Factors for Predicting Noninvasive Ventilation Failure in Elderly Patients with Respiratory Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Overview
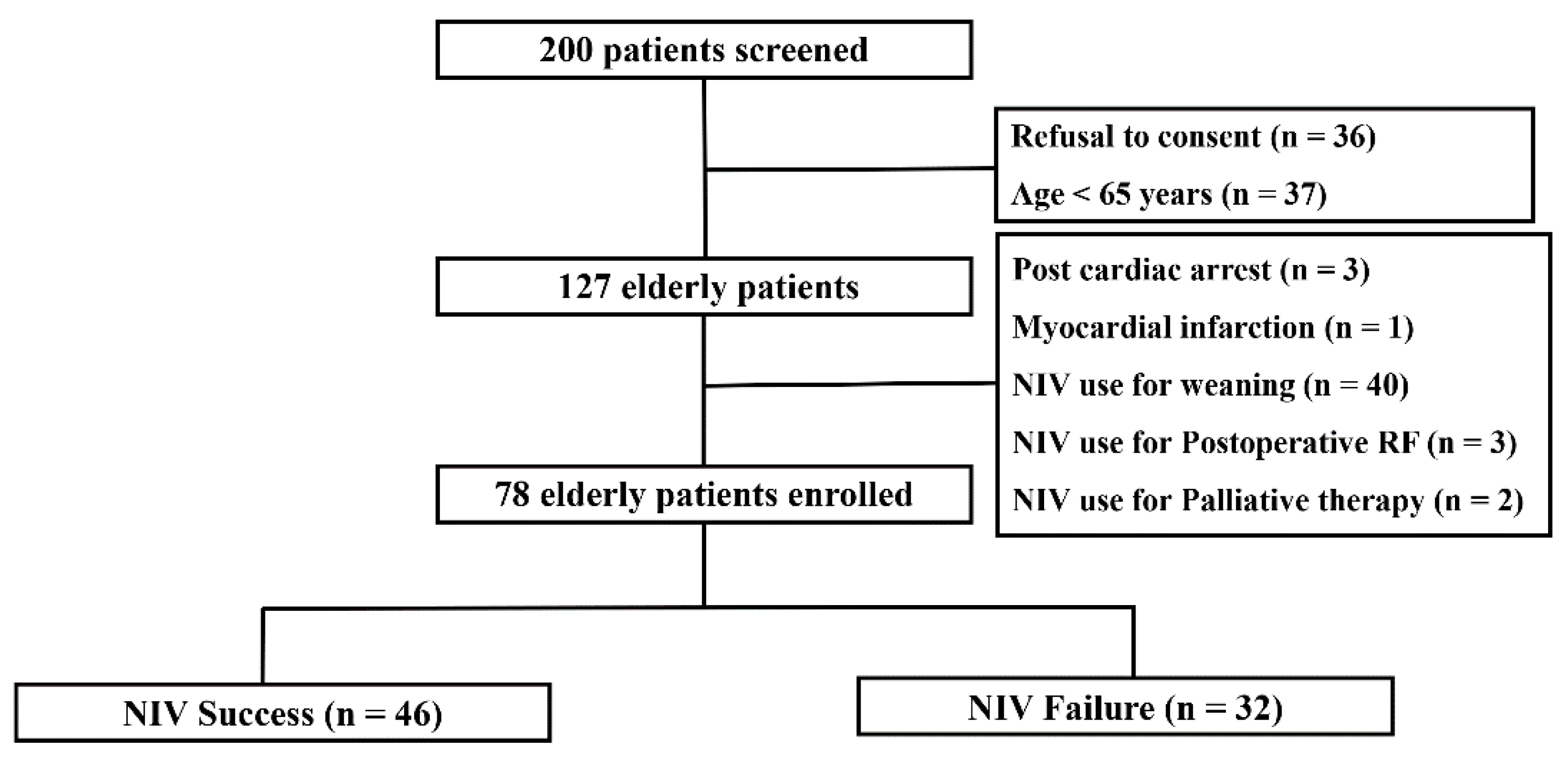
2.2. Patients
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Comparison of Treatment of Patients for Whom NIV Succeeded and Failed
3.3. Causes and Outcomes in Patients with NIV Failure
3.4. Factors Analyzed for Effects on NIV Success/Failure
3.5. Prognostic Utilities of Models Using Risk Factors for NIV Failure
3.6. Changes in Physiological Parameters after NIV Commencement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baudouin, S.; Blumenthal, S.; Cooper, B.; Davidson, C.; Davison, A.; Elliot, M.; Kinnear, W.; Paton, R.; Sawicka, E.; Turner, L. Noninvasive ventilation in acute respiratory failure. Thorax 2002, 57, 192–211. [Google Scholar] [CrossRef]
- Davidson, A.C.; Banham, S.; Elliott, M.; Kennedy, D.; Gelder, C.; Glossop, A.; Church, A.C.; Creagh-Brown, B.; Dodd, J.W.; Felton, T.; et al. BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax 2016, 71 (Suppl. 2), ii1–ii35. [Google Scholar] [CrossRef]
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; Hess, D.; Hill, N.S.; Nava, S.; Navalesi, P.M.O.T.S.C.; Antonelli, M.; Brozek, J.; Conti, G.; et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017, 50, 1602426. [Google Scholar] [CrossRef] [PubMed]
- Bourke, S.C.; Piraino, T.; Pisani, L.; Brochard, L.; Elliott, M.W. Beyond the guidelines for non-invasive ventilation in acute respiratory failure: Implications for practice. Lancet Respir. Med. 2018, 6, 935–947. [Google Scholar] [CrossRef]
- Girou, E.; Schortgen, F.; Delclaux, C.; Brun-Buisson, C.; Blot, F.; Lefort, Y.; Lemaire, F.; Brochard, L. Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients. JAMA 2000, 284, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Girou, E.; Brun-Buisson, C.; Taillé, S.; Lemaire, F.; Brochard, L. Secular trends in nosocomial infections and mortality associated with noninvasive ventilation in patients with exacerbation of COPD and pulmonary edema. JAMA 2003, 290, 2985–2991. [Google Scholar] [CrossRef] [PubMed]
- Grieco, D.L.; Menga, L.S.; Raggi, V.; Bongiovanni, F.; Anzellotti, G.M.; Tanzarella, E.S.; Bocci, M.G.; Mercurio, G.; Dell’Anna, A.M.; Eleuteri, D.; et al. Physiological Comparison of High-Flow Nasal Cannula and Helmet Noninvasive Ventilation in Acute Hypoxemic Respiratory Failure. Am. J. Respir. Crit. Care Med. 2020, 201, 303–312. [Google Scholar] [CrossRef]
- Ni, Y.N.; Luo, J.; Yu, H.; Liu, D.; Ni, Z.; Cheng, J.; Liang, B.M.; Liang, Z.A. Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients With Acute Respiratory Failure Compared With Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation?: A Systematic Review and Meta-analysis. Chest 2017, 151, 764–775. [Google Scholar] [CrossRef]
- Xu, X.P.; Zhang, X.C.; Hu, S.L.; Xu, J.Y.; Xie, J.F.; Liu, S.Q.; Liu, L.; Huang, Y.Z.; Guo, F.M.; Yang, Y.; et al. Noninvasive Ventilation in Acute Hypoxemic Nonhypercapnic Respiratory Failure: A Systematic Review and Meta-Analysis. Crit. Care Med. 2017, 45, e727–e733. [Google Scholar] [CrossRef]
- Frat, J.P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef]
- Li, J.; Jing, G.; Scott, J.B. Year in Review 2019: High-Flow Nasal Cannula Oxygen Therapy for Adult Subjects. Respir Care 2020, 65, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Flaatten, H.; de Lange, D.W.; Artigas, A.; Bin, D.; Moreno, R.; Christensen, S.; Joynt, G.M.; Bagshaw, S.M.; Sprung, C.L.; Benoit, D.; et al. The status of intensive care medicine research and a future agenda for very old patients in the ICU. Intensive Care Med. 2017, 43, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.A.F.; Camillo, C.A.; Langer, D.; Andrade, L.B.; Duarte, M.; Gosselink, R. Weaning failure and respiratory muscle function: What has been done and what can be improved? Respir. Med. 2018, 134, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Boles, J.M.; Bion, J.; Connors, A.; Herridge, M.; Marsh, B.; Melot, C.; Pearl, R.; Silverman, H.; Stanchina, M.; Vieillard-Baron, A.; et al. Weaning from mechanical ventilation. Eur. Respir. J. 2007, 29, 1033–1056. [Google Scholar] [CrossRef] [PubMed]
- Ely, E.W.; Wheeler, A.P.; Thompson, B.T.; Ancukiewicz, M.; Steinberg, K.P.; Bernard, G.R. Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann. Intern. Med. 2002, 136, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Funk, G.C.; Anders, S.; Breyer, M.K.; Burghuber, O.C.; Edelmann, G.; Heindl, W.; Hinterholzer, G.; Kohansal, R.; Schuster, R.; Schwarzmaier-D’Assie, A.; et al. Incidence and outcome of weaning from mechanical ventilation according to new categories. Eur. Respir. J. 2010, 35, 88–94. [Google Scholar] [CrossRef]
- Sellares, J.; Ferrer, M.; Cano, E.; Loureiro, H.; Valencia, M.; Torres, A. Predictors of prolonged weaning and survival during ventilator weaning in a respiratory ICU. Intensive Care Med. 2011, 37, 775–784. [Google Scholar] [CrossRef]
- Béduneau, G.; Pham, T.; Schortgen, F.; Piquilloud, L.; Zogheib, E.; Jonas, M.; Grelon, F.; Runge, I.; Nicolas, T.; Grangé, S.; et al. Epidemiology of Weaning Outcome according to a New Definition. The WIND Study. Am. J. Respir. Crit. Care Med. 2017, 195, 772–783. [Google Scholar] [CrossRef]
- Piroddi, I.M.G.; Barlascini, C.; Esquinas, A.; Braido, F.; Banfi, P.; Nicolini, A. Non-invasive mechanical ventilation in elderly patients: A narrative review. Geriatr. Gerontol. Int. 2017, 17, 689–696. [Google Scholar] [CrossRef]
- Scala, R. Challenges on non-invasive ventilation to treat acute respiratory failure in the elderly. BMC Pulm. Med. 2016, 16, 150. [Google Scholar] [CrossRef]
- Roussos, C.; Koutsoukou, A. Respiratory failure. Eur. Respir. J. Suppl. 2003, 47, 3–14. [Google Scholar] [CrossRef]
- Pontoppidan, H.; Geffin, B.; Lowenstein, E. Acute respiratory failure in the adult. 1. N. Engl. J. Med. 1972, 287, 690–698. [Google Scholar] [CrossRef]
- Guidet, B.; Leblanc, G.; Simon, T.; Woimant, M.; Quenot, J.P.; Ganansia, O.; Maignan, M.; Yordanov, Y.; Delerme, S.; Doumenc, B.; et al. Effect of Systematic Intensive Care Unit Triage on Long-term Mortality Among Critically Ill Elderly Patients in France: A Randomized Clinical Trial. JAMA 2017, 318, 1450–1459. [Google Scholar] [CrossRef]
- Farfel, J.M.; Franca, S.A.; Sitta Mdo, C.; Filho, W.J.; Carvalho, C.R. Age, invasive ventilatory support and outcomes in elderly patients admitted to intensive care units. Age Ageing 2009, 38, 515–520. [Google Scholar] [CrossRef][Green Version]
- Antonelli, M.; Conti, G.; Moro, M.L.; Esquinas, A.; Gonzalez-Diaz, G.; Confalonieri, M.; Pelaia, P.; Principi, T.; Gregoretti, C.; Beltrame, F.; et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: A multi-center study. Intensive Care Med. 2001, 27, 1718–1728. [Google Scholar] [CrossRef]
- Ozyilmaz, E.; Ugurlu, A.O.; Nava, S. Timing of noninvasive ventilation failure: Causes, risk factors, and potential remedies. BMC Pulm. Med. 2014, 14, 19. [Google Scholar] [CrossRef]
- Hong, Y.; Duan, J.; Bai, L.; Han, X.; Huang, S.; Guo, S. Noninvasive ventilation failure in pneumonia patients ≥ 65years old: The role of cough strength. J. Crit. Care 2018, 44, 149–153. [Google Scholar] [CrossRef]
- Petersson, J.; Glenny, R.W. Gas exchange and ventilation-perfusion relationships in the lung. Eur. Respir. J. 2014, 44, 1023–1041. [Google Scholar] [CrossRef]
- Sinha, P.; Fauvel, N.J.; Singh, S.; Soni, N. Ventilatory ratio: A simple bedside measure of ventilation. Br. J. Anaesth. 2009, 102, 692–697. [Google Scholar] [CrossRef]
- He, H.; Sun, B.; Liang, L.; Li, Y.; Wang, H.; Wei, L.; Li, G.; Guo, S.; Duan, J.; Li, Y.; et al. A multicenter RCT of noninvasive ventilation in pneumonia-induced early mild acute respiratory distress syndrome. Crit. Care 2019, 23, 300. [Google Scholar] [CrossRef] [PubMed]
- Carron, M.; Freo, U.; Zorzi, M.; Ori, C. Predictors of failure of noninvasive ventilation in patients with severe community-acquired pneumonia. J. Crit. Care 2010, 25, e549–e554. [Google Scholar] [CrossRef] [PubMed]
- Confalonieri, M.; Potena, A.; Carbone, G.; Porta, R.D.; Tolley, E.A.; Umberto Meduri, G. Acute respiratory failure in patients with severe community-acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation. Am. J. Respir. Crit. Care Med. 1999, 160, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, A.; Murad, A.; Li, P.Z.; Shahin, J. Outcomes and predictors of failure of non-invasive ventilation in patients with community acquired pneumonia in the ED. Am. J. Emerg. Med. 2018, 36, 347–351. [Google Scholar] [CrossRef]
- Murad, A.; Li, P.Z.; Dial, S.; Shahin, J. The role of noninvasive positive pressure ventilation in community-acquired pneumonia. J. Crit. Care 2015, 30, 49–54. [Google Scholar] [CrossRef]
- Valley, T.S.; Walkey, A.J.; Lindenauer, P.K.; Wiener, R.S.; Cooke, C.R. Association Between Noninvasive Ventilation and Mortality Among Older Patients with Pneumonia. Crit. Care Med. 2017, 45, e246–e254. [Google Scholar] [CrossRef]
- Stefan, M.S.; Priya, A.; Pekow, P.S.; Lagu, T.; Steingrub, J.S.; Hill, N.S.; Nathanson, B.H.; Lindenauer, P.K. The comparative effectiveness of noninvasive and invasive ventilation in patients with pneumonia. J. Crit. Care 2018, 43, 190–196. [Google Scholar] [CrossRef]
- Johnson, C.S.; Frei, C.R.; Metersky, M.L.; Anzueto, A.R.; Mortensen, E.M. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: A retrospective cohort study. BMC Pulm. Med. 2014, 14, 7. [Google Scholar] [CrossRef][Green Version]
| Variables | Total | Noninvasive Ventilation | p-Value | |
|---|---|---|---|---|
| n = 78 | Success (n = 46) | Failure (n = 32) | ||
| Age, years * | 77 (72–82) | 78 (73–82) | 76 (70–82) | 0.542 |
| Male | 43 (55.1) | 27 (58.7) | 16 (50) | 0.494 |
| Body mass index, kg/m2 * | 20 (17–24) | 20 (17–24) | 21 (17–25) | 0.699 |
| SOFA score at the start of NIV * | 4 (2–5) | 3 (2–5) | 4 (2–6) | 0.216 |
| Comorbidity | ||||
| Cardiovascular disease | 60 (76.9) | 26 (56.5) | 17 (53.1) | 0.820 |
| Diabetes mellitus | 21 (26.9) | 11 (23.9) | 10 (31.3) | 0.605 |
| Chronic kidney disease | 14 (17.9) | 8 (17.4) | 6 (18.8) | 1.000 |
| Liver cirrhosis | 3 (3.8) | 1 (2.2) | 2 (6.3) | 0.565 |
| Cerebrovascular disease | 9 (11.5) | 5 (10.9) | 4 (12.5) | 1.000 |
| Cancer | 7 (9) | 3 (6.5) | 4 (12.5) | 0.436 |
| Immunosuppression | 6 (7.7) | 1 (2.2) | 5 (15.6) | 0.040 |
| Underlying lung diseases | ||||
| No underlying lung disease | 20 (25.6) | 9 (19.6) | 11 (34.4) | 0.189 |
| COPD | 32 (41) | 21 (45.7) | 11 (34.4) | 0.357 |
| Bronchial asthma | 2 (2.6) | 1 (2.2) | 1 (3.1) | 1.000 |
| Interstitial lung disease | 5 (6.4) | 2 (4.3) | 3 (9.4) | 0.396 |
| Bronchiectasis | 3 (3.8) | 3 (6.5) | 0 (0) | 0.265 |
| TB destroyed lung disease | 8 (10.3) | 4 (8.7) | 4 (12.5) | 0.710 |
| Neuromuscular disease | 1 (1.3) | 0 (0) | 1 (3.1) | 0.410 |
| Obesity disease | 4 (5.1) | 4 (8.7) | 0 (0) | 0.140 |
| Scoliosis | 1 (1.3) | 1 (2.2) | 0 (0) | 1.000 |
| Others chest wall disease | 2 (2.6) | 1 (2.2) | 1 (3.1) | 1.000 |
| Total duration of NIV use, days * | 3 (2–8) | 4 (2–7) | 2 (1–5) | 0.042 |
| Duration of ICU stay, days * | 8 (5–20) | 7 (4–11) | 21 (7–40) | <0.001 |
| ICU mortality | 17 (21.8) | 0 (0) | 17 (53.1) | <0.001 |
| Variables | Total | Noninvasive Ventilation | p-Value | |
|---|---|---|---|---|
| n = 78 | Success (n = 46) | Failure (n = 32) | ||
| Cause of the NIV application | ||||
| Hypercapnic RF | 54 (69.2) | 35 (76.1) | 19 (59.4) | 0.139 |
| Hypoxic RF | 13 (16.7) | 3 (6.5) | 10 (31.3) | 0.006 |
| Acute on chronic RF | 11 (14.1) | 8 (17.4) | 3 (9.4) | 0.510 |
| Physiologic parameters on the application of NIV * | ||||
| Systolic BP (mmHg) | 125 (108–144) | 123 (106–142) | 126 (112–150) | 0.318 |
| Heart rate (beats/min) | 98 (83–110) | 92 (82–106) | 107 (85–117) | 0.051 |
| Respiratory rate (breaths/min) | 24 (20–29) | 24 (20–28) | 27 (23–32) | 0.030 |
| Arterial pH | 7.34 (7.28–7.39) | 7.34 (7.29–7.38) | 7.34 (7.27–7.43) | 0.830 |
| PaO2/FiO2 ratio | 211 (139–264) | 214 (142–270) | 199 (100–259) | 0.152 |
| PaCO2 (mmHg) | 65 (50–80) | 69 (59–83) | 53 (43–68) | 0.003 |
| Location of initial NIV application | 0.131 | |||
| Intensive Care Unit | 70 (89.7) | 39 (84.8) | 31 (96.9) | |
| General ward | 8 (10.3) | 7 (15.2) | 1 (3.1) | |
| Interface type | ||||
| Nasal type | 3 (3.8) | 2 (4.3) | 1 (3.1) | 1.000 |
| Oronasal type | 68 (87.2) | 41 (89.1) | 27 (84.4) | 0.732 |
| Total facial type | 1 (1.3) | 0 (0) | 1 (3.1) | 0.410 |
| Helmet type | 6 (7.7) | 3 (6.5) | 3 (9.4) | 0.685 |
| Initial mode | 0.119 | |||
| Assist control mode | 21 (26.9) | 9 (19.6) | 12 (37.5) | |
| Pressure support mode | 57 (73.1) | 37 (80.4) | 20 (62.5) | |
| Initial setting * | ||||
| IPAP (cmH2O) | 15 (12–18) | 14 (12–17) | 15 (13–20) | 0.199 |
| EPAP (cmH2O) | 5 (4–6) | 5 (4–5) | 5 (4–6) | 0.129 |
| Tidal volume (mL) | 400 (309–514) | 390 (303–505) | 410 (334–569) | 0.308 |
| Sedative use | 11 (14.1) | 5 (10.9) | 6 (18.8) | 0.344 |
| Complications during NIV | ||||
| Skin erythema | 12 (15.4) | 8 (17.4) | 4 (12.5) | 0.752 |
| Abdominal distension | 4 (6.4) | 3 (6.5) | 2 (6.3) | 1.000 |
| Dry mouth | 3 (3.8) | 2 (4.3) | 1 (3.1) | 1.000 |
| Aspiration | 2 (2.6) | 2 (4.3) | 0 (0) | 0.510 |
| Claustrophobia | 1 (1.3) | 1 (2.2) | 0 (0) | 1.000 |
| Nasal congestion | 1 (1.3) | 0 (0) | 1 (3.1) | 0.410 |
| Large leaks | 11 (14.1) | 5 (10.9) | 6 (18.8) | 0.344 |
| Duration of NIV (hours/day) * | 15 (7–22) | 16 (10–21) | 12 (3–24) | 0.449 |
| Variables | Number (%) |
|---|---|
| Cause of NIV failure | |
| Aggravated clinical conditions | 24 (75) |
| Agitation | 1 (3.1) |
| Aspiration | 2 (6.3) |
| Patients discomfort | 5 (15.6) |
| Outcomes after NIV failure | |
| Intubation & Mechanical ventilation | 22 (68.8) |
| Hopeless discharge with NIV | 1 (3.1) |
| Death during NIV treatment | 9 (28.1) |
| Variables | Odds Ratios | 95% CI | p-Value |
|---|---|---|---|
| Univariate Analysis | |||
| Age (years) | 0.99 | 0.929–1.055 | 0.753 |
| Male | 0.70 | 0.284–1.745 | 0.448 |
| Body mass index (kg/m2) | 0.99 | 0.912–1.072 | 0.787 |
| SOFA score at the start of NIV | 1.17 | 0.955–1.433 | 0.130 |
| Systolic blood pressure (mmHg) | 1.02 | 0.995–1.036 | 0.136 |
| Heart rate (beats/min) after NIV | 1.04 | 1.009–1.066 | 0.010 |
| Respiratory rate (breaths/min) after NIV | 1.09 | 1.001–1.187 | 0.047 |
| PaO2/FiO2 ratio after NIV | 0.99 | 0.988–1.001 | 0.074 |
| PaCO2 (mmHg) after NIV | 0.95 | 0.916–0.980 | 0.002 |
| The number of comorbidities | 1.29 | 0.849–1.966 | 0.232 |
| Assist control mode | 2.47 | 0.888–6.849 | 0.083 |
| IPAP | 1.06 | 0.943–1.185 | 0.344 |
| EPAP | 1.23 | 0.904–1.667 | 0.189 |
| Sedative use | 1.89 | 0.524–6.837 | 0.330 |
| Large leak | 1.89 | 0.524–6.837 | 0.330 |
| NIV application time during a day | 0.97 | 0.920–1.031 | 0.360 |
| The presence of pneumonia | 3.69 | 1.314–10.389 | 0.013 |
| Multivariate Analysis | |||
| PaCO2 (mmHg) after NIV | 0.95 | 0.910–0.982 | 0.004 |
| Heart rate (beats/min) after NIV | 1.05 | 1.013–1.080 | 0.006 |
| The presence of pneumonia | 3.32 | 1.026–10.719 | 0.045 |
| Models | C-Index | 95% CI | p-Value * | p-Value † |
|---|---|---|---|---|
| I | 0.639 | 0.534–0.744 | Reference | |
| II | 0.744 | 0.630–0.859 | 0.025 | Reference |
| III | 0.816 | 0.715–0.917 | 0.001 | 0.052 |
| Variables | NIV Application (n = 78) | p-Value | |
|---|---|---|---|
| NIV Success (n = 46) | Before | After | |
| Systolic blood pressure(mmHg) | 123 (106–142) | 117 (104–129) | 0.056 |
| Heart rate (beats/min) | 92 (82–106) | 89 (75–97) | 0.007 |
| Respiratory rate (breaths/min) | 24 (20–28) | 22 (19–25) | 0.298 |
| Arterial pH | 7.34 (7.29–7.38) | 7.37 (7.30–7.43) | <0.001 |
| PaO2/FiO2 ratio | 214 (142–270) | 233 (179–271) | 0.298 |
| PaCO2 (mmHg) | 69 (59–83) | 63 (51–75) | <0.001 |
| NIV failure (n = 32) | |||
| Systolic blood pressure(mmHg) | 126 (112–150) | 126 (107–145) | 0.462 |
| Heart rate (beats/min) | 107 (85–117) | 97 (89–114) | 0.296 |
| Respiratory rate (breaths/min) | 27 (23–32) | 24 (21–28) | 0.159 |
| Arterial pH | 7.34 (7.27–7.43) | 7.36 (7.31–7.43) | 0.082 |
| PaO2/FiO2 ratio | 199 (100–259) | 212 (120–280) | 0.247 |
| PaCO2 (mmHg) | 53 (43–68) | 48 (39–66) | 0.005 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.J.; Cho, J.H.; Chang, Y.; Moon, J.Y.; Park, S.; Park, T.S.; Lee, Y.S. Factors for Predicting Noninvasive Ventilation Failure in Elderly Patients with Respiratory Failure. J. Clin. Med. 2020, 9, 2116. https://doi.org/10.3390/jcm9072116
Park MJ, Cho JH, Chang Y, Moon JY, Park S, Park TS, Lee YS. Factors for Predicting Noninvasive Ventilation Failure in Elderly Patients with Respiratory Failure. Journal of Clinical Medicine. 2020; 9(7):2116. https://doi.org/10.3390/jcm9072116
Chicago/Turabian StylePark, Min Jeong, Jae Hwa Cho, Youjin Chang, Jae Young Moon, Sunghoon Park, Tai Sun Park, and Young Seok Lee. 2020. "Factors for Predicting Noninvasive Ventilation Failure in Elderly Patients with Respiratory Failure" Journal of Clinical Medicine 9, no. 7: 2116. https://doi.org/10.3390/jcm9072116
APA StylePark, M. J., Cho, J. H., Chang, Y., Moon, J. Y., Park, S., Park, T. S., & Lee, Y. S. (2020). Factors for Predicting Noninvasive Ventilation Failure in Elderly Patients with Respiratory Failure. Journal of Clinical Medicine, 9(7), 2116. https://doi.org/10.3390/jcm9072116





