Living Donor Kidney Transplantation Improves Graft and Recipient Survival in Patients with Multiple Kidney Transplants
Abstract
1. Introduction
2. Methods
Statistical Analysis
3. Results
3.1. Surgical Information
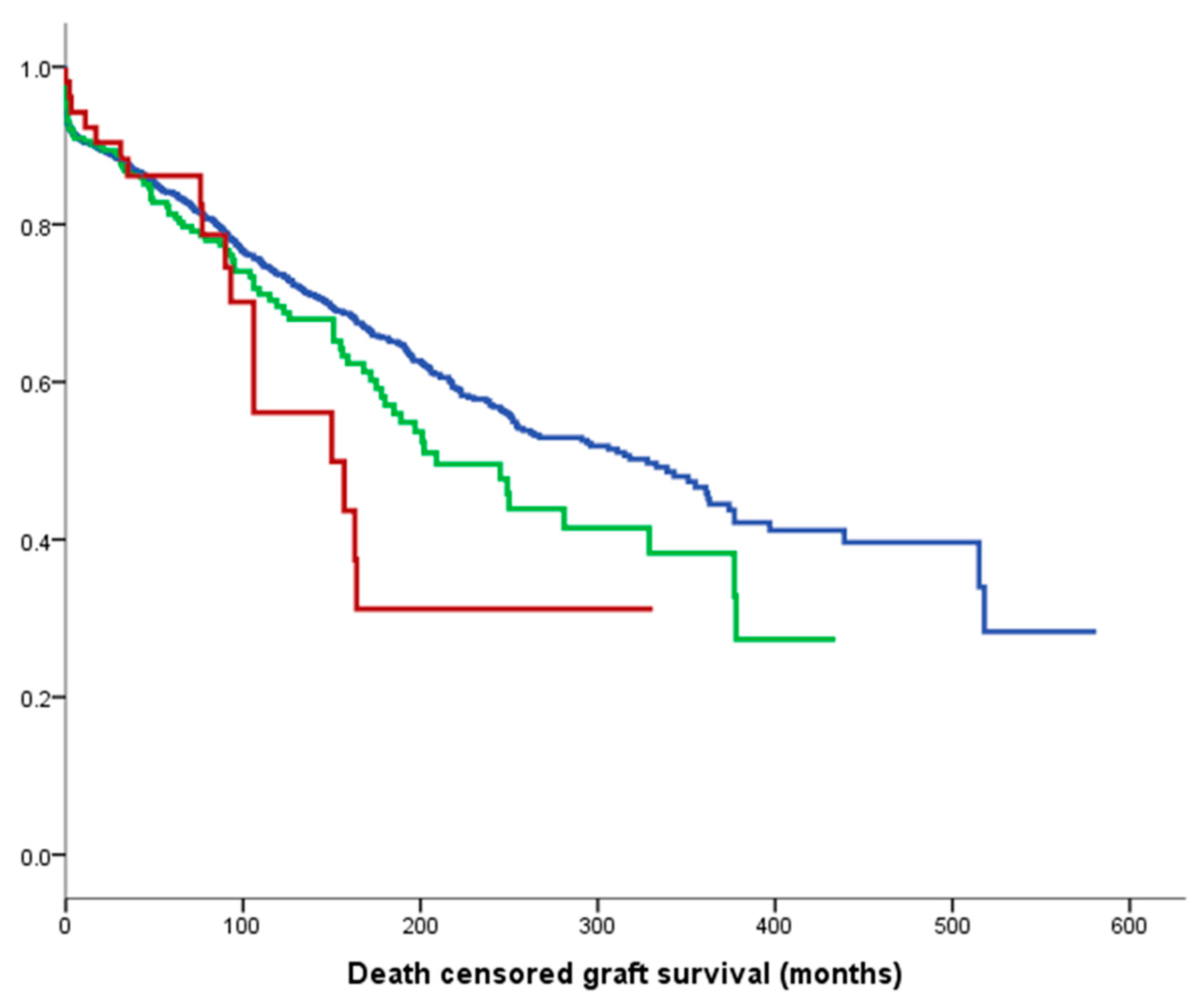
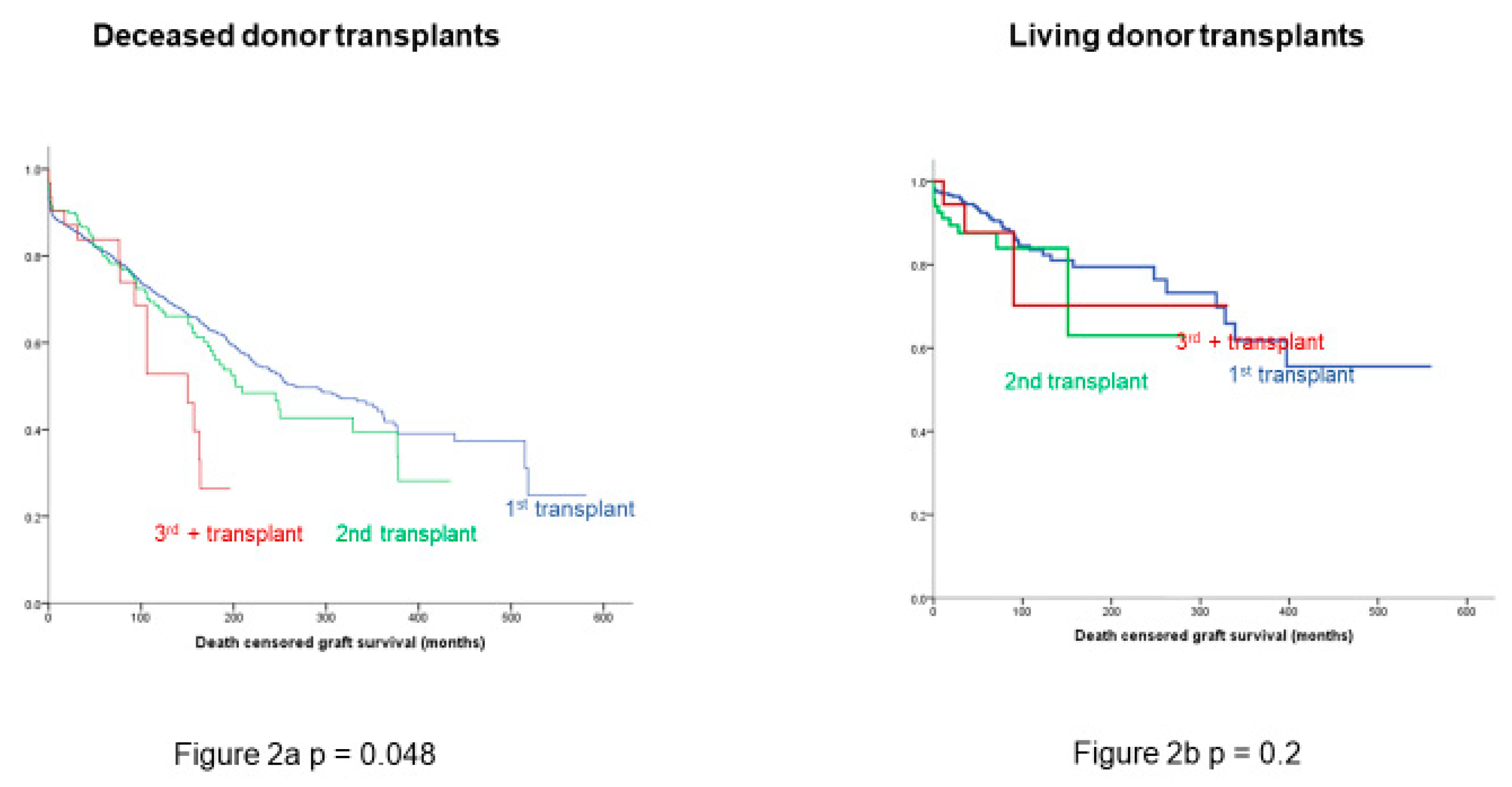
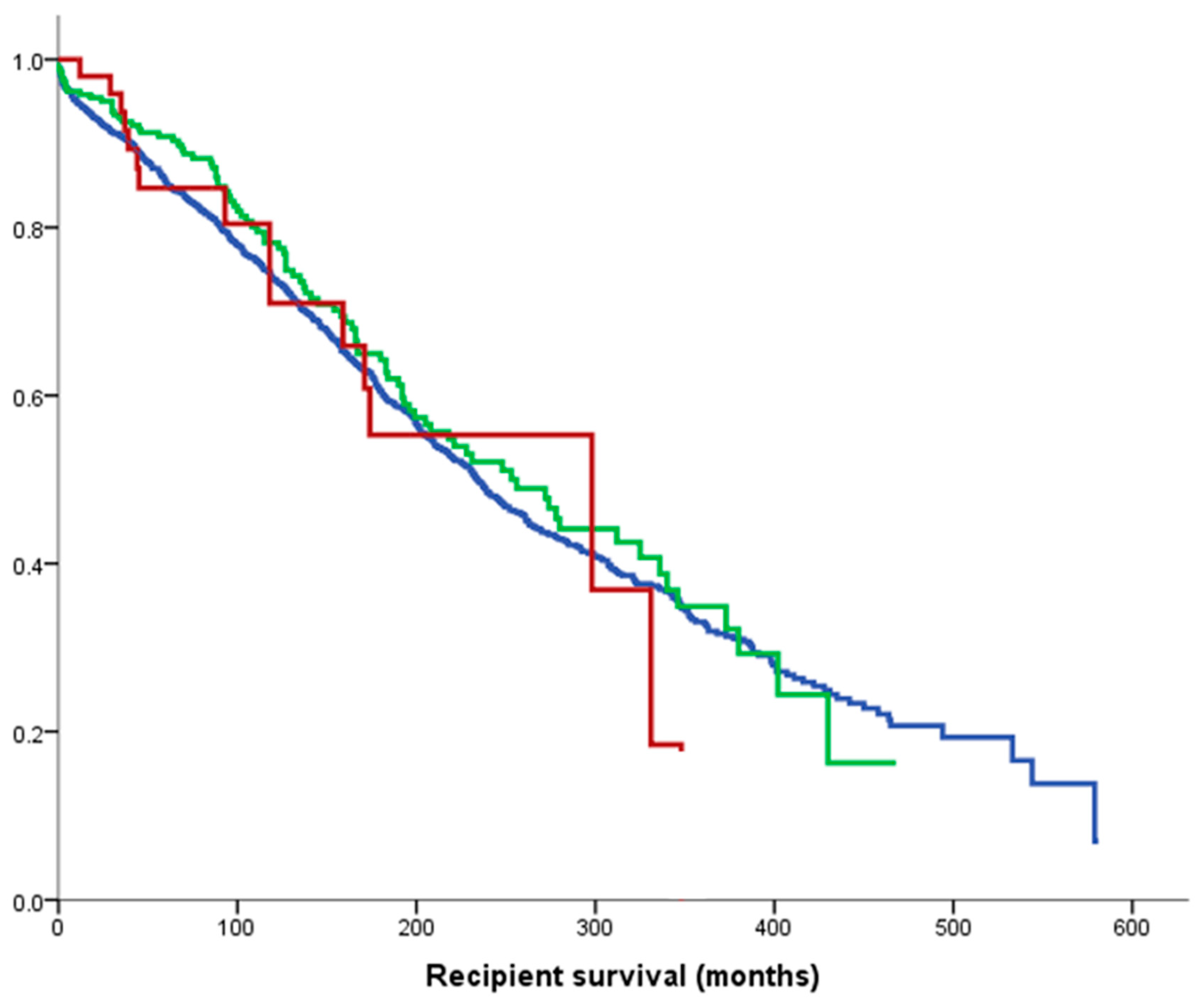
3.2. Death-Censored Graft and Recipient Survival
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbr. | Original Word |
| DSA | Donor-specific antibody |
| ESRD | End-stage renal disease |
| KTR | Kidney transplant recipient |
| PRA | Panel-reactive antibody |
| RRT | Renal replacement therapy (cumulative time) |
References
- U.S. Renal Data System, USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2009.
- U.S. Renal Data System, USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2010.
- Kaplan, B.; Meier-Kriesche, H.U. Death after graft loss: An important late study endpoint in kidney transplantation. Am. J. Transplant. 2002, 2, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, S.; Sohn, S.; Li, D.; Pankratz, J.J.; Therneau, T.; Sauver, J.L.S.; Liu, H.; Palakal, M. Temporal pattern and association discovery of diagnosis codes using deep learning. In Proceedings of the 2015 International Conference on Healthcare Informatics, Richardson, TX, USA, 21–23 October 2015; pp. 408–416. [Google Scholar]
- Fernandez Fresnedo, G.; Ruiz, J.C.; Gomez Alamillo, C.; de Francisco, A.L.; Arias, M. Survival after dialysis initiation: A comparison of transplant patients after graft loss versus nontransplant patients. Transplant. Proc. 2008, 40, 2889–2890. [Google Scholar] [CrossRef]
- McCaughan, J.A.; Patterson, C.C.; Maxwell, A.P.; Courtney, A.E. Factors influencing survival after kidney transplant failure. Transplant. Res. 2014, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Knoll, G.; Muirhead, N.; Trpeski, L.; Zhu, N.; Badovinac, K. Patient survival following renal transplant failure in Canada. Am. J. Transplant. 2005, 5, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Coupel, S.; Giral-Classe, M.; Karam, G.; Morcet, J.F.; Dantal, J.; Cantarovich, D.; Blancho, G.; Bignon, J.D.; Daguin, P.; Soulillou, J.P.; et al. Ten-year survival of second kidney transplants: Impact of immunologic factors and renal function at 12 months. Kidney Int. 2003, 64, 674–680. [Google Scholar] [CrossRef]
- Redfield, R.R.; Gupta, M.; Rodriguez, E.; Wood, A.; Abt, P.L.; Levine, M.H. Graft and patient survival outcomes of a third kidney transplant. Transplantation 2015, 99, 416–423. [Google Scholar] [CrossRef]
- Bellini, M.I.; Nozdrin, M.; Yiu, J.; Papalois, V. Machine perfusion for abdominal organ preservation: A systematic review of kidney and liver human grafts. J. Clin. Med. 2019, 8, 1221. [Google Scholar] [CrossRef]
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 2004, 364, 1814–1827. [Google Scholar] [CrossRef]
- Bellini, M.I.; Charalampidis, S.; Herbert, P.E.; Bonatsos, V.; Crane, J.; Muthusamy, A.; Dor, F.; Papalois, V. Cold pulsatile machine perfusion versus static cold storage in kidney transplantation: A single centre experience. BioMed. Res. Int. 2019, 2019, 7435248. [Google Scholar] [CrossRef]
- Redfield, R.R.; Scalea, J.R.; Zens, T.J.; Muth, B.; Kaufman, D.B.; Djamali, A.; Astor, B.C.; Mohamed, M. Predictors and outcomes of delayed graft function after living-donor kidney transplantation. Transpl. Int. 2016, 29, 81–87. [Google Scholar] [CrossRef]
- Lucisano, G.; Brookes, P.; Santos-Nunez, E.; Firmin, N.; Gunby, N.; Hassan, S.; Gueret-Wardle, A.; Herbert, P.; Papalois, V.; Willicombe, M.; et al. Allosensitization after transplant failure: The role of graft nephrectomy and immunosuppression—A retrospective study. Transpl. Int. 2019, 32, 949–959. [Google Scholar] [CrossRef]
- McCaughan, J.A.; Courtney, A.E. Successful kidney transplantation in highly sensitized, ultra-long-term dialysis patients. Transpl. Int. 2017, 30, 844–846. [Google Scholar] [CrossRef]
- Ooms, L.S.; Roodnat, J.I.; Dor, F.J.; Tran, T.C.; Kimenai, H.J.; Ijzermans, J.N.; Terkivatan, T. Kidney retransplantation in the ipsilateral iliac fossa: A surgical challenge. Am. J. Transplant. 2015, 15, 2947–2954. [Google Scholar] [CrossRef]
- Assfalg, V.; Selig, K.; Tolksdorf, J.; van Meel, M.; de Vries, E.; Ramsoebhag, A.M.; Rahmel, A.; Renders, L.; Novotny, A.; Matevossian, E.; et al. Repeated kidney re-transplantation-the Eurotransplant experience: A retrospective multicenter outcome analysis. Transpl. Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Morath, C.; Süsal, C. Three is not enough. Transpl. Int. 2020, 33, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Dabare, D.; Kassimatis, T.; Hodson, J.; Khurram, M.A.; Papadakis, G.; Rompianesi, G.; Shaw, O.; Karydis, N.; Callaghan, C.; Olsburgh, J.; et al. Outcomes in third and fourth kidney transplants based on the type of donor. Transplantation 2019, 103, 1494–1503. [Google Scholar] [CrossRef]
- Aubert, O.; Kamar, N.; Vernerey, D.; Viglietti, D.; Martinez, F.; Duong-Van-Huyen, J.P.; Eladari, D.; Empana, J.P.; Rabant, M.; Verine, J.; et al. Long term outcomes of transplantation using kidneys from expanded criteria donors: Prospective, population based cohort study. Br. Med. J. 2015, 351, h3557. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.I.; Charalampidis, S.; Stratigos, I.; Dor, F.; Papalois, V. The effect of Donors’ demographic characteristics in renal function post-living kidney donation. Analysis of a UK single centre cohort. J. Clin. Med. 2019, 8, 883. [Google Scholar] [CrossRef]
- Bellini, M.I.; Parisotto, C.; Dor, F.J.M.F.; Kessaris, N. Social Media Use Among Transplant Professionals in Europe: A Cross-Sectional Study From the European Society of Organ Transplantation. Exp. Clin. Transpl. 2020, 18, 169–176. [Google Scholar] [CrossRef]
- Bellini, M.I.; D’Andrea, V. Organ preservation: Which temperature for which organ? J. Int. Med. Res. 2019, 47, 2323–2325. [Google Scholar] [CrossRef]
- Chandak, P.; Byrne, N.; Coleman, A.; Karunanithy, N.; Carmichael, J.; Marks, S.D.; Stojanovic, J.; Kessaris, N.; Mamode, N. Patient-specific 3D printing: A novel technique for complex pediatric renal transplantation. Ann. Surg. 2019, 269, e18–e23. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Wilson, R.S.; Veitch, P.; Brown, T.; Courtney, A.; Maxwell, A.P.; D’Andrea, V.; McDaid, J. Hyperamylasemia post living donor nephrectomy does not relate to pain. Cureus 2020, 12, e8217. [Google Scholar] [CrossRef] [PubMed]
- The National Confidential Enquiry into Patient Outcome and Death. National Confidential Enquiry into Perioperative Outcome and Death. Who Operates when 1995/6? Available online: www.ncepod.org.uk/1995_6.html (accessed on 13 October 2019).
- Mazzucchi, E.; Danilovic, A.; Antonopoulos, I.M.; Piovesan, A.C.; Nahas, W.C.; Lucon, A.M.; Srougi, M. Surgical aspects of third and subsequent renal transplants performed by the extraperitoneal access. Transplantation 2006, 81, 840–844. [Google Scholar] [CrossRef]
- Bellini, M.I.; Charalmpidis, S.; Brookes, P.; Hill, P.; Dor, F.; Papalois, V. Bilateral nephrectomy for adult polycystic kidney disease does not affect the graft function of transplant patients and does not result in sensitisation. BioMed. Res. Int. 2019, 2019, 7423158. [Google Scholar] [CrossRef]
- British Transplantation Society guidelines. Available online: https://bts.org.uk/wp-content/uploads/2016/09/13_BTS_Failing_Graft-1.pdf (accessed on 13 October 2019).
- Lin, J.; Wang, R.; Xu, Y.; Chen, J. Impact of renal allograft nephrectomy on graft and patient survival following retransplantation: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2018, 33, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Yagmurdur, M.C.; Emiroğlu, R.; Ayvaz, I.; Sozen, H.; Karakayali, H.; Haberal, M. The effect of graft nephrectomy on long-term graft function and survival in kidney retransplantation. Transplant. Proc. 2005, 37, 2957–2961. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmed, K.; Mamode, N. Does nephrectomy of failed allograft influence graft survival after re-transplantation? Nephrol. Dial. Transplant. 2009, 24, 639–642. [Google Scholar] [CrossRef]
- McCaughan, J.A.; Courtney, A.E. The clinical course of kidney transplant recipients after 20 years of graft function. Am. J. Transplant. 2015, 15, 734–740. [Google Scholar] [CrossRef]
| 1st Transplant n = 2062 | 2nd Transplant n = 279 | 3rd+ Transplant n = 54 | p Value | |
|---|---|---|---|---|
| Recipient | ||||
| Recipient age (mean+SD, years) | 43.6 ± 16.3 | 39.9 ± 14.4 | 41.4 ± 11.5 | 0.001 |
| Recipient sex (male) | 1244 (60%) | 187 (67%) | 38 (73%) | 0.006 |
| Renal replacement therapy duration (mean+SD, months) | 22.5 ± 26.6 | 124.7 ± 95.8 | 207.3 ± 106.8 | <0.0001 |
| Calculated reaction frequency (cRF) (mean+SD, %) | 15.3 ± 29.7 | 54.1 ± 40.3 | 75.7 ± 34.5 | <0.0001 |
| Donor | ||||
| Donor age (mean+SD, years) | 49.7 ± 9 | 39.4 ± 16.1 | 43.5 ± 16 | 0.166 |
| Donor sex (male) | 1159 (56%) | 152 (54%) | 28 (54%) | 0.171 |
| Living donor | 549 (26%) | 82 (29%) | 22 (42%) | <0.0001 |
| Transplant | ||||
| Pre-emptive transplantation | 308 (14.9%) | 22 (7.9%) | 3 (5.6%) | <0.0001 |
| HLA-A, -B, -DR mismatch (mean+SD, number) | 2.4 ± 1.3 | 2 ± 1.4 | 1.4 ± 1.4 | <0.0001 |
| Ischaemic time (mean+SD, hours) | 17.5 + 18 | 16.6 + 16.5 | 13 + 9.9 | 0.197 |
| Covariate | HR | 95% CI | p Value |
|---|---|---|---|
| Decade of transplant | 0.84 | 0.78–0.90 | <0.001 |
| Recipient age (per decade) | 0.90 | 0.86–0.96 | <0.001 |
| Living donor | 0.61 | 0.45–0.83 | 0.002 |
| Donor age (per decade) | 1.14 | 1.08–1.20 | <0.001 |
| Transplant number | 1.23 | 1.03–1.48 | 0.02 |
| Ischaemic time (per 6h) | 1.09 | 1.01–1.17 | 0.02 |
| Covariate | HR | 95% CI | p Value |
|---|---|---|---|
| Decade of transplant | 0.98 | 0.49–1.96 | 0.9 |
| Recipient age (per decade) | 0.85 | 0.51–1.39 | 0.5 |
| Living donor | 0.10 | 0.01–0.89 | 0.04 |
| Donor age (per decade) | 0.76 | 0.63–1.40 | 0.8 |
| Ischaemic time (per 6h) | 0.57 | 0.30–1.10 | 0.08 |
| Covariate | HR | 95% CI | p Value |
|---|---|---|---|
| Decade of transplant | 0.60 | 0.56–0.65 | <0.001 |
| Recipient age (per decade) | 1.7 | 1.6–1.8 | <0.001 |
| Recipient primary disease diabetic nephropathy | 2.8 | 2.3–3.5 | <0.001 |
| RRT pre-transplant (per month) | 1.002 | 1.00–1.004 | 0.02 |
| Living donor | 0.75 | 0.56–1.00 | 0.05 |
| Donor age (per decade) | 1.07 | 1.02–1.1 | 0.03 |
| Ischaemic time (per 6h) | 1.1 | 1.04–1.2 | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, M.I.; Courtney, A.E.; McCaughan, J.A. Living Donor Kidney Transplantation Improves Graft and Recipient Survival in Patients with Multiple Kidney Transplants. J. Clin. Med. 2020, 9, 2118. https://doi.org/10.3390/jcm9072118
Bellini MI, Courtney AE, McCaughan JA. Living Donor Kidney Transplantation Improves Graft and Recipient Survival in Patients with Multiple Kidney Transplants. Journal of Clinical Medicine. 2020; 9(7):2118. https://doi.org/10.3390/jcm9072118
Chicago/Turabian StyleBellini, Maria Irene, Aisling E Courtney, and Jennifer A McCaughan. 2020. "Living Donor Kidney Transplantation Improves Graft and Recipient Survival in Patients with Multiple Kidney Transplants" Journal of Clinical Medicine 9, no. 7: 2118. https://doi.org/10.3390/jcm9072118
APA StyleBellini, M. I., Courtney, A. E., & McCaughan, J. A. (2020). Living Donor Kidney Transplantation Improves Graft and Recipient Survival in Patients with Multiple Kidney Transplants. Journal of Clinical Medicine, 9(7), 2118. https://doi.org/10.3390/jcm9072118






