A Multidisciplinary Approach for Improving Quality of Life and Self-Management in Diabetic Kidney Disease: A Crossover Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
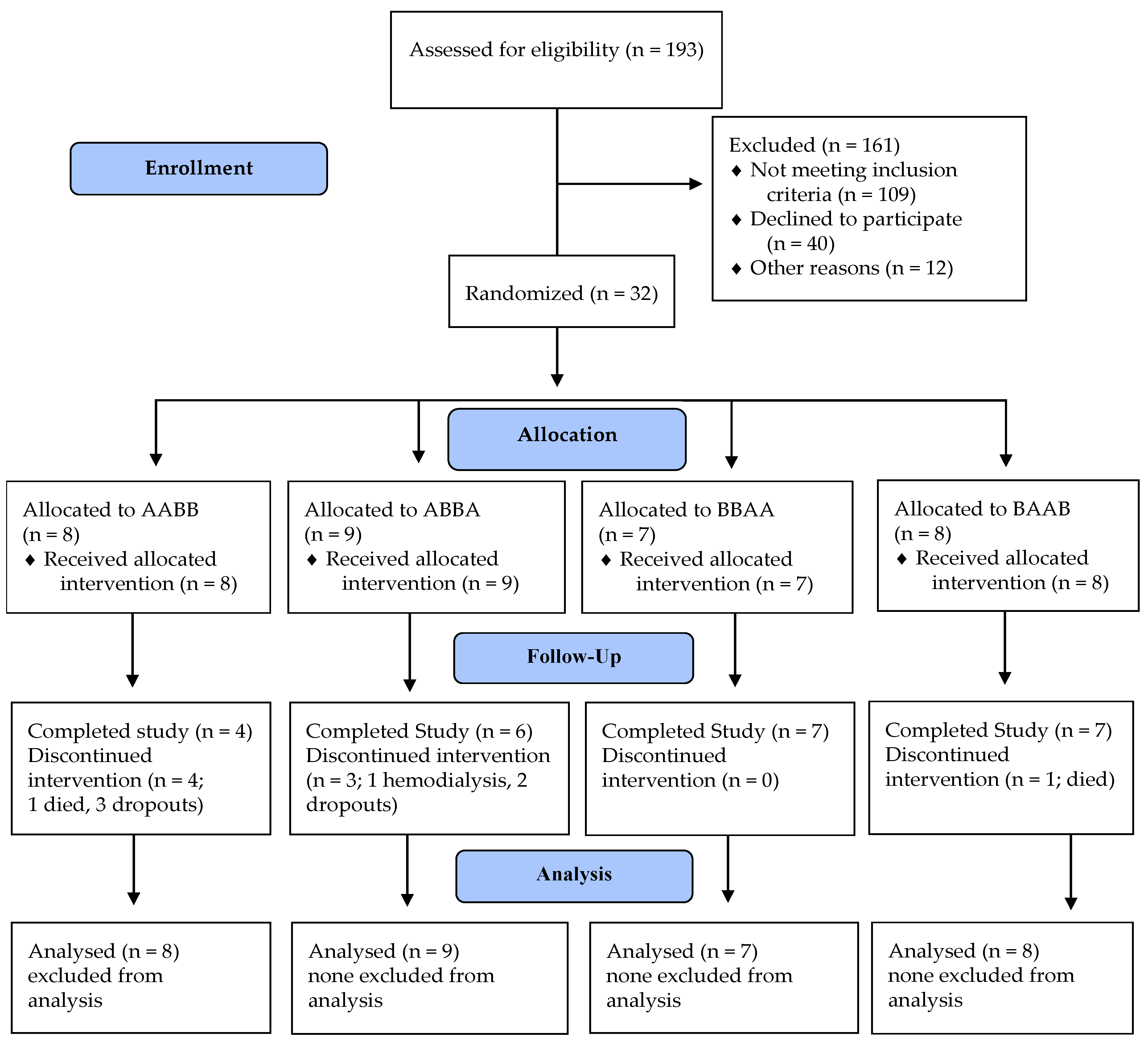
2.3. Randomization and Masking
2.4. Intervention
2.5. Outcome Measures
2.6. Statistical Analysis and Data Management
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afkarian, M.; Zelnick, L.R.; Hall, Y.N.; Heagerty, P.J.; Tuttle, K.; Weiss, N.S.; De Boer, I.H. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA 2016, 316, 602–610. [Google Scholar] [CrossRef]
- Lu, Y.; Stamm, C.; Nobre, D.; Pruijm, M.; Teta, D.; Cherpillod, A.; Halabi, G.; Phan, O.; Fumeaux, Z.; Bullani, R. Changing trends in end-stage renal disease patients with diabetes. Swiss Med. Wkly. 2017, 147, w14458. [Google Scholar]
- KDIGO. CKD Evaluation and Management–KDIGO. Available online: https://kdigo.org/guidelines/ckd-evaluation-and-management/ (accessed on 8 January 2020).
- United States Renal Data System. USRDS 2016 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2016.
- Tonelli, M.; Muntner, P.; Lloyd, A.; Manns, B.J.; Klarenbach, S.; Pannu, N.; James, M.T.; Hemmelgarn, B.R.; Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: A population-level cohort study. Lancet 2012, 380, 807–814. [Google Scholar] [CrossRef]
- Doshi, S.M.; Friedman, A.N. Diagnosis and Management of Type 2 Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 1366–1373. [Google Scholar] [CrossRef]
- Gorodetskaya, I.; Zenios, S.; McCulloch, C.E.; Bostrom, A.; Hsu, C.-Y.; Bindman, A.B.; Go, A.S.; Chertow, G.M. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005, 68, 2801–2808. [Google Scholar] [CrossRef]
- Ilyas, Z.; Chaiban, J.T.; Krikorian, A. Novel insights into the pathophysiology and clinical aspects of diabetic nephropathy. Rev. Endocr. Metab. Disord. 2017, 18, 21–28. [Google Scholar] [CrossRef]
- MacIsaac, R.J.; Jerums, G.; Ekinci, E.I. Effects of glycaemic management on diabetic kidney disease. World J. Diabetes 2017, 8, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; De Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D. Diabetic kidney disease: A report from an ADA Consensus Conference. Am. J. Kidney Dis. 2014, 64, 510–533. [Google Scholar] [CrossRef]
- Jain, K.; Mottl, A.K. Comprehensive Care for People with Diabetic Kidney Disease. Diabetes Spectr. 2015, 28, 187–192. [Google Scholar] [CrossRef][Green Version]
- Lopez-Vargas, P.A.; Tong, A.; Phoon, R.K.; Chadban, S.J.; Shen, Y.; Craig, J.C. Knowledge deficit of patients with stage 1–4 CKD: A focus group study. Nephrology 2014, 19, 234–243. [Google Scholar] [CrossRef]
- Helou, N.; Dwyer, A.; Shaha, M.; Zanchi, A. Multidisciplinary management of diabetic kidney disease: A systematic review and meta-analysis. JBI Database Syst. Rev. Implement. Rep. 2016, 14, 169–207. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Beanlands, H.; McCay, E.; Cattran, D.; Hladunewich, M.; Francis, D. The Self-Management Experience of People with Mild to Moderate Chronic Kidney Disease. Available online: https://pubmed.ncbi.nlm.nih.gov/18472683/ (accessed on 15 June 2020).
- Lindqvist, R.; Carlsson, M.; Sjödén, P.O. Perceived Consequences of Being a Renal Failure Patient. Available online: https://pubmed.ncbi.nlm.nih.gov/11249327/ (accessed on 15 June 2020).
- Teasdale, E.J.; Leydon, G.; Fraser, S.; Roderick, P.; Taal, M.W.; Tonkin-Crine, S. Patients’ Experiences after CKD Diagnosis: A Meta-ethnographic Study and Systematic Review. Am. J. Kidney Dis. 2017, 70, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.G.; Renpenning, K.M. Self-Care Science, Nursing Theory and Evidence-Based Practice; Springer Publishing Company: New York, NY, USA, 2011. [Google Scholar]
- Piantadosi, S. Crossover Designs. In Clinical Trials: A Methodologic Perspective, 2nd ed.; Wiley Blackwell: Hoboken, NJ, USA, 2005. [Google Scholar]
- Li, T.; Yu, T.; Hawkins, B.S.; Dickersin, K. Design, Analysis, and Reporting of Crossover Trials for Inclusion in a Meta-Analysis. PLoS ONE 2015, 10, e0133023. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.; Todd, C.; Gorton, T.; Symonds, E.; Martin, A.; Plowright, R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: The ADDQoL. Qual. Life Res. 1999, 8, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Toobert, D.J.; Hampson, S.E.; Glasgow, R.E. The summary of diabetes self-care activities measure: Results from 7 studies and a revised scale. Diabetes Care 2000, 23, 943–950. [Google Scholar] [CrossRef]
- Mallion, J.-M.; Dutrey-Dupagne, C.; Vaur, L.; Genes, N.; Renault, M.; Elkik, F.; Baguet, P.; Boutelant, S. Benefits of electronic pillboxes in evaluating treatment compliance of patients with mild to moderate hypertension. J. Hypertens. 1996, 14, 137–144. [Google Scholar]
- Hays, R.D.; Woolley, J.M. The Concept of Clinically Meaningful Difference in Health-Related Quality-of-Life Research. PharmacoEconomics 2000, 18, 419–423. [Google Scholar] [CrossRef]
- Ostini, R.; Dower, J.; Donald, M. The Audit of Diabetes-Dependent Quality of Life 19 (ADDQoL): Feasibility, reliability and validity in a population-based sample of Australian adults. Qual. Life Res. 2012, 21, 1471–1477. [Google Scholar] [CrossRef]
- Chan, J.C.; So, W.-Y.; Yeung, C.-Y.; Ko, G.T.; Lau, I.-T.; Tsang, M.-W.; Lau, K.-P.; Siu, S.-C.; Li, J.K.; Yeung, V.T.; et al. Effects of Structured Versus Usual Care on Renal Endpoint in Type 2 Diabetes: The SURE Study: A randomized multicenter translational study. Diabetes Care 2009, 32, 977–982. [Google Scholar] [CrossRef]
- Cueto-Manzano, A.M.; Martínez-Ramírez, H.R.; Cortés-Sanabria, L. Management of chronic kidney disease: Primary health-care setting, self-care and multidisciplinary approach. Clin. Nephrol. 2010, 74 (Suppl. 1), S99–S104. [Google Scholar] [CrossRef]
- Hotu, C.; Bagg, W.; Collins, J.; Harwood, L.; Whalley, G.; Doughty, R.; Gamble, G.; Braatvedt, G. A community-based model of care improves blood pressure control and delays progression of proteinuria, left ventricular hypertrophy and diastolic dysfunction in Māori and Pacific patients with type 2 diabetes and chronic kidney disease: A randomized controlled trial. Nephrol. Dial. Transplant. 2010, 25, 3260–3266. [Google Scholar] [CrossRef] [PubMed]
- Kazawa, K.; Takeshita, Y.; Yorioka, N.; Moriyama, M. Efficacy of a disease management program focused on acquisition of self-management skills in pre-dialysis patients with diabetic nephropathy: 24 months follow-up. J. Nephrol. 2015, 28, 329–338. [Google Scholar] [CrossRef]
- Steed, L.; Lankester, J.; Barnard, M.; Earle, K.; Hurel, S.; Newman, S. Evaluation of the UCL Diabetes Self-management Programme (UCL-DSMP): A Randomized Controlled Trial. J. Health Psychol. 2005, 10, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Vollenweider, P.; Waeber, G.; Marques-Vidal, P. Prevalence, awareness and treatment of type 2 diabetes mellitus in Switzerland: The CoLaus study. Diabet. Med. 2012, 29, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, U.; Bopp, M.; Holzer, B.M.; Djalali, S.; Kaplan, V. Prevalence of chronic medical conditions in Switzerland: Exploring estimates validity by comparing complementary data sources. BMC Public Health 2014, 14, 1157. [Google Scholar] [CrossRef] [PubMed]
- Odegard, P.S.; Capoccia, K. Medication Taking and Diabetes. Diabetes Educ. 2007, 33, 1014–1029. [Google Scholar] [CrossRef]
- Holtkamp, F.A.; de Zeeuw, D.; Thomas, M.C.; Cooper, M.E.; de Graeff, P.A.; Hillege, H.J.L.; Parving, H.-H.; Brenner, B.M.; Shahinfar, S.; Lambers Heerspink, H.J. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011, 80, 282–287. [Google Scholar] [CrossRef]
- Schell, J.O.; Patel, U.D.; Steinhauser, K.E.; Ammarell, N.; Tulsky, J.A. Discussions of the Kidney Disease Trajectory by Elderly Patients and Nephrologists: A Qualitative Study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2012, 59, 495–503. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, C.; Furtado, R.H.M.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, C.; et al. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Available online: https://pubmed.ncbi.nlm.nih.gov/30786725/ (accessed on 15 June 2020).
- Buse, J.; Wexler, D.; Tsapas, A.; Rossing, P.; Mingrone, M.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 Update To: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Available online: https://pubmed.ncbi.nlm.nih.gov/31857443/ (accessed on 15 June 2020).
- Ninčević, N.; Kolarić, T.O.; Roguljić, H.; Kizivat, T.; Smolić, M.; Bilić Ćurčić, I. Renal Benefits of SGLT 2 Inhibitors and GLP-1 Receptor Agonists: Evidence Supporting a Paradigm Shift in the Medical Management of Type 2 Diabetes. Available online: https://pubmed.ncbi.nlm.nih.gov/31757028/ (accessed on 15 June 2020).
- Sarafidis, P.; Ferro, C.J.; Morales, E.; Ortiz, A.; Malyszko, J.; Hojs, R.; Khazim, K.; Ekart, R.; Valdivielso, J.; Fouque, D.; et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol. Dial. Transplant. 2019, 34, 208–230. [Google Scholar] [CrossRef]
- Torre, C.; Guerreiro, G.; Longo, P.; Raposo, J.F.; Leufkens, H.; Martins, A.P. Health-related quality of life in adults with type 2 diabetes mellitus starting with new glucose lowering drugs: An inception cohort study. Prim. Care Diabetes 2019, 13, 221–232. [Google Scholar] [CrossRef]
- Lawn, S.; Schoo, A. Supporting self-management of chronic health conditions: Common approaches. Patient Educ. Couns. 2010, 80, 205–211. [Google Scholar] [CrossRef] [PubMed]
 | ||||
| Design | Period 1 0–3 Months | Period 2 3–6 Months | Period 3 6–9 Months | Period 4 9–12 Months |
| Sequence ABBA (n = 10) | UC | MSMP | MSMP | UC |
| Sequence BAAB (n = 10) | MSMP | UC | UC | MSMP |
| Sequence AABB (n = 10) | UC | UC | MSMP | MSMP |
| Sequence BBAA (n = 10) | MSMP | MSMP | UC | UC |
 | ||||
| A Multidisciplinary Self-Management Program Sequence BAAB | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weeks 1 and 41 | Weeks 3 and 43 | Weeks 5 and 45 | Weeks 7 and 47 | Weeks 9 and 49 | Weeks 11 and 51 | Weeks 12 and 52 | Weeks 13, 27, 28, 30 and 40, 52–54 | Week 52 | |||
| Assessment and/or Intervention | Medical visit and Screening | Nurse Home Visit | Nurse Telephone follow-up | Dietician Clinic Visit | Nurse Home Visit | Dietician Clinic Visit | Nurse Telephone Follow-up | Nurse Clinic Visit | Medical Visit nephrologist or diabetologist | End of the study | |
| Enrollme-nt | Inclusion/Exclusion criteria | X | |||||||||
| Information form | X | ||||||||||
| Randomization | X | ||||||||||
| Informed consent form | X | ||||||||||
| Treatment | 1. Comprehensive initial assessment and evaluation of patients’ self-care deficits | X | Medical follow-up | ||||||||
| Current Medications | X | ||||||||||
| Priority setting-one goal and contract signing | X | ||||||||||
| 2. Teaching and Training on self-care | X | X | |||||||||
| Education on DKD | X | ||||||||||
| Education on the risk of hypoglycemia | X | ||||||||||
| 3. Counseling on self-care development | X | X | |||||||||
| 4. Guiding and support | X | X | X | ||||||||
| 5. Coordination of Care | X | X | X | X | X | X | X | ||||
| 6. Follow-up and proactive monitoring | X | X | X | ||||||||
| Dietary plan and counseling | X | X | |||||||||
| Outcomes’ measurements | Demographics | X | |||||||||
| Self-management activities | X | X | X | ||||||||
| Medication adherence | X | X | X | ||||||||
| Quality of life | X | X | X | ||||||||
| Serum cr, eGFR, urinary albumin/cr ratio | X | X | X | ||||||||
| HbA1c | X | X | X | ||||||||
| Resource utilization | X | X | X | ||||||||
| Clinical Characteristics | Range | Mean (± SD)/Median | |
| Age (years) | 50–91 | 67.8 (±10.8)/67 | |
| HbA1c (%) | 5.7–10.5 | 7.7 (±1.3)/7.45 | |
| eGFR (mL/min/ 1.73 m2) | 15–108 | 41.3 (±21.5)/35 | |
| Sociodemographic Characteristics | Frequency | % | |
| Age Group | 50–64 | 14 | 43.8 |
| 65–80 | 13 | 40.6 | |
| ≥80 | 5 | 15.6 | |
| Sex | Men | 29 | 90.6 |
| Women | 3 | 9.4 | |
| Marital Status | Married/ Cohabitating | 21 | 65.6 |
| Divorced/Living alone | 11 | 34.4 | |
| Professional Status | Retired | 18 | 56.3 |
| Independent/Employed | 10 | 31.2 | |
| Medical cessation of work | 4 | 12.5 | |
| Educational Level | Academic | 11 | 34.4 |
| Short-cycle/Post-secondary | 12 | 37.5 | |
| Primary | 9 | 28.1 | |
| Comorbidities | Cardiovascular disease | 15 | 47 |
| Dyslipidemia | 20 | 63 | |
| Neuropathy | 14 | 44 | |
| Retinopathy | 14 | 44 | |
| Hypertension | 30 | 94 | |
| Peripheral vascular disease | 8 | 25 | |
| Gout | 3 | 9 | |
| Sleep apnea | 7 | 22 | |
| Medications | Insulin | 22 | 69 |
| ACE inhibitor or ARB | 27 | 84 | |
| GLP-1Ra | 2 | 6 | |
| SGLT2i | 0 | 0 | |
| Variable | Measure | Pre-Post Difference | n | Mean Rank | Rank Sum | Mean | SD | n | Mean Rank | Rank Sum | Mean | SD | M-U | Sig. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UC | MSMP | |||||||||||||
| Quality of Life | ADDQoL | Present QoL | 48 | 41.01 | 1968.5 | −0.19 | 0.87 | 44 | 52.49 | 2309.5 | 0.18 | 1.06 | 792.5 | 0.026 * |
| DM Impact on QoL | 48 | 47.24 | 2267.5 | 0.06 | 1.19 | 44 | 45.69 | 2010.5 | −0.05 | 0.96 | 1021 | 0.763 | ||
| DM Impact on Domains | 48 | 43.03 | 2065.5 | −0.11 | 0.95 | 44 | 50.28 | 2212.5 | −0.06 | 1.44 | 889.5 | 0.193 | ||
| Self-Management | R-SDSCA | General Diet Habits | 48 | 38.31 | 1839 | −0.46 | 1.94 | 44 | 55.43 | 2439 | 0.93 | 1.66 | 663.0 | 0.002 * |
| DM Specific Diet Habits | 48 | 37.02 | 1777 | −0.19 | 1.06 | 44 | 56.84 | 2501 | 0.82 | 1.85 | 601.0 | 0.000 * | ||
| Exercise Habits | 48 | 45.21 | 2170 | 0.30 | 2.66 | 44 | 47.91 | 2108 | 0.47 | 2.38 | 994.0 | 0.626 | ||
| Blood Sugar Testing | 48 | 39.77 | 1909 | −0.58 | 2.54 | 44 | 53.84 | 2369 | 0.80 | 2.22 | 733.0 | 0.008 * | ||
| Foot Care | 48 | 43.34 | 2080.5 | −0.14 | 1.88 | 44 | 49.94 | 2197.5 | 0.25 | 1.65 | 904.5 | 0.214 | ||
| % of Smoking Status | 48 | 47.90 | 2299 | 0.04 | 0.20 | 44 | 44.98 | 1979 | −0.02 | 0.15 | 989.0 | 0.089 | ||
| No· of cigarettes/ day | 9 | 10.22 | 92 | −0.44 | 4.10 | 9 | 8.78 | 79 | −1.22 | 6.22 | 34.00 | 0.558 | ||
| MEMS | Anti-HTN Medication | 26 | 21.79 | 566.5 | −3.08 | 12.1 | 22 | 27.71 | 609.5 | −1.32 | 6.44 | 215.5 | 0.140 | |
| Glycemic Control | % | HbA1c | 56 | 56.51 | 3164.5 | 0.06 | 0.64 | 50 | 50.13 | 2506.5 | −0.17 | 0.87 | 1232 | 0.285 |
| Renal Function | µmol/L | Serum Creatinine | 55 | 52.62 | 2894 | −3.44 | 32.3 | 51 | 54.45 | 2777 | 1.47 | 28.8 | 1354 | 0.759 |
| mL/min | eGFR (CKD_EPI) | 57 | 57.75 | 3291.5 | 1.51 | 12.4 | 51 | 50.87 | 2594.5 | −0.20 | 9.48 | 1269 | 0.254 | |
| mg/mmol | Urine Alb/Cr | 46 | 49.35 | 2270 | 9.89 | 103 | 46 | 43.65 | 2008 | −2.60 | 103 | 927.0 | 0.306 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helou, N.; Talhouedec, D.; Zumstein-Shaha, M.; Zanchi, A. A Multidisciplinary Approach for Improving Quality of Life and Self-Management in Diabetic Kidney Disease: A Crossover Study. J. Clin. Med. 2020, 9, 2160. https://doi.org/10.3390/jcm9072160
Helou N, Talhouedec D, Zumstein-Shaha M, Zanchi A. A Multidisciplinary Approach for Improving Quality of Life and Self-Management in Diabetic Kidney Disease: A Crossover Study. Journal of Clinical Medicine. 2020; 9(7):2160. https://doi.org/10.3390/jcm9072160
Chicago/Turabian StyleHelou, Nancy, Dominique Talhouedec, Maya Zumstein-Shaha, and Anne Zanchi. 2020. "A Multidisciplinary Approach for Improving Quality of Life and Self-Management in Diabetic Kidney Disease: A Crossover Study" Journal of Clinical Medicine 9, no. 7: 2160. https://doi.org/10.3390/jcm9072160
APA StyleHelou, N., Talhouedec, D., Zumstein-Shaha, M., & Zanchi, A. (2020). A Multidisciplinary Approach for Improving Quality of Life and Self-Management in Diabetic Kidney Disease: A Crossover Study. Journal of Clinical Medicine, 9(7), 2160. https://doi.org/10.3390/jcm9072160






