Clinical and Psychological Impact of COVID-19 Infection in Adult Patients with Eosinophilic Gastrointestinal Disorders during the SARS-CoV-2 Outbreak
Abstract
1. Introduction
2. Patients and Methods
Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Dellon, E.S.; Liacouras, C.A.; Molina-Infante, J.; Furuta, G.T.; Spergel, J.M.; Zevit, N.; Spechler, S.J.; Attwood, S.E.; Straumann, A.; Aceves, S.S.; et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018, 155, 1022–1033.e10. [Google Scholar] [CrossRef]
- Dellon, E.S.; Hirano, I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology 2018, 154, 319–332.e3. [Google Scholar] [CrossRef]
- De Bortoli, N.; Penagini, R.; Savarino, E.V.; Marchi, S. Eosinophilic esophagitis: Update in diagnosis and management. Position paper by the Italian Society of Gastroenterology and Gastrointestinal Endoscopy (SIGE). Dig. Liver Dis. 2017, 49, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Mukkada, V.A.; Falk, G.; Eichinger, C.S.; King, D.; Todorova, L.; Shaheen, N.J. Health-Related Quality of Life and Costs Associated With Eosinophilic Esophagitis: A Systematic Review. Clin. Gastroenterol. Hepatol. 2018, 16, 495–503.e8. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, K.M.; Aceves, S.S.; Dellon, E.S.; Gupta, S.K.; Spergel, J.M.; Furuta, G.T.; Rothenberg, M.E. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology 2017, 154, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Straumann, A.; Katzka, D.A. Diagnosis and Treatment of Eosinophilic Esophagitis. Gastroenterology 2018, 154, 346–359. [Google Scholar] [CrossRef]
- Wechsler, J.B.; Hirano, I. Biological therapies for eosinophilic gastrointestinal diseases. J. Allergy Clin. Immunol. 2018, 142, 24–31.e2. [Google Scholar] [CrossRef]
- Dellon, E.S. Management of refractory eosinophilic oesophagitis. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 479–490. [Google Scholar] [CrossRef]
- Bedell, A.; Taft, T.; Craven, M.R.; Guadagnoli, L.; Hirano, I.; Gonsalves, N. Impact on Health-Related Quality of Life in Adults with Eosinophilic Gastritis and Gastroenteritis: A Qualitative Assessment. Dig. Dis. Sci. 2018, 63, 1148–1157. [Google Scholar] [CrossRef]
- Available online: http://www.salute.gov.it/portale/nuovocoronavirus (accessed on 9 April 2020).
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Bezzio, C.; Saibeni, S.; Variola, A.; Allocca, M.; Massari, A.; Gerardi, V.; Casini, V.; Ricci, C.; Zingone, F.; Amato, A.; et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: An IG-IBD study. Gut 2020, 69, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Lukin, D.J.; Kumar, A.; Hajifathalian, K.; Sharaiha, R.Z.; Scherl, E.J.; Longman, R.S.; JRC Study Group; WCM-GI Study Group. Baseline Disease Activity and Steroid Therapy Stratify Risk of COVID-19 in Patients with Inflammatory Bowel Disease. Gastroenterology 2020. [Google Scholar] [CrossRef]
- Hanzel, J.; Ma, C.; Marshall, J.K.; Feagan, B.G.; Jairath, V. Managing Inflammatory Bowel Disease During COVID-19: Summary of Recommendations from Gastrointestinal Societies. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Geldsetzer, P. Knowledge and Perceptions of COVID-19 Among the General Public in the United States and the United Kingdom: A Cross-sectional Online Survey. Ann. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Molina-Infante, J.; Arias-Arias, Á.; Von Arnim, U.; Bredenoord, A.J.; Bussmann, C.; Dias, J.A.; Bove, M.; González-Cervera, J.; Larsson, H.; et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur. Gastroenterol. J. 2017, 5, 335–358. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Oouchi, S.; Fujisawa, T. Eosinophilic gastrointestinal diseases—Pathogenesis, diagnosis, and treatment. Allergol. Int. 2019, 68, 420–429. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Gutiérrez-Junquera, C.; Savarino, E.V.; Penagini, R.; Modolell, I.; Bartolo, O.; Prieto-García, A.; Mauro, A.; Alcedo, J.; Perello, A.; et al. Helicobacter Pylori Infection Does Not Protect Against Eosinophilic Esophagitis: Results From a Large Multicenter Case-Control Study. Am. J. Gastroenterol. 2018, 113, 972–979. [Google Scholar] [CrossRef]
- Roman, S.; Savarino, E.V.; Savarino, V.; Mion, F. Eosinophilic oesophagitis: From physiopathology to treatment. Dig. Liver Dis. 2013, 45, 871–878. [Google Scholar] [CrossRef]
- Frazzoni, M.; Penagini, R.; Frazzoni, L.; De Bortoli, N.; Mauro, A.; Tolone, S.; Bertani, H.; Conigliaro, R.; Savarino, E.V. Role of Reflux in the Pathogenesis of Eosinophilic Esophagitis—Comprehensive Appraisal with Off- and On-Ppi Impedance-Ph Monitoring. Am. J. Gastroenterol. 2019, 114, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Liacouras, C.A.; Wenner, W.J.; Brown, K.; Ruchelli, E. Primary Eosinophilic Esophagitis in Children: Successful Treatment with Oral Corticosteroids. J. Pediatr. Gastroenterol. Nutr. 1998, 26, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Laserna-Mendieta, E.J.; Casabona, S.; Savarino, E.V.; Perelló, A.; Pérez-Martínez, I.; Guagnozzi, D.; Barrio, J.; Guardiola, A.; Asensio, T.; De La Riva, S.; et al. Efficacy of Therapy for Eosinophilic Esophagitis in Real-World Practice. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Arias-Arias, Á.; Alcedo, J.; Garcia-Romero, R.; Casabona-Frances, S.; Prieto-García, A.; Modolell, I.; Gonzalez-Cordero, P.L.; Perez-Martinez, I.; Martin-Lorente, J.L.; et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: The 2-4-6 study. J. Allergy Clin. Immunol. 2018, 141, 1365–1372. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Blanchard, C.; Dawson, H.; Lucendo, A.; Mauro, A.; Ribi, C.; Safroneeva, E.; Savarino, E.V.; Penagini, R. Eosinophilic esophagitis: Latest insights from diagnosis to therapy. Ann. N. Y. Acad. Sci. 2018, 1434, 84–93. [Google Scholar] [CrossRef]
- Singh, S.; Facciorusso, A.; Dulai, P.S.; Jairath, V.; Sandborn, W.J. Comparative Risk of Serious Infections with Biologic and/or Immunosuppressive Therapy in Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 69–81.e3. [Google Scholar] [CrossRef]
- Savarino, V.; Dulbecco, P.; Savarino, E. Are proton pump inhibitors really so dangerous? Dig. Liver Dis. 2016, 48, 851–859. [Google Scholar] [CrossRef]
- Nussbaumer-Streit, B.; Mayr, V.; Dobrescu, A.I.; Chapman, A.; Persad, E.; Klerings, I.; Wagner, G.; Siebert, U.; Christof, C.; Zachariah, C.; et al. Quarantine alone or in combination with other public health measures to control COVID-19: A rapid review. Cochrane Database Syst. Rev. 2020, 4, CD013574. [Google Scholar] [CrossRef]
- Jnr, B.A. Use of Telemedicine and Virtual Care for Remote Treatment in Response to COVID-19 Pandemic. J. Med. Syst. 2020, 44, 13. [Google Scholar]
- Siniscalchi, M.; Zingone, F.; Savarino, E.V.; D’Odorico, A.; Ciacci, C. COVID-19 pandemic perception in adults with celiac disease: An impulse to implement the use of telemedicine. Dig. Liver Dis. 2020. [Google Scholar] [CrossRef]
- Safroneeva, E.; Coslovsky, M.; Kuehni, C.E.; Zwahlen, M.; Haas, N.A.; Panczak, R.; Taft, T.H.; Hirano, I.; Dellon, E.S.; Gonsalves, N.; et al. Eosinophilic oesophagitis: Relationship of quality of life with clinical, endoscopic and histological activity. Aliment. Pharmacol. Ther. 2015, 42, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Taft, T.; Zalewski, A.; Gonsalves, N.; Hirano, I. Prospective assessment of disease-specific quality of life in adults with eosinophilic esophagitis. Dis. Esophagus 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Taft, T.H.; Guadagnoli, L.; Edlynn, E. Anxiety and depression in eosinophilic esophagitis: A scoping review and recommendations for future research. J Asthma Allergy 2019, 12, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Ma, S.; Wang, Y.; Cai, Z.; Hu, J.; Wei, N.; Wu, J.; Du, H.; Chen, T.; Li, R.; et al. Factors Associated With Mental Health Outcomes Among Health Care Workers Exposed to Coronavirus Disease 2019. JAMA Netw. Open 2020, 3, e203976. [Google Scholar] [CrossRef]
- Kessler, R.C.; A McGonagle, K.; Zhao, S.; Nelson, C.B.; Hughes, M.; Eshleman, S.; Wittchen, H.U.; Kendler, K.S. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch. Gen. Psychiatry 1994, 51, 8–19. [Google Scholar] [CrossRef]
- Remes, O.; Brayne, C.; Van Der Linde, R.; LaFortune, L. A systematic review of reviews on the prevalence of anxiety disorders in adult populations. Brain Behav. 2016, 6, e00497. [Google Scholar] [CrossRef]
- Bjelland, I.; Krokstad, S.; Mykletun, A.; Dahl, A.A.; Tell, G.S.; Tambs, K. Does a higher educational level protect against anxiety and depression? The HUNT study. Soc. Sci. Med. 2008, 66, 1334–1345. [Google Scholar] [CrossRef]
- Kaleta, D.; Polanska, K.; Dziankowska-Zaborszczyk, E.; Hanke, W.; Drygas, W. Factors Influencing Self-perception of Health Status. Cent. Eur. J. Public Heal. 2009, 17, 122–127. [Google Scholar] [CrossRef]
- Schaefer, E.T.; Fitzgerald, J.F.; Molleston, J.P.; Croffie, J.M.B.; Pfefferkorn, M.D.; Corkins, M.R.; Lim, J.D.; Steiner, S.J.; Gupta, S.K. Comparison of Oral Prednisone and Topical Fluticasone in the Treatment of Eosinophilic Esophagitis: A Randomized Trial in Children. Clin. Gastroenterol. Hepatol. 2008, 6, 165–173. [Google Scholar] [CrossRef]
- Dellon, E.S.; Sheikh, A.; Speck, O.; Woodward, K.; Whitlow, A.B.; Hores, J.M.; Ivanovic, M.; Chau, A.; Woosley, J.T.; Madanick, R.D.; et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology 2012, 143, 321–324. [Google Scholar] [CrossRef]
- Miehlke, S.; Hruz, P.; Vieth, M.; Bussmann, C.; Von Arnim, U.; Bajbouj, M.; Schlag, C.; Madisch, A.; Fibbe, C.; Wittenburg, H.; et al. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut 2016, 65, 390–399. [Google Scholar] [CrossRef]
- Fardet, L.; Petersen, I.; Nazareth, I. Common Infections in Patients Prescribed Systemic Glucocorticoids in Primary Care: A Population-Based Cohort Study. PLoS Med. 2016, 13, e1002024. [Google Scholar] [CrossRef]
- Tinsley, A.; Navabi, S.; Williams, E.D.; Liu, G.; Kong, L.; Coates, M.D.; Clarke, K. Increased Risk of Influenza and Influenza-Related Complications Among 140,480 Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 369–376. [Google Scholar] [CrossRef]
- Ford, A.C.; Peyrin-Biroulet, L. Opportunistic Infections With Anti-Tumor Necrosis Factor-α Therapy in Inflammatory Bowel Disease: Meta-Analysis of Randomized Controlled Trials. Am. J. Gastroenterol. 2013, 108, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; HLH Across Speciality Collaboration. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- I Ritchie, A.; Singanayagam, A. Immunosuppression for hyperinflammation in COVID-19: A double-edged sword? Lancet 2020, 395, 1111. [Google Scholar] [CrossRef]
- Russell, C.D.; Millar, J.E.; Baillie, J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020, 395, 473–475. [Google Scholar] [CrossRef]
- Savarino, V.; Marabotto, E.; Furnari, M.; Zingone, F.; Zentilin, P.; Savarino, E. Latest insights into the hot question of proton pump inhibitor safety—A narrative review. Dig. Liver Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.F.; Yang, Y.-X.; Howden, C.W. Complications of Proton Pump Inhibitor Therapy. Gastroenterology 2017, 153, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Savarino, V.; Marabotto, E.; Zentilin, P.; Furnari, M.; Bodini, G.; De Maria, C.; Pellegatta, G.; Coppo, C.; Savarino, E.V. Proton pump inhibitors: Use and misuse in the clinical setting. Expert Rev. Clin. Pharmacol. 2018, 11, 1123–1134. [Google Scholar] [CrossRef]
- Gulmez, S.E.; Holm, A.; Frederiksen, H.; Jensen, T.G.; Pedersen, C.; Hallas, J. Use of Proton Pump Inhibitors and the Risk of Community-Acquired Pneumonia. Arch. Intern. Med. 2007, 167, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Laheij, R.J.; Sturkenboom, M.C.J.M.; Hassing, R.-J.; Dieleman, J.; Stricker, B.H.; Jansen, J.B.M.J. Risk of Community-Acquired Pneumonia and Use of Gastric Acid–Suppressive Drugs. JAMA 2004, 292, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Eom, C.-S.; Jeon, C.Y.; Lim, J.-W.; Cho, E.-G.; Park, S.M.; Lee, K.-S. Use of acid-suppressive drugs and risk of pneumonia: A systematic review and meta-analysis. Can. Med Assoc. J. 2010, 183, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Filion, K.B.; Chateau, D.; E Targownik, L.; Gershon, A.; Durand, M.; Tamim, H.; Teare, G.F.; Ravani, P.; Ernst, P.; Dormuth, C.R.; et al. Proton pump inhibitors and the risk of hospitalisation for community-acquired pneumonia: Replicated cohort studies with meta-analysis. Gut 2013, 63, 552–558. [Google Scholar] [CrossRef]
- Wang, C.-H.; Li, C.-H.; Hsieh, R.; Fan, C.-Y.; Hsu, T.-C.; Chang, W.-C.; Hsu, W.-T.; Lin, Y.-Y.; Lee, C.-C. Proton pump inhibitors therapy and the risk of pneumonia: A systematic review and meta-analysis of randomized controlled trials and observational studies. Expert Opin. Drug Saf. 2019, 18, 163–172. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2015, 65, 740–748. [Google Scholar] [CrossRef]
- Lo, W.-K.; Chan, W.W. Su2020 Proton Pump Inhibitor Use and the Risk of Small Intestinal Bacterial Overgrowth: A Meta-Analysis. Gastroenterology 2013, 11, 483–490. [Google Scholar] [CrossRef]
- Deshpande, A.; Pant, C.; Pasupuleti, V.; Rolston, D.D.; Jain, A.; Deshpande, N.; Thota, P.; Sferra, T.J.; Hernandez, A.V. Association Between Proton Pump Inhibitor Therapy and Clostridium difficile Infection in a Meta-Analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 225–233. [Google Scholar] [CrossRef]
- Kwok, C.S.; Arthur, A.K.; Anibueze, C.I.; Singh, S.; Cavallazzi, R.; Loke, Y.K.; Gupta, P.; Sinha, S.K.; Kochhar, R.; Debi, U. Corrigendum: Risk of Clostridium difficile Infection With Acid Suppressing Drugs and Antibiotics: Meta-Analysis. Am. J. Gastroenterol. 2012, 107, 1011–1019. [Google Scholar] [CrossRef]
- Janarthanan, S.; Ditah, I.; Adler, D.G.; Ehrinpreis, M.N. Clostridium difficile-Associated Diarrhea and Proton Pump Inhibitor Therapy: A Meta-Analysis. Am. J. Gastroenterol. 2012, 107, 1001–1010. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e3. [Google Scholar] [CrossRef] [PubMed]
- Coperture Della Vaccinazione Antinfluenzale in Italia—EpiCentro. Available online: https://www.epicentro.iss.it/influenza/coperture-vaccinali (accessed on 9 April 2020).
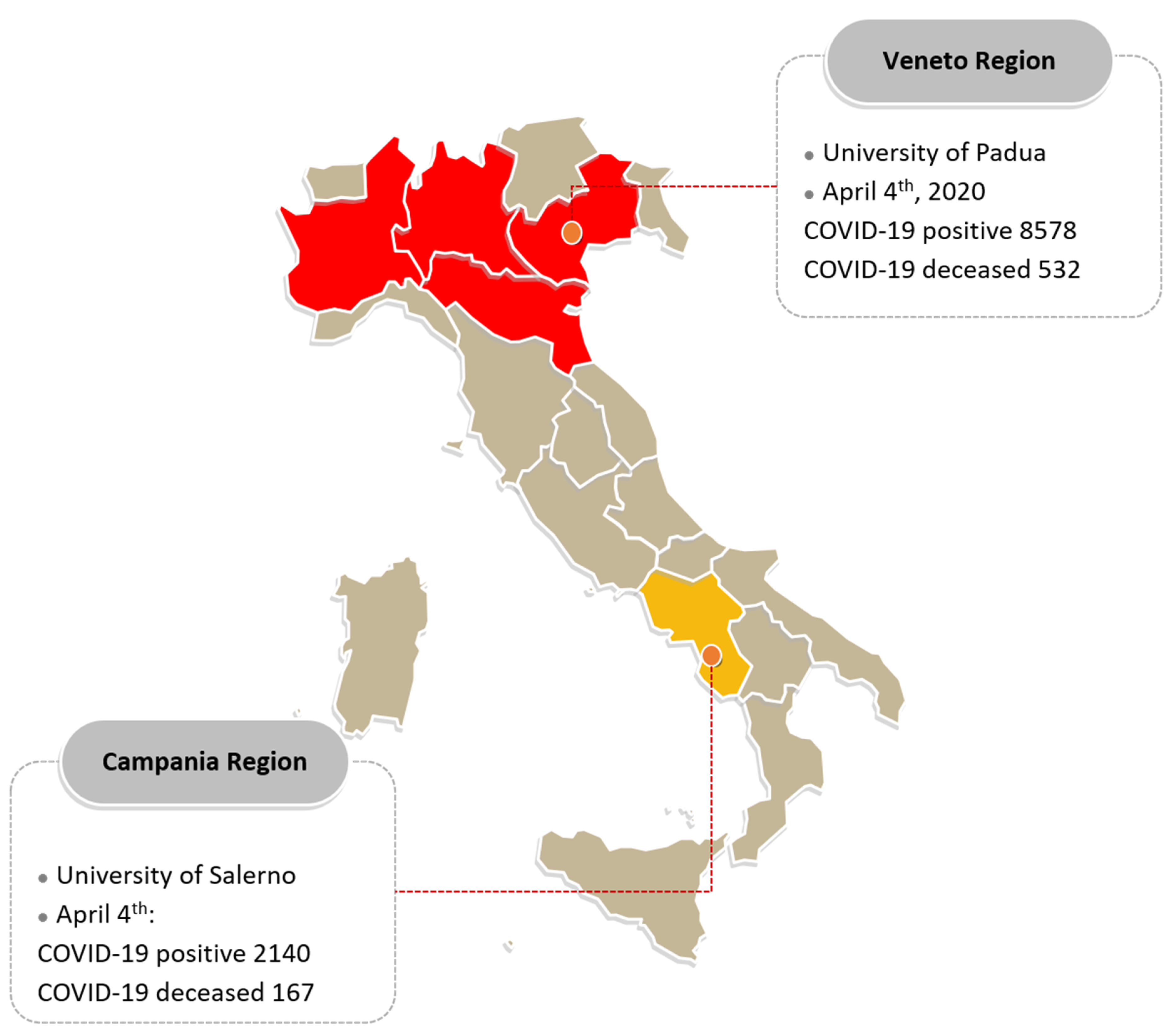

| Whole Population | Veneto Region | Campania Region | p-Value | |
|---|---|---|---|---|
| No. of questionnaires received /sent (%) | 102/157 (64.9%) | 82/132 (62.1%) | 20/25 (80.0%) | 0.11 |
| Female/Male, n (%) | 34/68 | 30/52 | 4/16 | 0.15 |
| (33.3%/66.7%) | (36.6%/63.4%) | (20.0%/80%) | ||
| Age groups, mean (SD) | 36.6 (12.4) | 35.5 (11.8) | 40.9 (14.1) | 0.08 |
| 51 (50.0) | 43 (52.3) | 8 (40.0) | 0.05 |
| 34 (33.3) | 29 (35.4) | 5 (20.0) | |
| 17 (16.7) | 10 (12.2) | 7 (35.0) | |
| Education degree, n (%) | ||||
| 12 (11.8) | 10 (12.2) | 2 (10) | 0.18 |
| 43 (42.1) | 31 (37.8) | 11 (60) | |
| 47 (46.1) | 41 (50) | 6 (30) | |
| Rural area, n (%) | 31 (34.4) | 27 (32.9) | 4 (20) | 0.26 |
| Urban area, n (%) | 71 (69.6) | 55 (67.1) | 16 (80) | |
| EGID phenotype, n (%) | ||||
| 89 (87.1) | 70 (85.4) | 19 (94.8) | 0.31 |
| 9 (8.9) | 9 (11.0) | 0 (0.0) | |
| 4 (4) | 3 (3.6) | 1 (5.2) | |
| Time from diagnosis, | ||||
| 75 (73.5) | 57 (69.5) | 18 (90) | 0.15 |
| 19 (18.6) | 17 (20.7) | 2 (10.0) | |
| 8 (7.4) | 8 (9.8) | 0 (0.0) | |
| Current therapy, n (%) | ||||
| 22 (21.6) | 10 (12.2) | 12 (60.0) | <0.001 |
| 55 (53.9) | 49 (59.7) | 6 (30.0) | |
| 48 (47.1) | 48 (58.5) | 0 (0.0) | |
| 10 (9.8) | 9 (11.0) | 1 (5.0) | |
| 2 (2.0) | 2 (2.4) | 0 (0.0) | |
| 2 (2.0) | 1 (1.2) | 1 (5.0) |
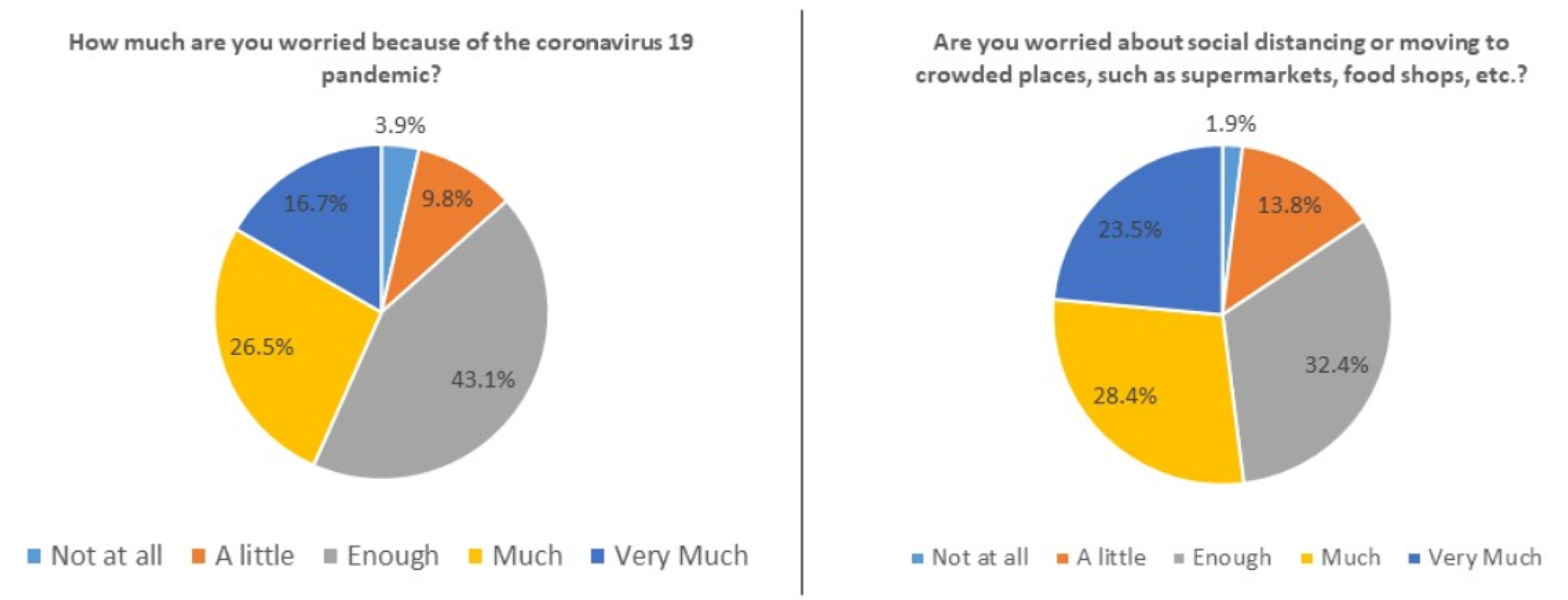
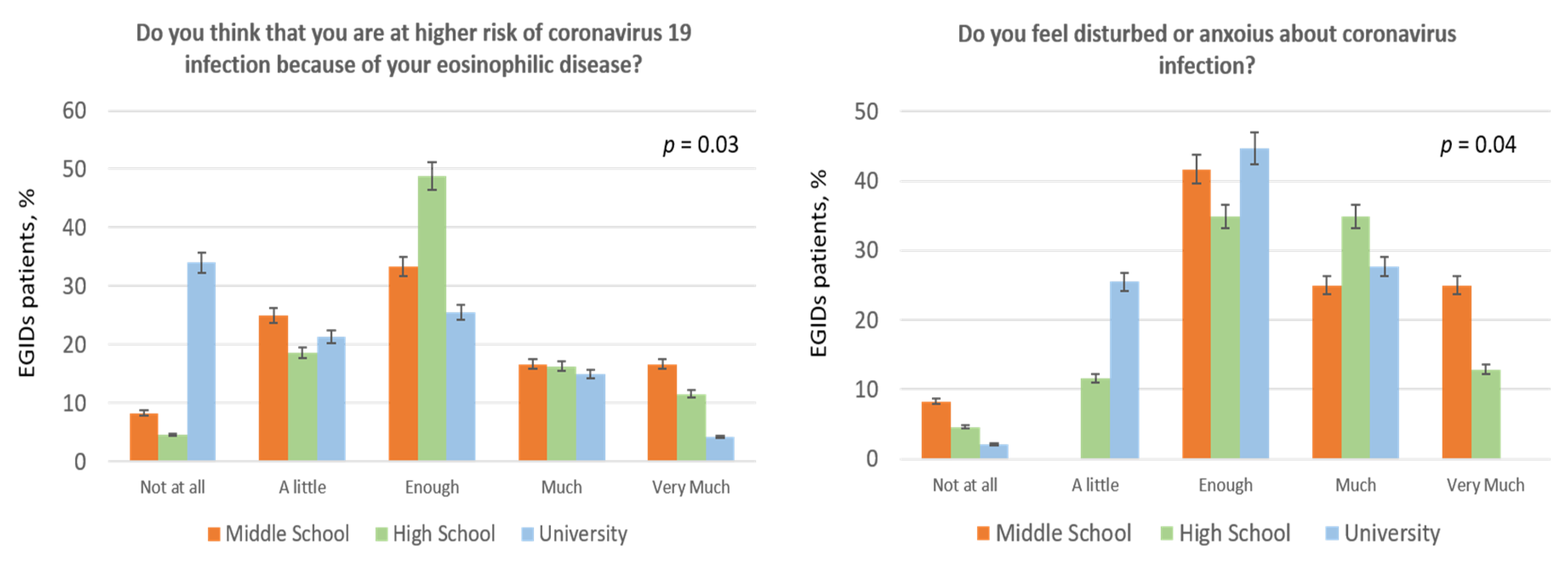
| Not at all | Little | Enough | Much | Very Much | |
|---|---|---|---|---|---|
| 1. How much are you worried because of the coronavirus 19 pandemic? | 3.9% | 9.8% | 43.1% | 26.5% | 16.7% |
| 2. Do you think that you are at higher risk of coronavirus 19 infection because of your eosinophilic disease? | 18.6% | 20.6% | 36.3% | 15.7% | 8.8% |
| 3. Are you worried about social distancing or moving to crowded places, such as supermarkets, food shops, hospitals? | 1.9% | 13.8% | 32.4% | 28.4% | 23.5% |
| 4. Do you think that coronavirus 19 information from social and mass media are excessive? | 31.4% | 37.2% | 21.6% | 8.8% | 1.0% |
| 5. Do you feel disturbed or anxious about coronavirus infection? | 3.9% | 16.7% | 40.2% | 30.4% | 8.8% |
| I would like to speak with doctors | No, I do not feel myself well followed-up | Yes, it is perfect | I am afraid. I cannot tell everything | ||
| 6. Do you agree with telemedicine consultations? | 8.8% | 1.9% | 87.4% | 1.9% | |
| No | Yes | Maybe | |||
| 7. Do you think that EGID renders you more susceptible to infection than general population? | 38.3% | 27.4% | 34.3% | ||
| 8. Are you afraid that pandemic reduces your care by physicians to less than it would be necessary? | 50.0% | 33.3% | 16.7% | ||
| 9. Are you reluctant to go to hospital, because of coronavirus infection? | 25.5% | 61.8% | 12.7% | ||
| 10. Do you think to be more susceptible to infection than general population because of your current therapy? | 47.0% | 11.8% | 41.2% | ||
| 11. Did you undergo seasonal flu vaccination? | 82.4% | 17.6% | N/A | ||
| 12. Would you like to undergo a coronavirus vaccination, when it will be available? | 6.9% | 46.1% | 47.0% | ||
| 13. Have you been diagnosed with COVID-19 infection (i.e., using nasopharyngeal swab)? | 93.1% | 0.0% | 6.9% * | ||
| 14. Have you been in contact with someone diagnosed with COVID-19 infection (i.e., using nasopharyngeal swab)? | 96.1% | 0.0% | 3.9% * | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savarino, E.V.; Iovino, P.; Santonicola, A.; Ghisa, M.; Laserra, G.; Barberio, B.; Maniero, D.; Lorenzon, G.; Ciacci, C.; Savarino, V.; et al. Clinical and Psychological Impact of COVID-19 Infection in Adult Patients with Eosinophilic Gastrointestinal Disorders during the SARS-CoV-2 Outbreak. J. Clin. Med. 2020, 9, 2011. https://doi.org/10.3390/jcm9062011
Savarino EV, Iovino P, Santonicola A, Ghisa M, Laserra G, Barberio B, Maniero D, Lorenzon G, Ciacci C, Savarino V, et al. Clinical and Psychological Impact of COVID-19 Infection in Adult Patients with Eosinophilic Gastrointestinal Disorders during the SARS-CoV-2 Outbreak. Journal of Clinical Medicine. 2020; 9(6):2011. https://doi.org/10.3390/jcm9062011
Chicago/Turabian StyleSavarino, Edoardo Vincenzo, Paola Iovino, Antonella Santonicola, Matteo Ghisa, Giorgio Laserra, Brigida Barberio, Daria Maniero, Greta Lorenzon, Carolina Ciacci, Vincenzo Savarino, and et al. 2020. "Clinical and Psychological Impact of COVID-19 Infection in Adult Patients with Eosinophilic Gastrointestinal Disorders during the SARS-CoV-2 Outbreak" Journal of Clinical Medicine 9, no. 6: 2011. https://doi.org/10.3390/jcm9062011
APA StyleSavarino, E. V., Iovino, P., Santonicola, A., Ghisa, M., Laserra, G., Barberio, B., Maniero, D., Lorenzon, G., Ciacci, C., Savarino, V., & Zingone, F. (2020). Clinical and Psychological Impact of COVID-19 Infection in Adult Patients with Eosinophilic Gastrointestinal Disorders during the SARS-CoV-2 Outbreak. Journal of Clinical Medicine, 9(6), 2011. https://doi.org/10.3390/jcm9062011








