. Microbiol. Infect. 2019, 25, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Barbero-Allende, J.M.; Garcia-Sanchez, M.; Montero-Ruiz, E.; Valles-Purroy, A.; Plasencia-Arriba, M.A.; Sanz-Moreno, J. Dual prophylaxis with teicoplanin and cefazolin in the prevention of prosthetic joint infection. Enferm. Infecc. Microbiol. Clin. 2019, 37, 588–591. [Google Scholar] [CrossRef]
- Huiras, P.; Logan, J.K.; Papadopoulos, S.; Whitney, D. Local antimicrobial administration for prophylaxis of surgical site infections. Pharmacotherapy 2012, 32, 1006–1019. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Meyer, C. Antibiotic elution from hip and knee acrylic bone cement spacers: A systematic review. Biomed. Res. Int. 2017, 2017, 4657874. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.Y.; Jiang, F.L.; Wu, Y.P.; Yang, B.B.; Liu, Z.Y.; Liu, D. Antibiotic-impregnated bone cement for preventing infection in patients receiving primary total hip and knee arthroplasty: A meta-analysis. Medicine 2019, 98, e18068. [Google Scholar] [CrossRef]
- Volejnikova, A.; Melichercik, P.; Nesuta, O.; Vankova, E.; Bednarova, L.; Rybacek, J.; Cerovsky, V. Antimicrobial peptides prevent bacterial biofilm formation on the surface of polymethylmethacrylate bone cement. J. Med. Microbiol. 2019, 68, 961–972. [Google Scholar] [CrossRef]
- Pontes, M.H.; Groisman, E.A. A physiological basis for nonheritable antibiotic resistance. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.A.; Samuel, L.T.; Umpierrez, E.; Swiergosz, A.; Rabin, J.; Mahmood, B.; Mont, M.A. Routine use of commercial antibiotic-loaded bone cement in primary total joint arthroplasty: A critical analysis of the current evidence. Ann. Transl. Med. 2019, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.E.; Politi, J.R.; Chambers, B.; Smith, C.A. Intrawound vancomycin powder reduces early prosthetic joint infections in revision hip and knee arthroplasty. Surg. Technol. Int. 2017, 30, 284–289. [Google Scholar] [PubMed]
- Sweet, F.A.; Forsthoefel, C.W.; Sweet, A.R.; Dahlberg, R.K. Local versus systemic antibiotics for surgical infection prophylaxis in a rat model. J. Bone Jt. Surg. 2018, 100, e120. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, N.D.; Mayfield, C.K.; Culvern, C.N.; Oakes, D.A.; Lieberman, J.R.; Della Valle, C.J. Systematic review and meta-analysis of intrawound vancomycin in total hip and total knee arthroplasty: A call for a prospective randomized trial. J. Arthroplast. 2019, 34, 1815–1822. [Google Scholar] [CrossRef]
- Hanada, M.; Nishikino, S.; Hotta, K.; Furuhashi, H.; Hoshino, H.; Matsuyama, Y. Intrawound vancomycin powder increases post-operative wound complications and does not decrease periprosthetic joint infection in primary total and unicompartmental knee arthroplasties. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2322–2327. [Google Scholar] [CrossRef]
- Egawa, S.; Hirai, K.; Matsumoto, R.; Yoshii, T.; Yuasa, M.; Okawa, A.; Sugo, K.; Sotome, S. Efficacy of antibiotic-loaded hydroxyapatite/collagen composites is dependent on adsorbability for treating staphylococcus aureus osteomyelitis in rats. J. Orthop. Res. 2020, 38, 843–851. [Google Scholar] [CrossRef]
- Bertsch, P.; Schneider, L.; Bovone, G.; Tibbitt, M.W.; Fischer, P.; Gstohl, S. Injectable biocompatible hydrogels from cellulose nanocrystals for locally targeted sustained drug release. ACS Appl. Mater. Interfaces 2019, 11, 38578–38585. [Google Scholar] [CrossRef]
- Boot, W.; Vogely, H.C.; Jiao, C.; Nikkels, P.G.; Pouran, B.; van Rijen, M.H.; Ekkelenkamp, M.B.; Hansch, G.M.; Dhert, W.J.; Gawlitta, D. Prophylaxis of implant-related infections by local release of vancomycin from a hydrogel in rabbits. Eur. Cell Mater. 2020, 39, 108–120. [Google Scholar] [CrossRef]
- Colding-Rasmussen, T.; Horstmann, P.; Petersen, M.M.; Hettwer, W. Antibiotic elution characteristics and pharmacokinetics of gentamicin and vancomycin from a mineral antibiotic carrier: An in vivo evaluation of 32 clinical cases. J. Bone Jt. Infect. 2018, 3, 234–240. [Google Scholar] [CrossRef]
- Loftus, R.W.; Dexter, F.; Goodheart, M.J.; McDonald, M.; Keech, J.; Noiseux, N.; Pugely, A.; Sharp, W.; Sharafuddin, M.; Lawrence, W.T.; et al. The effect of improving basic preventive measures in the perioperative arena on staphylococcus aureus transmission and surgical site infections: A randomized clinical trial. JAMA Netw. Open 2020, 3, e201934. [Google Scholar] [CrossRef] [PubMed]
- Paziuk, T.M.; Luzzi, A.J.; Fleischman, A.N.; Goswami, K.; Schwenk, E.S.; Levicoff, E.A.; Parvizi, J. General vs spinal anesthesia for total joint arthroplasty: A single-institution observational review. J. Arthroplast. 2020, 35, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Matharu, G.S.; Garriga, C.; Rangan, A.; Judge, A. Does regional anesthesia reduce complications following total hip and knee replacement compared with general anesthesia? An analysis from the national joint registry for england, wales, northern ireland and the isle of man. J. Arthroplast. 2020, 35, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.B.; Thomas, V.S.; Ismaily, S.K.; Muradov, P.I.; Noble, P.C.; Incavo, S.J. Hypothermia in total joint arthroplasty: A wake-up call. J. Arthroplast. 2018, 33, 1012–1018. [Google Scholar] [CrossRef]
- Matos, J.R.; McSwain, J.R.; Wolf, B.J.; Doty, J.W.; Wilson, S.H. Examination of intra-operative core temperature in joint arthroplasty: A single-institution prospective observational study. Int. Orthop. 2018, 42, 2513–2519. [Google Scholar] [CrossRef] [PubMed]
- Weenink, R.P.; de Jonge, S.W.; van Hulst, R.A.; Wingelaar, T.T.; van Ooij, P.A.M.; Immink, R.V.; Preckel, B.; Hollmann, M.W. Perioperative hyperoxyphobia: Justified or not? Benefits and harms of hyperoxia during surgery. J. Clin. Med. 2020, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Naranje, S.; Lendway, L.; Mehle, S.; Gioe, T.J. Does operative time affect infection rate in primary total knee arthroplasty? Clin. Orthop. Relat. Res. 2015, 473, 64–69. [Google Scholar] [CrossRef]
- Dicks, K.V.; Baker, A.W.; Durkin, M.J.; Anderson, D.J.; Moehring, R.W.; Chen, L.F.; Sexton, D.J.; Weber, D.J.; Lewis, S.S. Short operative duration and surgical site infection risk in hip and knee arthroplasty procedures. Infect. Control Hosp. Epidemiol. 2015, 36, 1431–1436. [Google Scholar] [CrossRef]
- Surace, P.; Sultan, A.A.; George, J.; Samuel, L.T.; Khlopas, A.; Molloy, R.M.; Stearns, K.L.; Mont, M.A. The association between operative time and short-term complications in total hip arthroplasty: An analysis of 89,802 surgeries. J. Arthroplast. 2019, 34, 426–432. [Google Scholar] [CrossRef]
- Bohl, D.D.; Ondeck, N.T.; Darrith, B.; Hannon, C.P.; Fillingham, Y.A.; Della Valle, C.J. Impact of operative time on adverse events following primary total joint arthroplasty. J. Arthroplast. 2018, 33, 2256–2262.E4. [Google Scholar] [CrossRef]
- Whiteside, L.A. Prophylactic peri-operative local antibiotic irrigation. Bone Jt. J. 2016, 98-B, 23–26. [Google Scholar] [CrossRef][Green Version]
- Calkins, T.E.; Culvern, C.; Nam, D.; Gerlinger, T.L.; Levine, B.R.; Sporer, S.M.; Della Valle, C.J. Dilute betadine lavage reduces the risk of acute postoperative periprosthetic joint infection in aseptic revision total knee and hip arthroplasty: A randomized controlled trial. J. Arthroplast. 2020, 35, 538–543.E1. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, N.M.; Hart, A.; Taunton, M.J.; Osmon, D.R.; Mabry, T.M.; Abdel, M.P.; Perry, K.I. Use of povidone-iodine irrigation prior to wound closure in primary total hip and knee arthroplasty: An analysis of 11,738 cases. J. Bone Jt. Surg. 2019, 101, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Sprowson, A.P.; Jensen, C.; Parsons, N.; Partington, P.; Emmerson, K.; Carluke, I.; Asaad, S.; Pratt, R.; Muller, S.; Ahmed, I.; et al. The effect of triclosan-coated sutures on the rate of surgical site infection after hip and knee arthroplasty: A double-blind randomized controlled trial of 2546 patients. Bone Jt. J. 2018, 100-B, 296–302. [Google Scholar] [CrossRef]
- Leaper, D.; Wilson, P.; Assadian, O.; Edmiston, C.; Kiernan, M.; Miller, A.; Bond-Smith, G.; Yap, J. The role of antimicrobial sutures in preventing surgical site infection. Ann. R Coll Surg. Engl. 2017, 99, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.J.; Crawford, E.J.; Syed, I.; Kim, P.; Rampersaud, Y.R.; Martin, J. Is the risk of infection lower with sutures than with staples for skin closure after orthopaedic surgery? A meta-analysis of randomized trials. Clin. Orthop. Relat. Res. 2019, 477, 922–937. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudre, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Wang, M.; Tang, T. Surface treatment strategies to combat implant-related infection from the beginning. J. Orthop. Translat. 2019, 17, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, M.C.; Foxall-Smith, M.; Roberton, A.; Beswick, A.; Kieser, D.C.; Whitehouse, M.R. The use of silver coating in hip megaprostheses: A systematic review. Hip Int. 2019, 29, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Zajonz, D.; Birke, U.; Ghanem, M.; Prietzel, T.; Josten, C.; Roth, A.; Fakler, J.K.M. Silver-coated modular megaendoprostheses in salvage revision arthroplasty after periimplant infection with extensive bone loss-a pilot study of 34 patients. BMC Musculoskelet. Disord. 2017, 18, 383. [Google Scholar] [CrossRef] [PubMed]
- Medellin, M.R.; Fujiwara, T.; Clark, R.; Stevenson, J.D.; Parry, M.; Jeys, L. Mechanisms of failure and survival of total femoral endoprosthetic replacements. Bone Jt. J. 2019, 101-B, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Tsuchiya, H.; Terauchi, R.; Tsuchida, S.; Mizoshiri, N.; Mori, Y.; Takeuchi, A.; Hayashi, K.; Yamamoto, N.; Ikoma, K.; et al. A retrospective study of antibacterial iodine-coated implants for postoperative infection. Medicine 2019, 98, e17932. [Google Scholar] [CrossRef] [PubMed]
- Shoji, M.M.; Chen, A.F. Biofilms in periprosthetic joint infections: A review of diagnostic modalities, current treatments, and future directions. J. Knee Surg. 2020, 33, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; Alt, V.; van der Mei, H.C.; Fagette, P.H.; Zimmerli, W.; Moriarty, T.F.; Parvizi, J.; Schmidmaier, G.; Raschke, M.J.; Gehrke, T.; et al. A trans-atlantic perspective on stagnation in clinical translation of antimicrobial strategies for the control of biomaterial-implant-associated infection. ACS Biomater. Sci. Eng. 2019, 5, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Kremers, K.; Leijtens, B.; Camps, S.; Tostmann, A.; Koeter, S.; Voss, A. Evaluation of early wound leakage as a risk factor for prosthetic joint infection. J. Am. Assoc. Nurse Pract. 2019, 31, 337–343. [Google Scholar] [CrossRef]
- Rudasill, S.; Gittings, D.J.; Elkassabany, N.M.; Liu, J.; Nelson, C.L.; Kamath, A.F. Preoperative risk factor score predicts malnutrition in total joint arthroplasty patients. J. Surg. Orthop. Adv. 2019, 28, 97–103. [Google Scholar]
- Li, Z.; Knetsch, M. Antibacterial strategies for wound dressing: Preventing infection and stimulating healing. Curr. Pharm. Des. 2018, 24, 936–951. [Google Scholar] [CrossRef]
- Matthews, C.N.; Chen, A.F.; Daryoush, T.; Rothman, R.H.; Maltenfort, M.G.; Hozack, W.J. Does an elastic compression bandage provide any benefit after primary tka? Clin. Orthop. Relat. Res. 2019, 477, 134–144. [Google Scholar] [CrossRef]
- Keeney, J.A.; Cook, J.L.; Clawson, S.W.; Aggarwal, A.; Stannard, J.P. Incisional negative pressure wound therapy devices improve short-term wound complications, but not long-term infection rate following hip and knee arthroplasty. J. Arthroplast. 2019, 34, 723–728. [Google Scholar] [CrossRef]
- Newman, J.M.; Siqueira, M.B.P.; Klika, A.K.; Molloy, R.M.; Barsoum, W.K.; Higuera, C.A. Use of closed incisional negative pressure wound therapy after revision total hip and knee arthroplasty in patients at high risk for infection: A prospective, randomized clinical trial. J. Arthroplast. 2019, 34, 554–559.E1. [Google Scholar] [CrossRef]
- Benito, N.; Mur, I.; Ribera, A.; Soriano, A.; Rodriguez-Pardo, D.; Sorli, L.; Cobo, J.; Fernandez-Sampedro, M.; Del Toro, M.D.; Guio, L.; et al. The different microbial etiology of prosthetic joint infections according to route of acquisition and time after prosthesis implantation, including the role of multidrug-resistant organisms. J. Clin. Med. 2019, 8, 673. [Google Scholar] [CrossRef] [PubMed]
- Zeller, V.; Kerroumi, Y.; Meyssonnier, V.; Heym, B.; Metten, M.A.; Desplaces, N.; Marmor, S. Analysis of postoperative and hematogenous prosthetic joint-infection microbiological patterns in a large cohort. J. Infect. 2018, 76, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, W.M.H.; Walenkamp, G.; Moojen, D.J.F.; Hendriks, J.G.E.; Goedendorp, T.A.; Rozema, F.R. Antibiotic prophylaxis is not indicated prior to dental procedures for prevention of periprosthetic joint infections. Acta Orthop. 2017, 88, 568–574. [Google Scholar] [CrossRef]
- Suda, K.J.; Calip, G.S.; Zhou, J.; Rowan, S.; Gross, A.E.; Hershow, R.C.; Perez, R.I.; McGregor, J.C.; Evans, C.T. Assessment of the appropriateness of antibiotic prescriptions for infection prophylaxis before dental procedures, 2011 to 2015. JAMA Netw. Open 2019, 2, e193909. [Google Scholar] [CrossRef]
- Slullitel, P.A.; Onativia, J.I.; Piuzzi, N.S.; Higuera-Rueda, C.; Parvizi, J.; Buttaro, M.A. Is there a role for antibiotic prophylaxis prior to dental procedures in patients with total joint arthroplasty? A systematic review of the literature. J. Bone Jt. Infect. 2020, 5, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Sollecito, T.P.; Abt, E.; Lockhart, P.B.; Truelove, E.; Paumier, T.M.; Tracy, S.L.; Tampi, M.; Beltran-Aguilar, E.D.; Frantsve-Hawley, J. The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints: Evidence-based clinical practice guideline for dental practitioners—A report of the american dental association council on scientific affairs. J. Am. Dent. Assoc. 2015, 146, 11–16.E8. [Google Scholar] [CrossRef] [PubMed]
- Jahoda, D.; Nyc, O.; Simsa, J.; Kucera, E.; Hanek, P.; Chrz, P.; Pokorny, D.; Tawa, N.; Landor, I.; Sosna, A. Late hematogenous infection of prosthetic joint. Acta Chir. Orthop. Traumatol. Cechoslov. 2008, 75, 88–92. (In Czech) [Google Scholar]
- Jahoda, D.; Nyc, O.; Simsa, J.; Kucera, E.; Hanek, P.; Chrz, P.; Pokorny, D.; Tawa, N.; Landor, I.; Sosna, A. Late hematogenous infection of prosthetic joints in our patients and proposal for a system of prevention. Acta Chir. Orthop. Traumatol. Cechoslov. 2007, 74, 397–400. (In Czech) [Google Scholar]
- Rakow, A.; Perka, C.; Trampuz, A.; Renz, N. Origin and characteristics of haematogenous periprosthetic joint infection. Clin. Microbiol Infect. 2019, 25, 845–850. [Google Scholar] [CrossRef]
- Tomas, T. Patient-related risk factors for infected total arthroplasty. Acta Chir. Orthop. Traumatol. Cechoslov. 2008, 75, 451–456. (In Czech) [Google Scholar]
- Wouthuyzen-Bakker, M.; Lora-Tamayo, J.; Senneville, E.; Scarbourough, M.; Ferry, T.; Uckay, I.; Salles, M.J.; O’Connell, K.; Iribarren, J.A.; Vigante, D.; et al. Erysipelas or cellulitis with a prosthetic joint in situ. J. Bone Jt. Infect. 2018, 3, 222–225. [Google Scholar] [CrossRef]
- Stevignon, T.; Mouton, A.; Meyssonnier, V.; Kerroumi, Y.; Yazigi, A.; Aubert, T.; Lhotellier, L.; Le Strat, V.; Passeron, D.; Graff, W.; et al. Haematogenous prosthetic knee infections: Prospective cohort study of 58 patients. Orthop. Traumatol. Surg. Res. 2019, 105, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Salt, E.; Wiggins, A.T.; Rayens, M.K.; Morris, B.J.; Mannino, D.; Hoellein, A.; Donegan, R.P.; Crofford, L.J. Moderating effects of immunosuppressive medications and risk factors for post-operative joint infection following total joint arthroplasty in patients with rheumatoid arthritis or osteoarthritis. Semin. Arthritis Rheum. 2017, 46, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Holzer, R.J.; Dayton, J.D. Registries, risk calculators, and risk-adjusted outcomes: Current usage, limitations, and future prospects. Pediatr. Cardiol. 2020, 41, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Backhouse, A.; Ogunlayi, F. Quality improvement into practice. BMJ 2020, 368, m865. [Google Scholar] [CrossRef] [PubMed]
- Eckhoff, M.D.; Bader, J.M.; Nesti, L.J.; Dunn, J.C. Acute complications in total wrist arthroplasty: A national surgical quality improvement program review. J. Wrist Surg. 2020, 9, 124–128. [Google Scholar] [CrossRef]
- Childers, C.P.; Siletz, A.E.; Singer, E.S.; Faltermeier, C.; Hu, Q.L.; Ko, C.Y.; Golladay, G.J.; Kates, S.L.; Wick, E.C.; Maggard-Gibbons, M. Surgical technical evidence review for elective total joint replacement conducted for the ahrq safety program for improving surgical care and recovery. Geriatr. Orthop. Surg. Rehabil. 2018, 9, 2151458518754451. [Google Scholar] [CrossRef]
- Curtin, A.G.; Anderson, V.; Brockhus, F.; Cohen, D.R. Novel team-based approach to quality improvement effectively engages staff and reduces adverse events in healthcare settings. BMJ Open Qual. 2020, 9, e000741. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Beswick, A.D.; Whitehouse, M.R.; Blom, A.W.; Lenguerrand, E. Implant fixation and risk of prosthetic joint infection following primary total hip replacement: Meta-analysis of observational cohort and randomised intervention studies. J. Clin. Med. 2019, 8, 722. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Wylde, V.; Whitehouse, M.R.; Beswick, A.D.; Lenguerrand, E.; Blom, A.W. Influence of fixation methods on prosthetic joint infection following primary total knee replacement: Meta-analysis of observational cohort and randomised intervention studies. J. Clin. Med. 2019, 8, 828. [Google Scholar] [CrossRef]
- Lenguerrand, E.; Whitehouse, M.R.; Beswick, A.D.; Kunutsor, S.K.; Burston, B.; Porter, M.; Blom, A.W. Risk factors associated with revision for prosthetic joint infection after hip replacement: A prospective observational cohort study. Lancet Infect. Dis. 2018, 18, 1004–1014. [Google Scholar] [CrossRef]
- Lamplot, J.D.; Luther, G.; Mawdsley, E.L.; Luu, H.H.; Manning, D. Modified protocol decreases surgical site infections after total knee arthroplasty. J. Knee Surg. 2015, 28, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.J.; Kesselheim, A.S.; Vokinger, K.N. Lifecycle regulation of artificial intelligence- and machine learning-based software devices in medicine. JAMA 2019, 322, 2285–2286. [Google Scholar] [CrossRef] [PubMed]
- Loftus, T.J.; Tighe, P.J.; Filiberto, A.C.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Rashidi, P.; Upchurch, G.R., Jr.; Bihorac, A. Artificial intelligence and surgical decision-making. JAMA Surg. 2019. [Google Scholar] [CrossRef]
- Greenland, P.; Hassan, S. Precision preventive medicine-ready for prime time? JAMA Intern. Med. 2019, 179, 605–606. [Google Scholar] [CrossRef]
- Lenguerrand, E.; Whitehouse, M.R.; Beswick, A.D.; Kunutsor, S.K.; Foguet, P.; Porter, M.; Blom, A.W.; National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. Risk factors associated with revision for prosthetic joint infection following knee replacement: An observational cohort study from england and wales. Lancet Infect. Dis. 2019, 19, 589–600. [Google Scholar] [CrossRef]
- Alamanda, V.K.; Springer, B.D. The prevention of infection: 12 modifiable risk factors. Bone Jt. J. 2019, 101-B, 3–9. [Google Scholar] [CrossRef]
- Blanco, J.F.; Diaz, A.; Melchor, F.R.; da Casa, C.; Pescador, D. Risk factors for periprosthetic joint infection after total knee arthroplasty. Arch. Orthop. Trauma. Surg. 2020, 140, 239–245. [Google Scholar] [CrossRef]
- Karas, V.; Kildow, B.J.; Baumgartner, B.T.; Green, C.L.; Attarian, D.E.; Bolognesi, M.P.; Seyler, T.M. Preoperative patient profile in total hip and knee arthroplasty: Predictive of increased medicare payments in a bundled payment model. J. Arthroplast. 2018, 33, 2728–2733.E3. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Kamdar, N.; Kim, N.; Gonzales, G.; Singh, K.; Waljee, A.K. Use of electronic medical records in development and validation of risk prediction models of hospital readmission: Systematic review. BMJ 2020, 369, m958. [Google Scholar] [CrossRef]
| Variable | Level of Evidence | Estimated Size Effect | Population Effect |
|---|---|---|---|
| Systemic malignancy | Individual study [38] SR [39] | OR 3.1; 95% CI, 1.3–7.2 RR 1.52; 95% CI, 0.98–2.34 | Limited |
| Chronic kidney insufficiency | Individual studies [40,41,42,43] | 5-year PJI rates, 8.5%; in transplant patients, 4.5% [44] | Low # |
| Severe hepatic disease | SR [45] | Revision rate, 4% (vs. 0.2% in controls); PJI in 70%; mean infection rate, 4.1% (for urgent cases, 8.6%) ¶ | Limited |
| Rheumatoid arthritis | SR, MA [46] | OR 1.89, 95% CI, 1.34–2.66 ¶¶ | Moderate |
| Risk of biologics | Individual study [47] | Compared to a 2.14% 1-year cumulative incidence of PJI with abatacept, predicted incidence ranged from 0.35% (95% CI, 0.11–1.12) with rituximab to 3.67% (95% CI, 1.69–7.88) with tocilizumab | Low to moderate |
| BMI (≥30) BMI (≥40/<30) | SR, MA [48] | RR 2.22; 95% CI, 1.67–2.96 RR 8.48; 95% CI, 3.47–20.71 | Strong |
| Malnutrition | SR + MA [49] | OR 3.58, 95% CI, 1.82–7.03 | Moderate |
| Diabetes | SR/MA for SSI/for PJI [39] | RR 1.74; 95% CI, 1.45–2.09 | Strong |
| Smoking | SR/MA [50] | OR 2.02; 95% CI, 1.47–2.77 | Strong * |
| Lymphedema | Individual study [51] | Revision HR 6.19 (95% CI, 2.22–17.23) | Limited to low # |
| HIV + patients | SR [52] | Risk ratio 3.31; 95% CI, 1.18–9.29 | Limited |
| Hypothyroidism | Individual study [53] Individual study [54] | OR 2.04; 95% CI, 1.02–4.08 TEA OR 1.32; 95% CI, 1.03–1.69 TAA | Low |
| Prior joint surgery | SR [39] | RR 2.98; 95% CI, 1.49–5.93 | Strong |
| Prior PJI in another joint | Individual study [55] | HR 3.3, 95% CI, 1.18–8.97 (patients on chronic suppression: HR 15) | NR |
| Previous steroid administration | SR [39] | RR 1.68; 95% CI, 1.26–2.25 | Low to moderate |
| Peripheral vascular disease | Individual study [54] | OR 2.46; 95% CI, 1.87–3.22 | Low to moderate # |
| Intervention | Description of Effect |
|---|---|
| Pre-/perioperative serum glucose control, hemoglobin A1c, fructosamine | Optimizing for patients with unstable diabetes could target (i) wound healing; (ii) SSI; and (iii) re-admission rate [60,61,62,63]. |
| Reducing BMI | Optimizing weight for patients with overweight could target (i) wound healing; (ii) SSI; and (iii) PJI rate [64,65,66]. Patients with BMI >35 are advised to lose weight prior to surgery; however, candidacy restriction is not appropriate because these patients have no other options for pain relief. |
| Treatment of all preoperative infections | To reduce the chance of the development of hematogenous and/or directly spread PJIs. |
| Staphylococcal decolonization (nasal, skin) | Elimination of S. aureus (MRSA) carriers from TJA surgery could contribute to reductions in PJI [57]; however, some patients may be carriers even after decontamination [67,68]. |
| Discontinuation of immunosuppressive therapy | To reduce the effect of therapy on the capacity and efficacy of the immune system; various recommendations: (i) methotrexate off (1 wk preop.; 2 wks postop.); (ii) anti-TNF agents off (2–8 wks preop.; 2–4 wks postop.) [7]; described in detail elsewhere [69]. |
| Preoperative wash/cloth with antibacterial substances | To reduce bacterial skin load; all chlorhexidine, dilute povidone-iodine solutions can contribute to reduced risk for SSI/PJI [70,71,72]. |
| Antibacterial incisional drape | To eliminate the residual bacterial skin load after routine skin preparation [73,74,75]. |
| Novel strategies for systemic/local antibiotics/antimicrobials | Extension and/or prolongation of the antibacterial effect of ATBs via dual [76,77] or extended ATBs [78,79]; limited evidence for intrawound ATBs [80]. |
| The best operating rooms/surgeons * | To decrease the intraoperative bacterial load via an experienced surgeon, team, and aseptic theater [81,82,83]. |
| Intraoperative wound wash of antimicrobials | To decrease the intraoperative bacterial load [84,85]. |
| Implants with antibacterial surfaces | To improve resistance of an implant against bacterial adhesion via antibacterial hydrogel [86], other antibacterial carriers [87,88] or silver coating [89]. |
| Anti-staphylococcal vaccine | To increase efficacy of the immune system to eradicate Staphylococcus spp. intra-/postoperatively [90,91]. |
| Event | Recommendation | Ref. |
|---|---|---|
| Dental intervention | Evidence against routine ATB prophylaxis after TJA, only in special clinical situations | [193,195,196] |
| Abdominal surgery | Inconclusive evidence, ATBs are indicated, apart from situations always requiring ATB therapy, also for advanced forms of acute appendicitis, perirectal abscess, invasive endoscopy procedures on the colon, soft tissue phlegmon or abscess, surgical treatment of venous ulceration and pressure sores, and limb amputation | [197,198,199] |
| Cardiological interventions | Patients with TJA should not receive ATBs before cardiovascular interventions | [199] |
| Dialysis | No recommendation in relation to a combined risk from the chronic vascular approach and end-stage renal insufficiency | [40] |
| Urinary tract intervention, infection | Inconclusive evidence, however, a risk-associated procedure on the urogenital system (endoscopic or open surgery, prostate gland biopsy, extracorporeal lithotripsy) could be subject to ATB prophylaxis | [197,198,199] |
| Skin infections | Treat emergently all erysipelas as it can affect previously healthy TKA | [199,200,201,202] |
| Postoperative immunosuppressive therapy | Reduce doses of glucocorticoids because prednisone increases risk for a postoperative infection (OR 1.59, P < 0.001) | [203] |
| Propensity-adjusted HR 1.36 (95% CI, 0.90–2.04) for 5–10 mg and 1.86 (95% CI, 1.02–3.37) for >10 mg | [47] | |
| described in detail elsewhere | [69] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, J.; Nieslanikova, E. Prevention of Prosthetic Joint Infection: From Traditional Approaches towards Quality Improvement and Data Mining. J. Clin. Med. 2020, 9, 2190. https://doi.org/10.3390/jcm9072190
Gallo J, Nieslanikova E. Prevention of Prosthetic Joint Infection: From Traditional Approaches towards Quality Improvement and Data Mining. Journal of Clinical Medicine. 2020; 9(7):2190. https://doi.org/10.3390/jcm9072190
Chicago/Turabian StyleGallo, Jiri, and Eva Nieslanikova. 2020. "Prevention of Prosthetic Joint Infection: From Traditional Approaches towards Quality Improvement and Data Mining" Journal of Clinical Medicine 9, no. 7: 2190. https://doi.org/10.3390/jcm9072190
APA StyleGallo, J., & Nieslanikova, E. (2020). Prevention of Prosthetic Joint Infection: From Traditional Approaches towards Quality Improvement and Data Mining. Journal of Clinical Medicine, 9(7), 2190. https://doi.org/10.3390/jcm9072190







