Impact of Pancreas Transplantation on the Patient Survival—An Analysis of the Japanese Pancreas Transplants Registry
Abstract
1. Introduction
2. Patients and Methods
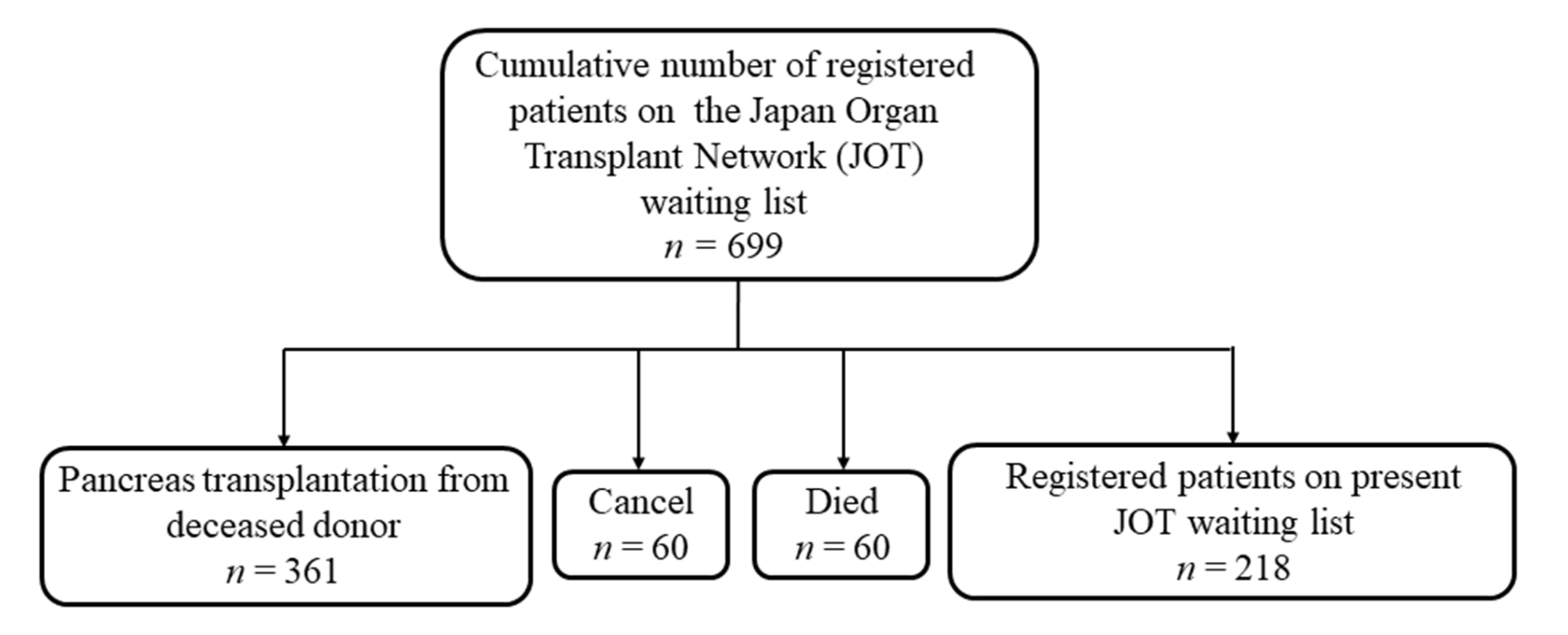
2.1. Enrolled Patients
2.2. Study Design
2.3. Statistical Analyses
2.4. Ethical Considerations
3. Results
3.1. Patient Background
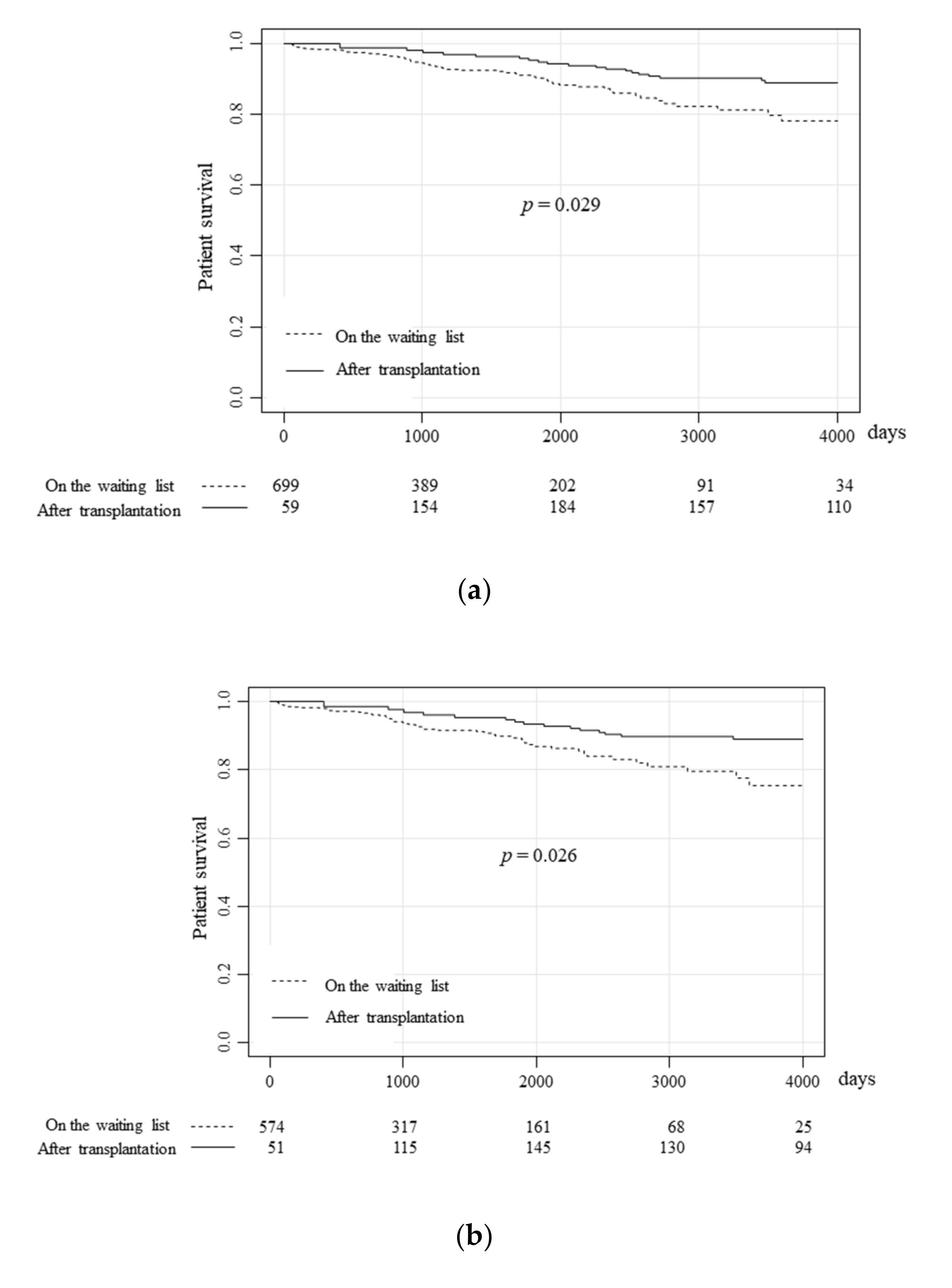
3.2. The Patient Prognoses of Patients on the Waiting List and Pancreas Transplantation Recipients
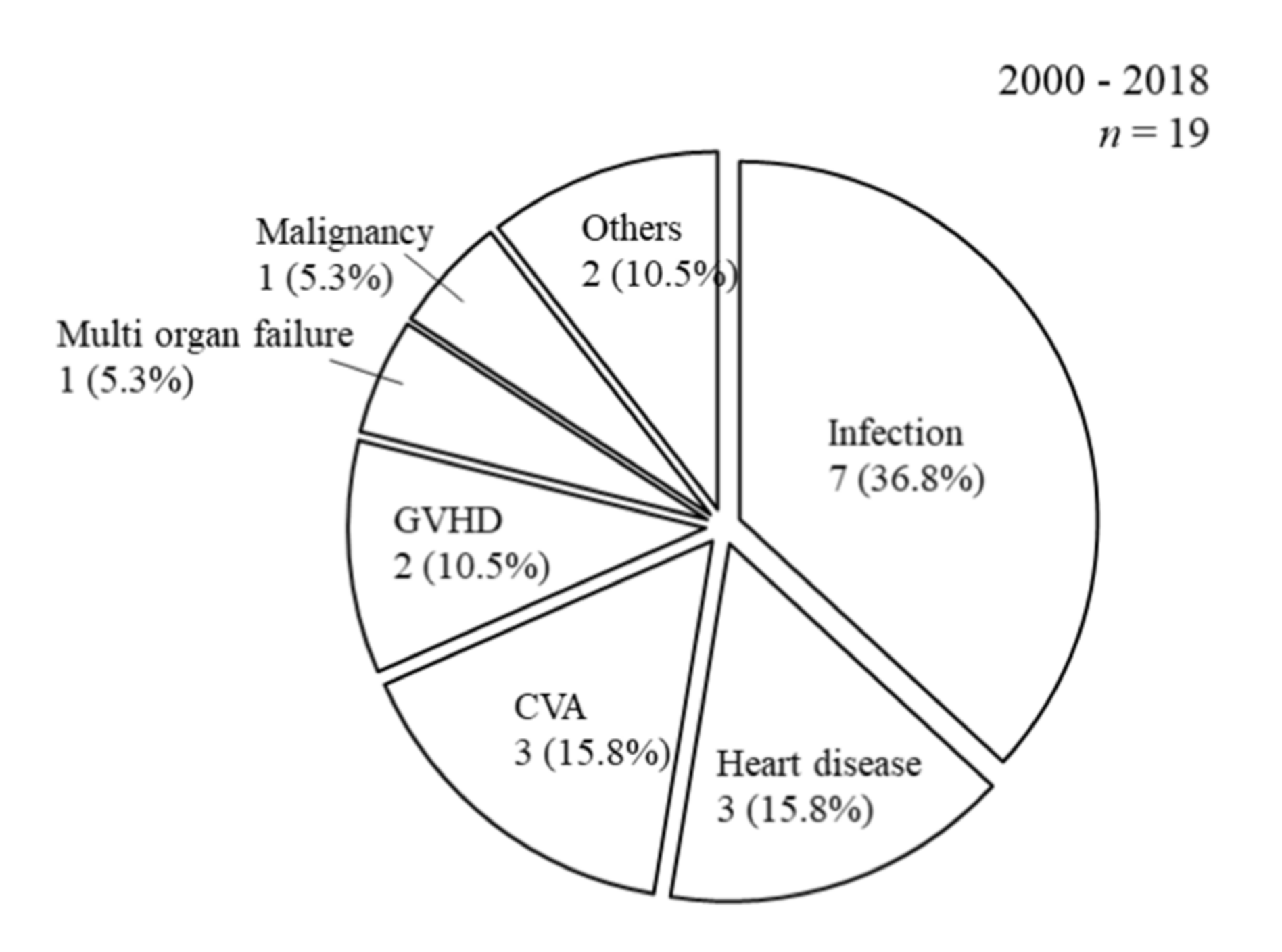
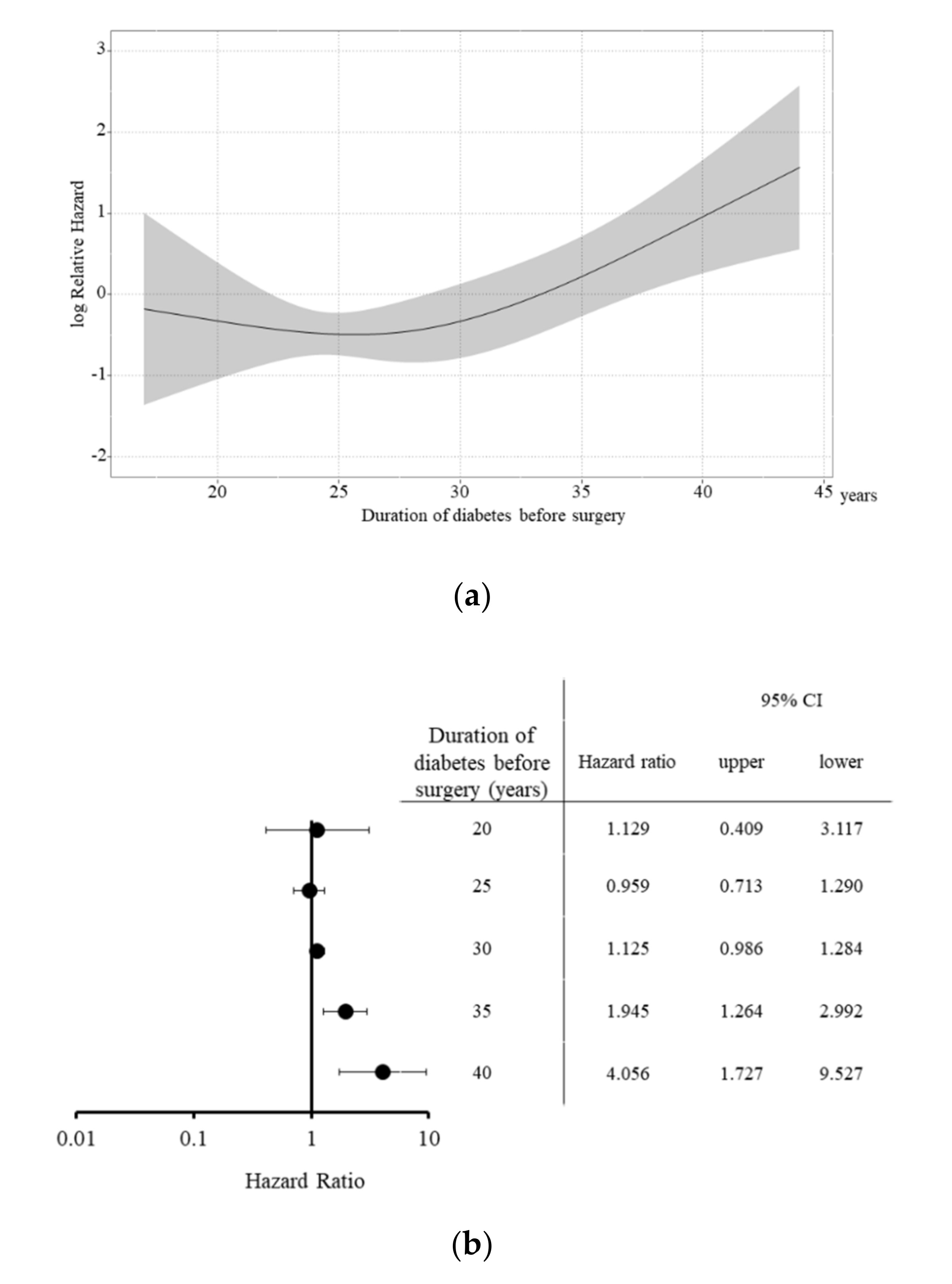
3.3. Background Factors Affecting Patient Survival after SPK
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kelly, W.D.; Lillehei, R.C.; Merkel, F.K.; Idezuki, Y.; Goetz, F.C. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967, 61, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, A.C.; Gruessner, R.W. Pancreas Transplantation of US and Non-US Cases from 2005 to 2014 as Reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Rev. Diabet. Stud. 2016, 13, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kenmochi, T.; Maruyama, M.; Saigo, K.; Akutsu, N.; Iwashita, C.; Otsuki, K.; Miyazaki, M. Evaluation of Quality of Life After Simultaneous Pancreas and Kidney Transplantation from Living Donors Using Short Form 36. Transplant Proc. 2008, 40, 2565–2567. [Google Scholar] [CrossRef] [PubMed]
- Nyumura, I.; Babazono, T.; Tauchi, E.; Yamashita, S.; Toyonaga, A.; Yoshida, N.; Takemura, S.; Takagi, M.; Yoshida, N.; Hanai, K.; et al. Quality of life in Japanese patients with type 1 diabetes and end-stage renal disease undergoing simultaneous pancreas and kidney transplantation. Diabetol. Int. 2017, 8, 268–274. [Google Scholar] [CrossRef]
- Adang, E.M.; Engel, G.L.; Van Hooff, J.P.; Kootstra, G. comparison before and after transplantation of pancreas-kidney and pancreas-kidney with loss of pancreas-A prospective controlled quality of life study1,2. Transplant 1996, 62, 754–758. [Google Scholar] [CrossRef]
- Smith, G.C.; Trauer, T.; Kerr, P.G.; Chadban, S.J. Prospective Quality-of-Life Monitoring of Simultaneous Pancreas and Kidney Transplant Recipients Using the 36-Item Short Form Health Survey. Am. J. Kidney Dis. 2010, 55, 698–707. [Google Scholar] [CrossRef]
- Fioretto, P.; Steffes, M.W.; Sutherland, D.E.; Goetz, F.C.; Mauer, M. Reversal of Lesions of Diabetic Nephropathy after Pancreas Transplantation. N. Engl. J. Med. 1998, 339, 69–75. [Google Scholar] [CrossRef]
- Gaber, A.O.; el-Gebely, S.O.L.I.M.A.N.; Sugathan, P.R.A.S.A.N.N.A.; Elmer, D.S.; Hathaway, D.K.; McCuLLY, R.B.; Shokouh-Amiri, M.H.; Burlew, B.S. Early improvement in cardiac function occurs for pancreas-kidney but not diabetic kidney-alone transplant recipients. Transplantation 1995, 59, 1105–1112. [Google Scholar] [CrossRef]
- Cheung, A.T.W.; Perez, R.V.; Chen, P.C.Y. improvements in diabetic microangiopathy after successful simultaneous PANCREAS-kidney transplantation: A computer-assisted Intravital microscopy study on the conjunctival microcirculation1,2. Transplantation 1999, 68, 927–932. [Google Scholar] [CrossRef]
- Nankivell, B.; Ai-Harbi, I.; Morris, J.; Clouston, P.; O’Connell, P.; Chapman, J.; Allen, R. Recovery of diabetic neuropathy after pancreas transplantation. Transplant. Proc. 1997, 29, 658–659. [Google Scholar] [CrossRef]
- Allen, R.D.; Al-Harbi, I.S.; Morris, J.G.; Clouston, P.D.; O’Connell, P.J.; Chapman, J.R.; Nankivell, B.J. Diabetic neuropathy after pancreas transplantation: Determinants of recovery. Transplantation 1997, 63, 830–838. [Google Scholar] [CrossRef]
- Föger, B.; Königsrainer, A.; Palos, G.; Brandstätter, E.; Ritsch, A.; König, P.; Miesenboeck, G.; Lechleitner, M.; Margreiter, R.; Patsch, J.R. Effect of pancreas transplantation on lipoprotein lipase, postprandial lipemia, and hdl cholesterol. Transplantation 1994, 58, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, R.; Sutherland, D.E.; Gruessner, A.C. Mortality Assessment for Pancreas Transplants. Arab. Archaeol. Epigr. 2004, 4, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, T.; Ito, T.; Kenmochi, T. The registry of Japanese pancreas and islet transplantation 2019. Ishoku 2019, 54, 111–119. [Google Scholar]
- Nakamoto, H. The Current Status and Future of Peritoneal Dialysis in Japan. Contrib. Nephrol. 2019, 198, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Toida, T.; Sato, Y.; Ogata, S.; Wada, A.; Masakane, I.; Fujimoto, S. Synergic Impact of Body Mass Index, Diabetes, and Age on Long-Term Mortality in Japanese Incident Hemodialysis Patients: A Cohort Study on a Large National Dialysis Registry. J. Ren. Nutr. 2019. [Google Scholar] [CrossRef]
- Ito, T.; Kenmochi, T.; Aida, N.; Kurihara, K.; Kawai, A.; Suzuki, A.; Shibata, M.; Hiratsuka, I.; Hasegawa, M. The Outcomes of Pancreatic Transplantation from Pediatric Donors–A Single Institution Experience. J. Clin. Med. 2019, 8, 1386. [Google Scholar] [CrossRef]
- Ito, T.; Kenmochi, T.; Aida, N.; Kurihara, K.; Asaoka, T.; Ito, T. Are the outcomes of Japanese pancreas transplantation utilizing extended-criteria donors acceptable? A propensity score matching analysis for donors < 50 or ≥ 50 years old. Transpl. Int. 2020. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2012, 48, 452–458. [Google Scholar] [CrossRef]
- Fridell, J.; Niederhaus, S.; Urban, R.; Fox, A.; Odorico, J. Yes, we do need to demonstrate the survival advantage of pancreas after kidney transplantation. Arab. Archaeol. Epigr. 2019, 19, 823–830. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jung, J.H.; Shin, S.; Kim, Y.H.; Han, D.J. Association between the pancreas transplantation and survival of patients with diabetes: A single center experience. PLoS ONE 2017, 12, e0186827. [Google Scholar] [CrossRef] [PubMed]
- Kaku, K.; Kitada, H.; Noguchi, H.; Kurihara, K.; Kawanami, S.; Nakamura, U.; Tanaka, M. Living Donor Kidney Transplantation Preceding Pancreas Transplantation Reduces Mortality in Type 1 Diabetics with End-stage Renal Disease. Transplant. Proc. 2015, 47, 733–737. [Google Scholar] [CrossRef]
- Ito, T.; Kenmochi, T.; Aida, N.; Kurihara, K.; Kawai, A. Effectiveness of Preceding Solo Kidney Transplantation for Type 1 Diabetes with End-Stage Renal Failure. Transplant. Proc. 2018, 50, 3249–3254. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P.; Safi, K.; Little, D.M.; Donohoe, J.; Conlon, P.; Walshe, J.; O’Kelly, P.; Thompson, C.J.; Hickey, D.P. Improved patient survival in recipients of simultaneous pancreas-kidney transplant compared with kidney transplant alone in patients with type 1 diabetes mellitus and end-stage renal disease. BJS 2003, 90, 1137–1141. [Google Scholar] [CrossRef]
- Yagisawa, T.; Mieno, M.; Ichimaru, N.; Morita, K.; Nakamura, M.; Hotta, K.; Kenmochi, T. Annual Progress Report from the Japanese Renal Transplant Registry: Number of Renal Transplantations in 2018 and Follow-up Survey. Ishoku 2019, 54, 61–80. [Google Scholar]
- Sollinger, H.W.; Odorico, J.S.; Becker, Y.T.; D’alessandro, A.M.; Pirsch, J.D. One Thousand Simultaneous Pancreas-Kidney Transplants at a Single Center With 22-Year Follow-Up. Trans. Meet. Am. Surg. Assoc. 2009, 127, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Tydén, G.; Tollemar, J.; Bolinder, J. Combined pancreas and kidney transplantation improves survival in patients with end-stage diabetic nephropathy. Clin. Transplant. 2000, 14, 505–508. [Google Scholar] [CrossRef]
- Knoll, G.A.; Nichol, G. Dialysis, kidney transplantation, or pancreas transplantation for patients with diabetes mellitus and renal failure: A decision analysis of treatment options. J. Am. Soc. Nephrol. 2003, 14, 500–515. [Google Scholar] [CrossRef]
- Reddy, K.; Stablein, D.; Taranto, S.; Stratta, R.J.; Johnston, T.D.; Waid, T.H.; McKeown, J.; Lucas, B.A.; Ranjan, D. Long-term survival following simultaneous kidney-pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Am. J. Kidney Dis. 2003, 41, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Rayhill, S.C.; D’Alessandro, A.M.; Odorico, J.S.; Knechtle, S.; Pirsch, J.D.; Heisey, D.M.; Kirk, A.D.; Van Der Werf, W.; Sollinger, H.W. Simultaneous Pancreas-Kidney Transplantation and Living Related Donor Renal Transplantation in Patients With Diabetes: Is There a Difference in Survival? Ann. Surg. 2000, 231, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.O.; Meier-Kriesche, H.-U.; Hanson, J.A.; Leichtman, A.; Magee, J.C.; Cibrik, D.; Wolfe, R.A.; Port, F.K.; Agodoa, L.; Kaufman, D.B.; et al. The impact of simultaneous pancreas-kidney transplantation on long-term patient survival1. Transplantation 2001, 71, 82–89. [Google Scholar] [CrossRef]
- Morath, C.; Zeier, M.; Döhler, B.; Schmidt, J.; Nawroth, P.P.; Opelz, G. Metabolic control improves long-term renal allograft and patient survival in type 1 diabetes. J. Am. Soc. Nephrol. 2008, 19, 1557–1563. [Google Scholar] [CrossRef]
- Gruessner, A.C.; Gruessner, R.W.G. Pancreas Transplantation for Patients with Type 1 and Type 2 Diabetes Mellitus in the United States: A Registry Report. Gastroenterol. Clin. North Am. 2018, 47, 417–441. [Google Scholar] [CrossRef]
- Dorman, J.S.; Laporte, R.E.; Kuller, L.H.; Cruickshanks, K.J.; Orchard, T.J.; Wagener, D.K.; Becker, D.J.; Cavender, D.E.; Drash, A.L. The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study. Mortality results. Diabetes 1984, 33, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Asao, K.; Sarti, C.; Forsen, T.; Hyttinen, V.; Nishimura, R.; Matsushima, M.; Reunanen, A.; Tuomilehto, J.; Tajima, N. Long-Term Mortality in Nationwide Cohorts of Childhood-Onset Type 1 Diabetes in Japan and Finland. Diabetes Care 2003, 26, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Auinger, M.; Anderwald, C.H.; Kästenbauer, T.; Kramar, R.; Feinböck, C.; Irsigler, K.; Kronenberg, F.; Prager, R. Long-Term Mortality and Incidence of Renal Dialysis and Transplantation in Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2006, 91, 3814–3820. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rossing, P.; Hougaard, P.; Borch-Johnsen, K.; Parving, H.-H. Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ 1996, 313, 779–784. [Google Scholar] [CrossRef]
- Muhlhauser, I.; Overmann, H.; Bender, R.; Jorgens, V.; Berger, M. Predictors of mortality and end-stage diabetic complications in patients with Type 1 diabetes mellitus on intensified insulin therapy. Diabet. Med. 2000, 17, 727–734. [Google Scholar] [CrossRef]
- Finne, P.; Reunanen, A.; Stenman, S.; Groop, P.-H.; Grönhagen-Riska, C. Incidence of End-stage Renal Disease in Patients With Type 1 Diabetes. JAMA 2005, 294, 1782. [Google Scholar] [CrossRef] [PubMed]
- Pambianco, G.; Costacou, T.; Ellis, D.; Becker, D.J.; Klein, A.P.; Orchard, T.J. The 30-Year Natural History of Type 1 Diabetes Complications: The Pittsburgh Epidemiology of Diabetes Complications Study Experience. Diabetes 2006, 55, 1463–1469. [Google Scholar] [CrossRef]
- Lindahl, J.P.; Hartmann, A.; Horneland, R.; Holdaas, H.; Reisæter, A.V.; Midtvedt, K.; Leivestad, T.; Øyen, O.; Jenssen, T. Improved patient survival with simultaneous pancreas and kidney transplantation in recipients with diabetic end-stage renal disease. Diabetologia 2013, 56, 1364–1371. [Google Scholar] [CrossRef] [PubMed]

| Median (Range) or Ratio | ||||
|---|---|---|---|---|
| Overall | SPK | PAK | PTA | |
| n | 699 | 574 | 97 | 28 |
| Age | 41.0 (16–64) | 42.0 (25–64) | 40.0 (28–62) | 39.0 (16–62) |
| Sex (male:female) | 271:428 | 227:347 | 34:63 | 10:18 |
| Period of DM (years) | 25.0 (0–52) | 25.0 (0–52) | 26.0 (2–42) | 17.0 (2–42) |
| Period of HD (years) | 2.0 (0–23) | 2.0 (0–23) | N/A | N/A |
| HbA1c (%) | 7.40 (4.1–20.1) | 7.40 (4.1–20.1) | 7.25 (5.6–16.7) | 9.00 (6.2–14.5) |
| Median (Min-Max) or Ratio | ||||||
|---|---|---|---|---|---|---|
| Overall | SPK | PAK | PTA | p Value | ||
| n | 361 | 298 | 48 | 15 | ||
| Donor factors | Age | 43.0 (5–72) | 43.0 (5–72.0) | 42.5 (8–65) | 43.0 (5–58) | 0.983 |
| Gender (male:female) | 205:156 | 163:135 | 31:17 | 11:4 | 0.183 | |
| BMI (kg/m2) | 21.8 (11.4–34.3) | 21.7 (11.4–34.3) | 22.1 (16.0–35.6) | 22.0 (16.3–25.4) | 0.940 | |
| HbA1c (%) | 5.4 (4.3–7.7) | 5.4 (4.3–6.5) | 5.4 (4.5–7.7) | 5.4 (4.8–6.5) | 0.913 | |
| Cre (mg/dL) | 0.75 (0.12–10.33) | 0.75 (0.12–10.33) | 0.71 (0.14–8.85) | 0.97 (0.20–6.93) | 0.178 | |
| Cause of death (CVA:Others) | 183:178 | 149:149 | 26:22 | 8:7 | 0.848 | |
| CIT (min) | 720 (270–1383) | 731 (271–1383) | 650 (349–950) | 697 (338–1038) | <0.001 | |
| Recipient factors | Age | 44.0 (24–69) | 44.0 (29–69) | 42.5 (31–53) | 40.0 (24–57) | 0.041 |
| Gender (male:female) | 136:225 | 111:187 | 19:29 | 6:9 | 0.936 | |
| BMI (kg/m2) | 20.6 (14.6–30.4) | 20.6 (14.6–30.4) | 20.0 (15.8–28.5) | 22.1 (17.6–28.9) | 0.104 | |
| Period of DM (years) | 28.0 (6–49) | 28.0 (6–49) | 29.0 (16–40) | 18.0 (6–26) | <0.001 | |
| Induction of dialysis (yes:preemptive) | 292:6 | 292:6 | N/A | N/A | N/A | |
| Type of hemodialysis (HD:PD) | 14:278 | 14:278 | N/A | N/A | N/A | |
| Period of HD (years) | 6.2 (0–30.0) | 6.2 (0–30.0) | N/A | N/A | N/A | |
| Waiting period (days) | 975.0 (10–5740) | 1064.0 (11–5740) | 749.0 (10–4453) | 409.0 (45–3279) | 0.023 | |
| HbA1c (%) | 7.50 (4.8–15.2) | 7.40 (4.8–15.2) | 7.45 (5.4–13.6) | 9.59 (6.2–14.7) | 0.005 | |
| Cre (mg/dL) | 7.54 (0.36–17.29) | 8.20 (2.26–17.29) | 1.19 (0.53–2.58) | 0.68 (0.36–1.41) | <0.001 | |
| Diabetic retinopathy (with:without) | 323:38 | 273:25 | 43:5 | 7:8 | <0.001 | |
| Diabetic neuropathy (with:without) | 293:68 | 243:55 | 42:6 | 8:7 | 0.012 | |
| Hypertension (with:without) | 233:128 | 204:94 | 26:22 | 3:12 | <0.001 | |
| Cardiovascular disorders (with:without) | 34:327 | 31:267 | 3:45 | 0:15 | 0.292 | |
| Number of HLA mismatch (0:1:2:3:4:5:6) | 10:39:122:109:57:24:0 | 9:33:112:94:39:11:0 | 0:4:9:12:13:10:0 | 1:2:1:3:5:3:0 | <0.001 | |
| Inductio therapy (Anti-CD25:T-cell duplete) | 275:86 | 242:56 | 24:24 | 9:6 | <0.001 | |
| Type of CNI (Tacrolimus: Cyclosporine) | 356:5 | 295:3 | 46:2 | 15:0 | 0.198 | |
| Univariate Analysis | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p Value | Hazard Ratio | 95% CI | * p Value | p Value for Recipient Age | ||
| Donor factors | Age | 1.013 | 0.979–1.049 | 0.453 | 1.012 | 0.978–1.047 | 0.488 | <0.001 |
| Sex (male) | 1.897 | 0.720–4.998 | 0.195 | 1.752 | 0.662–4.630 | 0.258 | <0.001 | |
| BMI (kg/m2) | 0.957 | 0.843–1.085 | 0.491 | 0.964 | 0.857–1.085 | 0.542 | <0.001 | |
| HbA1c (%) | 1.038 | 0.305–3.531 | 0.951 | 0.831 | 0.240–2.880 | 0.771 | <0.001 | |
| Cre (mg/dL) | 0.798 | 0.435–1.467 | 0.468 | 0.778 | 0.429–1.412 | 0.409 | <0.001 | |
| Cause of death (Others) | 0.971 | 0.394–2.395 | 0.949 | 0.914 | 0.370–2.259 | 0.845 | <0.001 | |
| CIT (min) | 1.002 | 0.999–1.005 | 0.075 | 1.002 | 0.999–1.004 | 0.136 | 0.001 | |
| Recipient factors | Age | 1.098 | 1.041–1.159 | <0.001 | - | - | - | - |
| Sex (male) | 0.763 | 0.289–2.012 | 0.585 | 0.617 | 0.231–1.649 | 0.336 | <0.001 | |
| BMI (kg/m2) | 0.853 | 0.701–1.039 | 0.114 | 0.893 | 0.736–1.084 | 0.254 | 0.001 | |
| Period of DM (years) | 1.129 | 1.061–1.201 | <0.001 | 1.095 | 1.020–1.175 | 0.012 | 0.097 | |
| Induction of dialysis (yes) | 9,212,000 | 0-Inf | 0.998 | 10,770,000 | 0-Inf | 0.998 | <0.001 | |
| Type of dialysis (Peritoneal dialysis) | <0.001 | 0-Inf | 0.997 | <0.001 | 0-Inf | 0.997 | <0.001 | |
| Period of HD (years) | 1.125 | 1.043–1.213 | 0.002 | 1.069 | 0.982–1.165 | 0.125 | 0.023 | |
| Waiting period (years) | 1.070 | 0.938–1.221 | 0.312 | 0.983 | 0.851–1.135 | 0.811 | 0.001 | |
| HbA1c (%) at transplant | 1.028 | 0.751–1.407 | 0.862 | 1.178 | 0.828–1.677 | 0.362 | <0.001 | |
| Cre (mg/dL) | 0.946 | 0.805–1.111 | 0.499 | 0.97 | 0.823–1.144 | 0.719 | 0.001 | |
| Diabetic retinopathy (with) | 4.534 | 0.603–34.09 | 0.142 | 1.637 | 0.216- 12.43 | 0.634 | <0.001 | |
| Diabetic neuropathy (with) | 4.534 | 0.603–34.09 | 0.142 | 4.754 | 0.627–36.06 | 0.132 | <0.001 | |
| Hypertension (with) | 0.926 | 0.350–2.445 | 0.876 | 0.996 | 0.375–2.643 | 0.993 | <0.001 | |
| Cardiovascular disorders (with) | <0.001 | 0-Inf | 0.998 | <0.001 | 0-Inf | 1.052 | <0.001 | |
| HLA mismatch | 0.839 | 0.547–1.287 | 0.421 | 0.852 | 0.557–1.302 | 0.459 | <0.001 | |
| Induction therapy (T-cell depleting antibody) | 0.306 | 0.041–2.313 | 0.251 | 0.32 | 0.042–2.427 | 0.270 | <0.001 | |
| Type of CNI (Tacrolimus) | 9,521,000 | 0-Inf | 0.997 | 9,159,000 | 0-Inf | 0.997 | <0.001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, T.; Kenmochi, T.; Aida, N.; Matsushima, H.; Kurihara, K.; Ishihara, T.; Shintani, A.; Asaoka, T.; Ito, T. Impact of Pancreas Transplantation on the Patient Survival—An Analysis of the Japanese Pancreas Transplants Registry. J. Clin. Med. 2020, 9, 2134. https://doi.org/10.3390/jcm9072134
Ito T, Kenmochi T, Aida N, Matsushima H, Kurihara K, Ishihara T, Shintani A, Asaoka T, Ito T. Impact of Pancreas Transplantation on the Patient Survival—An Analysis of the Japanese Pancreas Transplants Registry. Journal of Clinical Medicine. 2020; 9(7):2134. https://doi.org/10.3390/jcm9072134
Chicago/Turabian StyleIto, Taihei, Takashi Kenmochi, Naohiro Aida, Hajime Matsushima, Kei Kurihara, Takuma Ishihara, Ayumi Shintani, Tadafumi Asaoka, and Toshinori Ito. 2020. "Impact of Pancreas Transplantation on the Patient Survival—An Analysis of the Japanese Pancreas Transplants Registry" Journal of Clinical Medicine 9, no. 7: 2134. https://doi.org/10.3390/jcm9072134
APA StyleIto, T., Kenmochi, T., Aida, N., Matsushima, H., Kurihara, K., Ishihara, T., Shintani, A., Asaoka, T., & Ito, T. (2020). Impact of Pancreas Transplantation on the Patient Survival—An Analysis of the Japanese Pancreas Transplants Registry. Journal of Clinical Medicine, 9(7), 2134. https://doi.org/10.3390/jcm9072134





