Both Low and High PAPP-A Concentrations in the First Trimester of Pregnancy Are Associated with Increased Risk of Delivery before 32 Weeks in Twin Gestation
Abstract
:1. Introduction
2. Materials and Methods
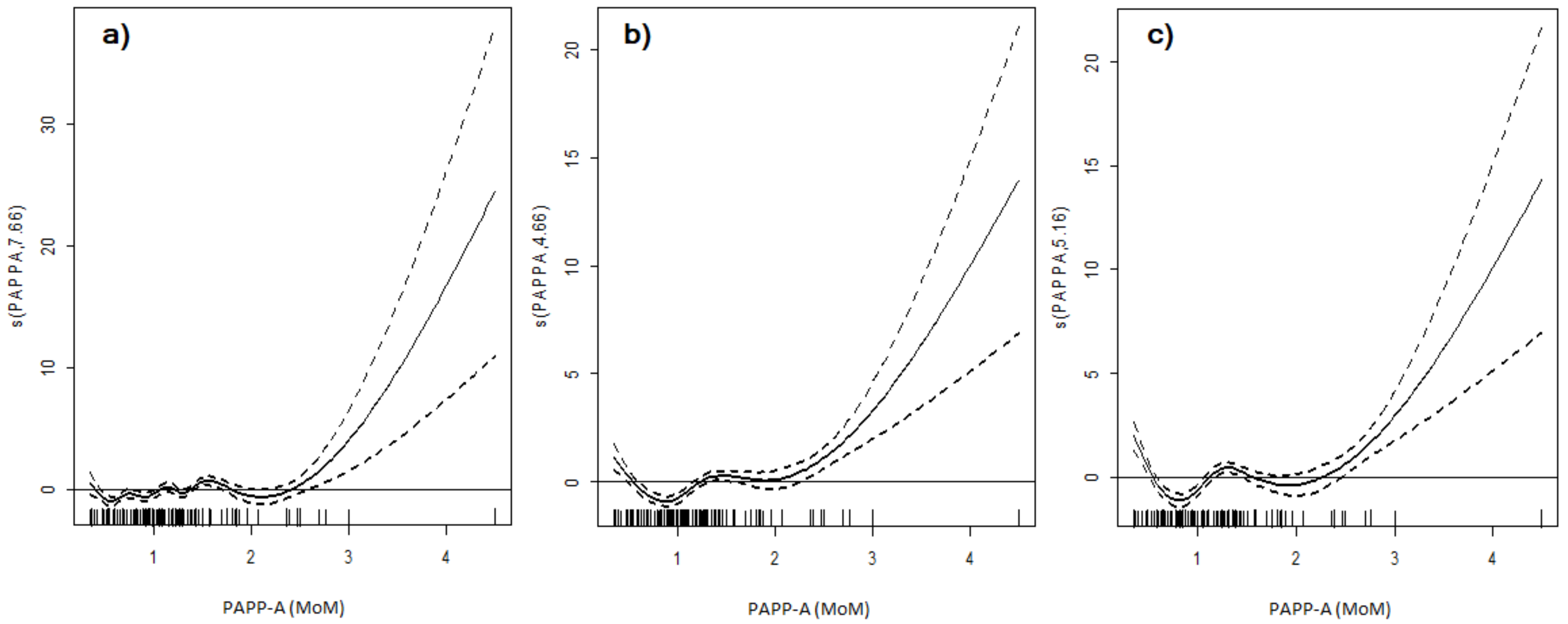
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lopriore, E.; Stroeken, H.; Sueters, M.; Meerman, R.J.; Walther, F.; Vandenbussche, F. Term perinatal mortality and morbidity in monochorionic and dichorionic twin pregnancies: A retrospective study. Acta Obstet. Gynecol. Scand. 2008, 87, 541–545. [Google Scholar] [CrossRef]
- Hack, K.E.; Derks, J.B.; Elias, S.G.; Franx, A.; Roos, E.J.; Voerman, S.K.; Bode, C.L.; Koopman-Esseboom, C.; Visser, G.H. Increased perinatal mortality and morbidity in monochorionic versus dichorionic twin pregnancies: Clinical implications of a large Dutch cohort study. BJOG 2008, 115, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kosińska-Kaczyńska, K.; Szymusik, I.; Bomba-Opoń, D.; Olejek, A.; Sławska, H.; Zimmer, M.; Pomorski, M.; Bręborowicz, G.; Drews, K.; Seremak-Mrozikiewicz, A.; et al. Perinatal outcome according to chorionicity in twins—A Polish multicenter study. Ginekol. Pol. 2016, 87, 384–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagnon, A.; Wilson, R.D. SOCIETY OF OBSTETRICIANS AND GYNAECOLOGISTS OF CANADA GENETICS COMMITTEE. Obstetrical complications associated with abnormal maternal serum markers analytes. J. Obstet. Gynaecol. Can. 2008, 30, 918–932. [Google Scholar] [CrossRef]
- Shin, J.E.; Shin, J.C.; Kim, S.J.; Lee, Y.; Park, I.Y.; Lee, S. Early midtrimester serum insulin-like factors and cervical length to predict preterm delivery. Taiwan J. Obstet. Gynecol. 2016, 55, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Santolaya-Forgas, J.; De Leon, J.A.; Cullen Hopkins, R.; Castracane, V.D.; Kauffman, R.P.; Sifuentes, G.A. Low pregnancy-associated plasma protein-a at 10(+1) to 14(+6) weeks of gestation and a possible mechanism leading to miscarriage. Fetal Diagn. Ther. 2004, 19, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.M.; Kumar, S. Low First Trimester Pregnancy-Associated Plasma Protein-A Levels Are Not Associated with an Increased Risk of Intrapartum Fetal Compromise or Adverse Neonatal Outcomes: A Retrospective Cohort Study. J. Clin. Med. 2020, 9, 1108. [Google Scholar] [CrossRef]
- Rosner, J.Y.; Fox, N.S.; Saltzman, D.; Klauser, C.K.; Rebarber, A.; Gupta, S. Abnormal Biochemical Analytes Used for Aneuploidy Screening and Adverse Pregnancy Outcomes in Twin Gestations. Am. J. Perinatol. 2015, 32, 1331–1335. [Google Scholar]
- Laughon, S.K.; Rebarber, A.; Rolnitzky, L.; Fink, L.; Saltzman, D.H. Decreased first-trimester maternal serum free-beta subunit human chorionic gonadotropin and preterm birth in twin gestations. Am. J. Perinatol. 2009, 26, 491–494. [Google Scholar] [CrossRef]
- Iskender, C.; Tarım, E.; Çok, T.; Yalcınkaya, C.; Kalaycı, H.; Yanık, F.B. Obstetrical complications associated with first-trimester screening markers in twin pregnancies. J. Obstet. Gynaecol. Res. 2013, 39, 1495–1499. [Google Scholar] [CrossRef]
- Fathian, A.; Miller, R.; Wolf, E. Analysis of first trimester markers, PAPP-A and free-βhCG, and adverse outcomes in twin pregnancies. Am. J. Obstet. Gynecol. 2014, 214, S135. [Google Scholar] [CrossRef]
- Chasen, S.T.; Martinucci, S.; Perni, S.C.; Kalish, R.B. First-trimester biochemistry and outcomes in twin pregnancy. J. Reprod. Med. 2009, 54, 312–314. [Google Scholar]
- Ghi, T.; Prefumo, F.; Fichera, A.; Lanna, M.; Periti, E.; Persico, N.; Viora, E.; Rizzo, G.; Società Italiana di Ecografia Ostetrica e Ginecologica Working Group on Fetal Biometric Charts. Development of customized fetal growth charts in twins. Am. J. Obstet. Gynecol. 2017, 216, e1–e17. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2019, 133, e1–e25. [Google Scholar] [CrossRef]
- Wender-Ożegowska, E.; Bomba-Opoń, D.; Brązert, J.; Celewicz, Z.; Czajkowski, K.; Gutaj, P.; Malinowska-Polubiec, A.; Zawiejska, A.; Wielgoś, M. Standards of Polish Society of Gynecologists and Obstetricians in management of women with diabetes. Ginekol. Pol. 2018, 89, 341–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conde-Agudelo, A.; Romero, R. Prediction of preterm birth in twin gestations using biophysical and biochemical tests. Am. J. Obstet. Gynecol. 2014, 211, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Mesdaghi-Nia, E.; Behrashi, M.; Saeidi, A.; Abedzadeh Kalahroodi, M.; Sehat, M. Association between PAPP-A and placental thickness. Int. J. Reprod. Biomed. 2016, 14, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Brizot, M.L.; Hyett, J.A.; Mckie, A.T.; Bersinger, N.A.; Farzaneh, F.; Nicolaides, K.H. Gene expression of human pregnancy-associated plasma protein-A in placenta from trisomic pregnancies. Placenta 1996, 17, 33–36. [Google Scholar] [CrossRef]
- Pummara, P.; Tongsong, T.; Wanapirak, C.; Sirichotiyakul, S.; Luewan, S. Association of first-trimester pregnancy-associated plasma protein A levels and idiopathic preterm delivery: A population-based screening study. Taiwan J. Obstet. Gynecol. 2016, 55, 72–75. [Google Scholar] [CrossRef] [Green Version]
- Morris, R.K.; Bilagi, A.; Devani, P.; Kilby, M.D. Association of Serum PAPP-A Levels in First Trimester with Small for Gestational Age and Adverse Pregnancy Outcomes: Systematic Review and Meta-Analysis. Prenat. Diagn. 2017, 37, 253–265. [Google Scholar] [CrossRef]
- Paelez, L.; Chasen, S.; Baergen, R. Relationship between first trimester maternal serum PAPP-A levels and placental lesions in twin gestations. Am. J. Obstet. Gynecol. 2008, 6, S95. [Google Scholar] [CrossRef]
- Wang, H.S.; Perry, L.A.; Kanisius, J.; Iles, R.K.; Holly, J.M.; Chard, T. Purification and assay of insulin-like growth factor-binding protein-1: Measurement of circulating levels throughout pregnancy. J. Endocrinol. 1991, 128, 161–168. [Google Scholar] [CrossRef] [PubMed]
| Study Group n = 304 | PAPP-A <10th pc n = 31 | PAPP-A 10–90th pc n = 245 | p | PAPP-A >90th pc n = 28 | p | |
|---|---|---|---|---|---|---|
| Means ± SD /n (%) | Means ± SD /n (%) | Means ± SD /n (%) | Means ± SD /n (%) | |||
| age (years) * | 34.02 ± 3.06 | 33.51 ± 4.1 | 34.12 ± 3.41 | 0.6 | 30.91 ± 3.89 | 0.08 |
| Primiparity ** | 168 (55.3) | 18 (58.1) | 136 (55.51) | 0.8 | 14 (50) | 0.7 |
| Monochorionicity ** | 142 (46.7) | 16 (51.62) | 108 (44.08) | 0.4 | 18 (64.28) | 0.047 |
| BMI (kg/m2) * | 22.94 ± 2.56 | 23.7 ± 1.62 | 22.97 ± 1.98 | 0.7 | 22.01 ± 2.25 | 0.8 |
| Smoker ** | 17 (5.6) | 3 (9.7) | 14 (5.71) | 0.4 | 0 | 0.3 |
| ART ** | 50 (16.4) | 2 (6.5) | 46 (18.78) | 0.1 | 2 (7.14) | 0.2 |
| gestational age at delivery (weeks) * | 34.98 ± 3.08 | 32.65 ± 1.37 | 35.12 ± 3.23 | 0.03 | 32.82 ± 1.57 | 0.04 |
| 1st twin birtweight (g) * | 2383 ± 582 | 2309 ± 378 | 2415 ± 498 | 0.1 | 2366 ± 404 | 0.2 |
| 2nd twin birtweight (g) * | 2289 ± 538 | 2345 ± 214 | 2278 ± 642 | 0.3 | 2054 ± 225 | 0.08 |
| 1st twin SGA ** | 19 (6.3) | 2 (6.5) | 17 (6.94) | 1 | 0 | 0.3 |
| 2nd twin SGA ** | 25 (8.2) | 6 (19.35) | 17 (6.94) | 0.03 | 2 (7.14) | 1 |
| PAPP-A <10th pc n (%) | PAPP-A 10–90th pc n (%) | p | OR (95% CI) | RR (95% CI) | PAPP-A >90th pc n (%) | p | OR (95% CI) | RR (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| Delivery < 37 weeks | 28 (87.5) | 130 (53.3) | <0.001 | 3.14 (2.1–18) | 2.47 (1.1–5.3) | 20 (71.4) | 0.07 | 2.19 (0.9–5.2) | 2.05 (0.9–4.9) |
| Delivery < 34 weeks | 14 (43.8) | 60 (24.6) | 0.2 | 2.39 (1.1–5.1) | 2.25 (1.1–4.6) | 16 (57.1) | 0.001 | 4.09 (1.8–9.1) | 3.46 (1.6–7.5) |
| Delivery < 32 weeks | 12 (37.5) | 40 (16.4) | 0.005 | 3.06 (1.4–6.8) | 2.72 (1.3–5.5) | 10 (35.7) | 0.02 | 2.83 (1.2–6.6) | 2.48 (1.1–5.3) |
| Delivery < 28 weeks | 4 (12.5) | 26 (10.7) | 0.7 | 1.2 (0.4–3.7) | 1.22 (0.4–3.3) | 8 (28.6) | 0.01 | 3.35 (1.3–8.4) | 2.18 (1.1–4.2) |
| PPROM | 5 (16.1) | 26 (10.6) | 0.4 | 1.6 (0.6–4.6) | 1.52 (0.5–3.7) | 4 (14.3) | 0.7 | 0.6 (0.1–2.9) | 1.35 (0.4–3.6) |
| Spontaneous uterine contractions resulting in delivery | 10 (32.3) | 42 (17.1) | 0.049 | 2.3 (1–5.2) | 2.05 (1–4.2) | 7 (25%) | 0.3 | 1.6 (0.6–4) | 1.5 (0.6–3.5) |
| Cervix insufficiency | 0 (0) | 3 (1) | 0.8 | 0.1 (0.1–4.2) | 0.21 (0–6.4) | 0 (0) | 0.9 | 0.23 (0.1–3.1) | 0.2 (0–3.8) |
| GDM | 12 (37.5) | 30 (12.3) | 0.001 | 4.28 (1.9–9.6) | 3.5 (1.7–6.9) | 6 (21.4) | 0.2 | 1.9 (0.7–5.2) | 1.79 (0.6–4.2) |
| GH and PE | 8 (25) | 36 (14.7) | 0.2 | 1.93 (0.8–4.6) | 1.84 (0.6–3.9) | 2 (7.1) | 0.4 | 1.44 (0.1–1.9) | 3.52 (1.7–6.9) |
| IUD | 0 | 4 (1.6) | 1 | - | 0 (0–8.6) | 4 (14.3) | 0.005 | 10 (2.4–42.5) | 8.9 (3.1–11.5) |
| SGA | 8 (25) | 34 (13.9) | 0.1 | 2.1 (0.9–4.9) | 1.91 (0.8–4.1) | 2 (7.1) | 0.5 | 0.47 (0.1–2.1) | 0.51 (0.1–2) |
| >25% BW | 6 (18.8) | 24 (9.8) | 0.1 | 2.11 (0.8–5.7) | 1.91 (0.7–4.4) | 4 (14.3) | 0.5 | 1.53 (0.5–4.8) | 1.46 (0.4–3.9) |
| Monochorionic n = 142 | Dichorionic n = 162 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PAPP-A <10th pc n = 15 | PAPP-A 10–90th pc n = 114 | PAPP-A >90th pc n = 13 | PAPP-A <10th pc n = 16 | p * | PAPP-A 10–90th pc n = 131 | p ** | PAPP-A >90th pc n = 15 | p *** | |
| delivery < 37 weeks | 13 (86.67) | 68 (59.65) | 8 (61.54) | 15 (93.75) | 0.5 | 62 (47.33) | 0.06 | 12 (80) | 0.3 |
| delivery < 34 weeks | 7 (46.67) | 31 (27.19) | 7 (53.85) | 7 (43.75) | 0.8 | 29 (22.14) | 0.4 | 9 (60) | 1 |
| delivery < 32 weeks | 5 (33.33) | 21 (18.42) | 4 (30.8) | 7 (43.75) | 0.7 | 19 (14.5) | 0.5 | 6 (40) | 0.7 |
| delivery < 28 weeks | 2 (13.33) | 12 (10.53) | 4 (30.77) | 2 (12.5) | 0.8 | 14 (10.69) | 0.8 | 4 (26.67) | 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saletra-Bielińska, A.; Kosińska-Kaczyńska, K.; Szymusik, I.; Kaczyński, B.; Brawura-Biskupski-Samaha, R.; Kozłowski, S.; Jarmużek, P.; Walasik, I.; Wielgoś, M. Both Low and High PAPP-A Concentrations in the First Trimester of Pregnancy Are Associated with Increased Risk of Delivery before 32 Weeks in Twin Gestation. J. Clin. Med. 2020, 9, 2099. https://doi.org/10.3390/jcm9072099
Saletra-Bielińska A, Kosińska-Kaczyńska K, Szymusik I, Kaczyński B, Brawura-Biskupski-Samaha R, Kozłowski S, Jarmużek P, Walasik I, Wielgoś M. Both Low and High PAPP-A Concentrations in the First Trimester of Pregnancy Are Associated with Increased Risk of Delivery before 32 Weeks in Twin Gestation. Journal of Clinical Medicine. 2020; 9(7):2099. https://doi.org/10.3390/jcm9072099
Chicago/Turabian StyleSaletra-Bielińska, Aleksandra, Katarzyna Kosińska-Kaczyńska, Iwona Szymusik, Bartosz Kaczyński, Robert Brawura-Biskupski-Samaha, Szymon Kozłowski, Patrycja Jarmużek, Izabela Walasik, and Mirosław Wielgoś. 2020. "Both Low and High PAPP-A Concentrations in the First Trimester of Pregnancy Are Associated with Increased Risk of Delivery before 32 Weeks in Twin Gestation" Journal of Clinical Medicine 9, no. 7: 2099. https://doi.org/10.3390/jcm9072099
APA StyleSaletra-Bielińska, A., Kosińska-Kaczyńska, K., Szymusik, I., Kaczyński, B., Brawura-Biskupski-Samaha, R., Kozłowski, S., Jarmużek, P., Walasik, I., & Wielgoś, M. (2020). Both Low and High PAPP-A Concentrations in the First Trimester of Pregnancy Are Associated with Increased Risk of Delivery before 32 Weeks in Twin Gestation. Journal of Clinical Medicine, 9(7), 2099. https://doi.org/10.3390/jcm9072099






