Dominantly Inherited Hereditary Nonpolyposis Colorectal Cancer Not Caused by MMR Genes
Abstract
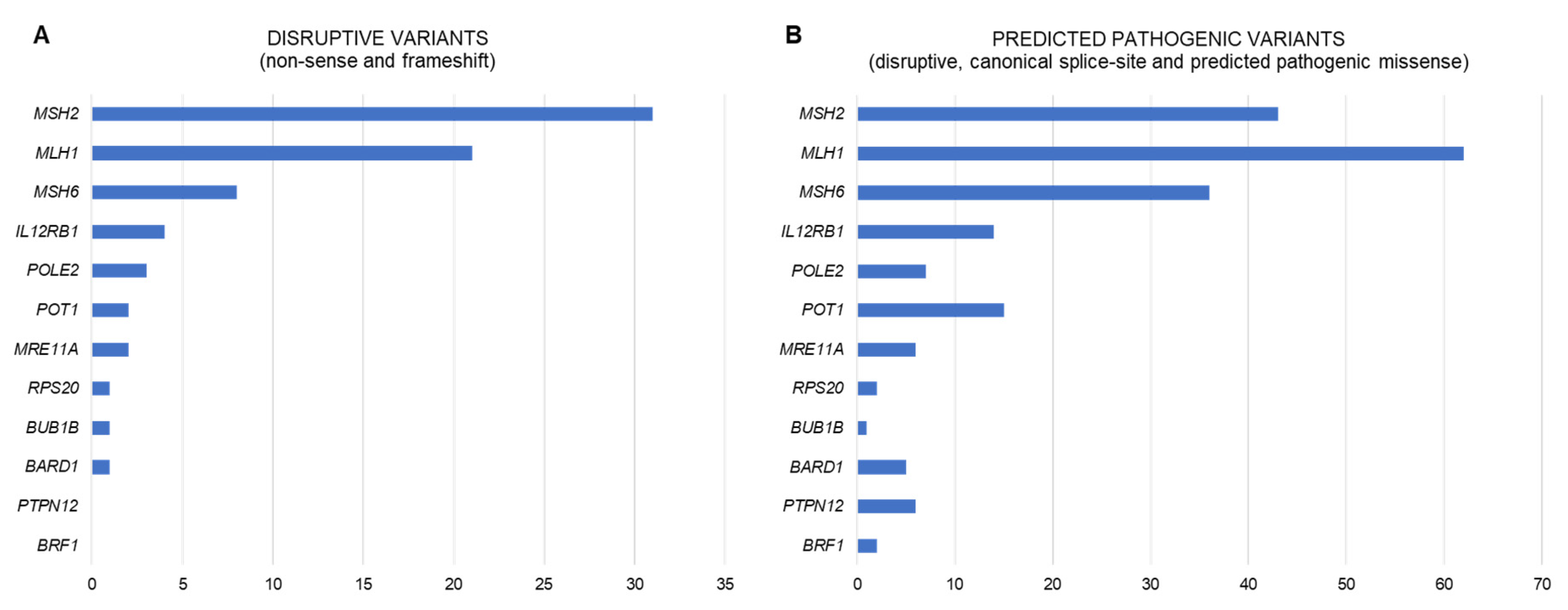
:1. Introduction
2. RPS20 Mutations as a Rare Cause of Hereditary Nonpolyposis Colorectal Cancer
3. Candidate Causal Genes for Mismatch Repair Proficient Hereditary Nonpolyposis Colorectal Cancer
3.1. DNA Damage Response
3.1.1. DNA Repair
Base Excision Repair
Nucleotide Excision Repair and MGMT
Double-Strand Break Repair
Fanconi Anemia Pathway
3.1.2. Telomere Maintenance
3.1.3. Cell Cycle—Checkpoint and Chromosome-Associated Proteins
3.2. DNA Replication, Transcription, and Translation
3.3. Wnt and TGF-beta Pathways
3.4. Additional Candidates
4. Non-CRC Hereditary Cancer Genes
5. Conclusions/Final Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frank, C.; Fallah, M.; Sundquist, J.; Hemminki, A.; Hemminki, K. Population Landscape of Familial Cancer. Sci. Rep. 2015, 5, 12891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, C.; Sundquist, J.; Yu, H.; Hemminki, A.; Hemminki, K. Concordant and discordant familial cancer: Familial risks, proportions and population impact. Int. J. Cancer 2017, 140, 1510–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeRycke, M.S.; Gunawardena, S.; Balcom, J.R.; Pickart, A.M.; Waltman, L.A.; French, A.J.; McDonnell, S.; Riska, S.M.; Fogarty, Z.C.; Larson, M.C.; et al. Targeted sequencing of 36 known or putative colorectal cancer susceptibility genes. Mol. Genet. Genom. Med. 2017, 5, 553–569. [Google Scholar] [CrossRef] [Green Version]
- Yurgelun, M.B.; Kulke, M.H.; Fuchs, C.S.; Allen, B.A.; Uno, H.; Hornick, J.L.; Ukaegbu, C.I.; Brais, L.K.; McNamara, P.G.; Mayer, R.J.; et al. Cancer Susceptibility Gene Mutations in Individuals With Colorectal Cancer. J. Clin. Oncol. 2017, 35, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- AlDubayan, S.H.; Giannakis, M.; Moore, N.D.; Han, G.C.; Reardon, B.; Hamada, T.; Mu, X.J.; Nishihara, R.; Qian, Z.; Liu, L.; et al. Inherited DNA-Repair Defects in Colorectal Cancer. Am. J. Hum. Genet. 2018, 102, 401–414. [Google Scholar] [CrossRef] [Green Version]
- You, Y.N.; Borras, E.; Chang, K.; Price, B.A.; Mork, M.; Chang, G.J.; Rodriguez-Bigas, M.A.; Bednarski, B.K.; Meric-Bernstam, F.; Vilar, E. Detection of Pathogenic Germline Variants Among Patients With Advanced Colorectal Cancer Undergoing Tumor Genomic Profiling for Precision Medicine. Dis. Colon Rectum 2019, 62, 429–437. [Google Scholar] [CrossRef]
- Valle, L. Mismatch Repair-Proficient Hereditary Nonpolyposis Colorectal Cancer. In Hereditary Colorectal Cancer: Genetic Basis and Clinical Implications; Valle, L., Gruber, S.B., Capellá, G., Eds.; Springer: Cham, Switzerland, 2018; pp. 55–66. [Google Scholar]
- Nieminen, T.T.; O’Donohue, M.F.; Wu, Y.; Lohi, H.; Scherer, S.W.; Paterson, A.D.; Ellonen, P.; Abdel-Rahman, W.M.; Valo, S.; Mecklin, J.P.; et al. Germline mutation of RPS20, encoding a ribosomal protein, causes predisposition to hereditary nonpolyposis colorectal carcinoma without DNA mismatch repair deficiency. Gastroenterology 2014, 147, 595–598.e95. [Google Scholar] [CrossRef] [Green Version]
- Broderick, P.; Dobbins, S.E.; Chubb, D.; Kinnersley, B.; Dunlop, M.G.; Tomlinson, I.; Houlston, R.S. Validation of Recently Proposed Colorectal Cancer Susceptibility Gene Variants in an Analysis of Families and Patients—A Systematic Review. Gastroenterology 2017, 152, 75–77.e74. [Google Scholar] [CrossRef] [Green Version]
- Belhadj, S.; Terradas, M.; Munoz-Torres, P.M.; Aiza, G.; Navarro, M.; Capellá, G.; Valle, L. Candidate genes for hereditary colorectal cancer: Mutational screening and systematic review. Hum. Mutat. 2020. [Google Scholar] [CrossRef]
- Thompson, B.A.; Snow, A.K.; Koptiuch, C.; Kohlmann, W.K.; Mooney, R.; Johnson, S.; Huff, C.D.; Yu, Y.; Teerlink, C.C.; Feng, B.J.; et al. A novel ribosomal protein S20 variant in a family with unexplained colorectal cancer and polyposis. Clin. Genet. 2020, 97, 943–944. [Google Scholar] [CrossRef]
- Arora, S.; Yan, H.; Cho, I.; Fan, H.Y.; Luo, B.; Gai, X.; Bodian, D.L.; Vockley, J.G.; Zhou, Y.; Handorf, E.A.; et al. Genetic Variants That Predispose to DNA Double-Strand Breaks in Lymphocytes From a Subset of Patients With Familial Colorectal Carcinomas. Gastroenterology 2015, 149, 1872–1883.e1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteban-Jurado, C.; Vila-Casadesus, M.; Garre, P.; Lozano, J.J.; Pristoupilova, A.; Beltran, S.; Munoz, J.; Ocana, T.; Balaguer, F.; Lopez-Ceron, M.; et al. Whole-exome sequencing identifies rare pathogenic variants in new predisposition genes for familial colorectal cancer. Genet. Med. 2015, 17, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reilly, N.M.; Novara, L.; Di Nicolantonio, F.; Bardelli, A. Exploiting DNA repair defects in colorectal cancer. Mol. Oncol. 2019, 13, 681–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurz, E.U.; Lees-Miller, S.P. DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair 2004, 3, 889–900. [Google Scholar] [CrossRef]
- Di Leonardo, A.; Linke, S.P.; Clarkin, K.; Wahl, G.M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994, 8, 2540–2551. [Google Scholar] [CrossRef] [Green Version]
- Bakkenist, C.J.; Kastan, M.B. Initiating cellular stress responses. Cell 2004, 118, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Kastan, M.B.; Onyekwere, O.; Sidransky, D.; Vogelstein, B.; Craig, R.W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991, 51, 6304–6311. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Al-Tassan, N.; Chmiel, N.H.; Maynard, J.; Fleming, N.; Livingston, A.L.; Williams, G.T.; Hodges, A.K.; Davies, D.R.; David, S.S.; Sampson, J.R.; et al. Inherited variants of MYH associated with somatic G:C→T:A mutations in colorectal tumors. Nat. Genet. 2002, 30, 227–232. [Google Scholar] [CrossRef]
- Weren, R.D.; Ligtenberg, M.J.; Kets, C.M.; de Voer, R.M.; Verwiel, E.T.; Spruijt, L.; van Zelst-Stams, W.A.; Jongmans, M.C.; Gilissen, C.; Hehir-Kwa, J.Y.; et al. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat. Genet. 2015, 47, 668–671. [Google Scholar] [CrossRef]
- Kim, I.J.; Ku, J.L.; Kang, H.C.; Park, J.H.; Yoon, K.A.; Shin, Y.; Park, H.W.; Jang, S.G.; Lim, S.K.; Han, S.Y.; et al. Mutational analysis of OGG1, MYH, MTH1 in FAP, HNPCC and sporadic colorectal cancer patients: R154H OGG1 polymorphism is associated with sporadic colorectal cancer patients. Hum. Genet. 2004, 115, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Farrington, S.M.; Tenesa, A.; Barnetson, R.; Wiltshire, A.; Prendergast, J.; Porteous, M.; Campbell, H.; Dunlop, M.G. Germline susceptibility to colorectal cancer due to base-excision repair gene defects. Am. J. Hum. Genet. 2005, 77, 112–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garre, P.; Briceno, V.; Xicola, R.M.; Doyle, B.J.; de la Hoya, M.; Sanz, J.; Llovet, P.; Pescador, P.; Puente, J.; Diaz-Rubio, E.; et al. Analysis of the oxidative damage repair genes NUDT1, OGG1, and MUTYH in patients from mismatch repair proficient HNPCC families (MSS-HNPCC). Clin. Cancer Res. 2011, 17, 1701–1712. [Google Scholar] [CrossRef] [Green Version]
- Morak, M.; Massdorf, T.; Sykora, H.; Kerscher, M.; Holinski-Feder, E. First evidence for digenic inheritance in hereditary colorectal cancer by mutations in the base excision repair genes. Eur. J. Cancer 2011, 47, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.G.; West, H.; Harris, R.; Idziaszczyk, S.; Maughan, T.S.; Kaplan, R.; Richman, S.; Quirke, P.; Seymour, M.; Moskvina, V.; et al. Role of the oxidative DNA damage repair gene OGG1 in colorectal tumorigenesis. J. Natl. Cancer Inst. 2013, 105, 1249–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinnersley, B.; Buch, S.; Castellvi-Bel, S.; Farrington, S.M.; Forsti, A.; Hampe, J.; Hemminki, K.; Hofstra, R.M.; Northwood, E.; Palles, C.; et al. Re: Role of the oxidative DNA damage repair gene OGG1 in colorectal tumorigenesis. J. Natl. Cancer Inst. 2014, 7, 10611. [Google Scholar] [CrossRef] [Green Version]
- Chubb, D.; Broderick, P.; Dobbins, S.E.; Frampton, M.; Kinnersley, B.; Penegar, S.; Price, A.; Ma, Y.P.; Sherborne, A.L.; Palles, C.; et al. Rare disruptive mutations and their contribution to the heritable risk of colorectal cancer. Nat. Commun. 2016, 7, 11883. [Google Scholar] [CrossRef]
- Mur, P.; Jemth, A.S.; Bevc, L.; Amaral, N.; Navarro, M.; Valdés-Mas, R.; Pons, T.; Aiza, G.; Urioste, M.; Valencia, A.; et al. Germline variation in the oxidative DNA repair genes NUDT1 and OGG1 is not associated with hereditary colorectal cancer or polyposis. Hum. Mutat. 2018, 39, 1214–1225. [Google Scholar] [CrossRef]
- Broderick, P.; Bagratuni, T.; Vijayakrishnan, J.; Lubbe, S.; Chandler, I.; Houlston, R.S. Evaluation of NTHL1, NEIL1, NEIL2, MPG, TDG, UNG and SMUG1 genes in familial colorectal cancer predisposition. BMC Cancer 2006, 6, 243. [Google Scholar] [CrossRef] [Green Version]
- Dallosso, A.R.; Dolwani, S.; Jones, N.; Jones, S.; Colley, J.; Maynard, J.; Idziaszczyk, S.; Humphreys, V.; Arnold, J.; Donaldson, A.; et al. Inherited predisposition to colorectal adenomas caused by multiple rare alleles of MUTYH but not OGG1, NUDT1, NTH1 or NEIL 1, 2 or 3. Gut 2008, 57, 1252–1255. [Google Scholar] [CrossRef]
- Martin-Morales, L.; Rofes, P.; Diaz-Rubio, E.; Llovet, P.; Lorca, V.; Bando, I.; Perez-Segura, P.; de la Hoya, M.; Garre, P.; Garcia-Barberan, V.; et al. Novel genetic mutations detected by multigene panel are associated with hereditary colorectal cancer predisposition. PLoS ONE 2018, 13, e0203885. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gay, M.; Franch-Expósito, S.; Arnau-Collell, C.; Park, S.; Supek, F.; Muñoz, J.; Bonjoch, L.; Gratacós-Mulleras, A.; Sánchez-Rojas, P.A.; Esteban-Jurado, C.; et al. Integrated Analysis of Germline and Tumor DNA Identifies New Candidate Genes Involved in Familial Colorectal Cancer. Cancer 2019, 11, 362. [Google Scholar] [CrossRef] [Green Version]
- Lind, G.E.; Thorstensen, L.; Løvig, T.; Meling, G.I.; Hamelin, R.; Rognum, T.O.; Esteller, M.; Lothe, R.A. A CpG island hypermethylation profile of primary colorectal carcinomas and colon cancer cell lines. Mol. Cancer 2004, 3, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Kondo, Y.; Rosner, G.L.; Xiao, L.; Hernandez, N.S.; Vilaythong, J.; Houlihan, P.S.; Krouse, R.S.; Prasad, A.R.; Einspahr, J.G.; et al. MGMT promoter methylation and field defect in sporadic colorectal cancer. J. Natl. Cancer Inst. 2005, 97, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, S.; Moutinho, C.; Mur, P.; Setien, F.; Llinàs-Arias, P.; Pérez-Salvia, M.; Pons, T.; Pineda, M.; Brunet, J.; Navarro, M.; et al. Germline variation in O6-Methylguanine-DNA Methyltransferase (MGMT) as cause of hereditary colorectal cancer. Cancer Lett. 2019, 447, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Gisselsson, D.; Pettersson, L.; Höglund, M.; Heidenblad, M.; Gorunova, L.; Wiegant, J.; Mertens, F.; Dal Cin, P.; Mitelman, F.; Mandahl, N. Chromosomal breakage-fusion-bridge events cause genetic intratumor heterogeneity. Proc. Natl. Acad. Sci. USA. 2000, 97, 5357–5362. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Rahman, W.M.; Ollikainen, M.; Kariola, R.; Järvinen, H.J.; Mecklin, J.P.; Nyström-Lahti, M.; Knuutila, S.; Peltomäki, P. Comprehensive characterization of HNPCC-related colorectal cancers reveals striking molecular features in families with no germline mismatch repair gene mutations. Oncogene 2005, 24, 1542–1551. [Google Scholar] [CrossRef] [Green Version]
- Bellido, F.; Pineda, M.; Sanz-Pamplona, R.; Navarro, M.; Nadal, M.; Lázaro, C.; Blanco, I.; Moreno, V.; Capellá, G.; Valle, L. Comprehensive molecular characterisation of hereditary non-polyposis colorectal tumours with mismatch repair proficiency. Eur. J. Cancer 2014, 50, 1964–1972. [Google Scholar] [CrossRef]
- Ihara, K.; Yamaguchi, S.; Ueno, N.; Tani, Y.; Shida, Y.; Ogata, H.; Domeki, Y.; Okamoto, K.; Nakajima, M.; Sasaki, K.; et al. Expression of DNA double-strand break repair proteins predicts the response and prognosis of colorectal cancer patients undergoing oxaliplatin-based chemotherapy. Oncol. Rep. 2016, 35, 1349–1355. [Google Scholar] [CrossRef] [Green Version]
- Situ, Y.; Chung, L.; Lee, C.S.; Ho, V. MRN (MRE11-RAD50-NBS1) Complex in Human Cancer and Prognostic Implications in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 816. [Google Scholar] [CrossRef] [Green Version]
- Gruber, S.B.; Ellis, N.A.; Scott, K.K.; Almog, R.; Kolachana, P.; Bonner, J.D.; Kirchhoff, T.; Tomsho, L.P.; Nafa, K.; Pierce, H.; et al. BLM heterozygosity and the risk of colorectal cancer. Science 2002, 297, 2013. [Google Scholar] [CrossRef] [PubMed]
- Sokolenko, A.P.; Iyevleva, A.G.; Preobrazhenskaya, E.V.; Mitiushkina, N.V.; Abysheva, S.N.; Suspitsin, E.N.; Kuligina, E.S.; Gorodnova, T.V.; Pfeifer, W.; Togo, A.V.; et al. High prevalence and breast cancer predisposing role of the BLM c.1642 C>T (Q548X) mutation in Russia. Int. J. Cancer 2012, 130, 2867–2873. [Google Scholar] [CrossRef]
- Thompson, E.R.; Doyle, M.A.; Ryland, G.L.; Rowley, S.M.; Choong, D.Y.; Tothill, R.W.; Thorne, H.; Barnes, D.R.; Li, J.; Ellul, J.; et al. Exome sequencing identifies rare deleterious mutations in DNA repair genes FANCC and BLM as potential breast cancer susceptibility alleles. PLoS Genet. 2012, 8, e1002894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prokofyeva, D.; Bogdanova, N.; Dubrowinskaja, N.; Bermisheva, M.; Takhirova, Z.; Antonenkova, N.; Turmanov, N.; Datsyuk, I.; Gantsev, S.; Christiansen, H.; et al. Nonsense mutation p.Q548X in BLM, the gene mutated in Bloom’s syndrome, is associated with breast cancer in Slavic populations. Breast Cancer Res. Treat. 2013, 137, 533–539. [Google Scholar] [CrossRef] [PubMed]
- De Voer, R.M.; Hahn, M.M.; Mensenkamp, A.R.; Hoischen, A.; Gilissen, C.; Henkes, A.; Spruijt, L.; van Zelst-Stams, W.A.; Kets, C.M.; Verwiel, E.T.; et al. Deleterious Germline BLM Mutations and the Risk for Early-onset Colorectal Cancer. Sci. Rep. 2015, 5, 14060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, E.M.; Halley, N.S.; Gimenez, T.M.; Rangel-Santos, A.; Azambuja, A.M.; Brumatti, M.; Pereira, P.L.; Vince, C.S.; Giorgi, R.R.; Bendit, I.; et al. BLM germline and somatic PKMYT1 and AHCY mutations: Genetic variations beyond MYCN and prognosis in neuroblastoma. Med. Hypotheses 2016, 97, 22–25. [Google Scholar] [CrossRef]
- Raskin, L.; Guo, Y.; Du, L.; Clendenning, M.; Rosty, C.; Lindor, N.M.; Gruber, S.B.; Buchanan, D.D.; Colon Cancer Family Registry (CCFR). Targeted sequencing of established and candidate colorectal cancer genes in the Colon Cancer Family Registry Cohort. Oncotarget 2017, 8, 93450–93463. [Google Scholar] [CrossRef] [Green Version]
- Schayek, H.; Laitman, Y.; Katz, L.H.; Pras, E.; Ries-Levavi, L.; Barak, F.; Friedman, E. Colorectal and Endometrial Cancer Risk and Age at Diagnosis in BLMAsh Mutation Carriers. Isr. Med. Assoc. J. 2017, 19, 365–367. [Google Scholar]
- Walker, L.C.; Pearson, J.F.; Wiggins, G.A.; Giles, G.G.; Hopper, J.L.; Southey, M.C. Increased genomic burden of germline copy number variants is associated with early onset breast cancer: Australian breast cancer family registry. Breast Cancer Res. 2017, 19, 30. [Google Scholar] [CrossRef] [Green Version]
- Cleary, S.P.; Zhang, W.; Di Nicola, N.; Aronson, M.; Aube, J.; Steinman, A.; Haddad, R.; Redston, M.; Gallinger, S.; Narod, S.A.; et al. Heterozygosity for the BLM(Ash) mutation and cancer risk. Cancer Res. 2003, 63, 1769–1771. [Google Scholar]
- Baris, H.N.; Kedar, I.; Halpern, G.J.; Shohat, T.; Magal, N.; Ludman, M.D.; Shohat, M. Prevalence of breast and colorectal cancer in Ashkenazi Jewish carriers of Fanconi anemia and Bloom syndrome. Isr. Med. Assoc. J. 2007, 9, 847–850. [Google Scholar]
- Antczak, A.; Kluźniak, W.; Wokołorczyk, D.; Kashyap, A.; Jakubowska, A.; Gronwald, J.; Huzarski, T.; Byrski, T.; Dębniak, T.; Masojć, B.; et al. A common nonsense mutation of the BLM gene and prostate cancer risk and survival. Gene 2013, 532, 173–176. [Google Scholar] [CrossRef]
- Laitman, Y.; Boker-Keinan, L.; Berkenstadt, M.; Liphsitz, I.; Weissglas-Volkov, D.; Ries-Levavi, L.; Sarouk, I.; Pras, E.; Friedman, E. The risk for developing cancer in Israeli ATM, BLM, and FANCC heterozygous mutation carriers. Cancer Genet. 2016, 209, 70–74. [Google Scholar] [CrossRef]
- De Voer, R.M.; Hahn, M.M.; Weren, R.D.; Mensenkamp, A.R.; Gilissen, C.; van Zelst-Stams, W.A.; Spruijt, L.; Kets, C.M.; Zhang, J.; Venselaar, H.; et al. Identification of Novel Candidate Genes for Early-Onset Colorectal Cancer Susceptibility. PLoS Genet. 2016, 12, e1005880. [Google Scholar] [CrossRef]
- Bellido, F.; Sowada, N.; Mur, P.; Lazaro, C.; Pons, T.; Valdes-Mas, R.; Pineda, M.; Aiza, G.; Iglesias, S.; Soto, J.L.; et al. Association Between Germline Mutations in BRF1, a Subunit of the RNA Polymerase III Transcription Complex, and Hereditary Colorectal Cancer. Gastroenterology 2018, 154, 181–194–e20. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, Y.; Halpern, N.; Hubert, A.; Adler, S.N.; Cohen, S.; Plesser-Duvdevani, M.; Pappo, O.; Shaag, A.; Meiner, V. Mutated MCM9 is associated with predisposition to hereditary mixed polyposis and colorectal cancer in addition to primary ovarian failure. Cancer Genet. 2015, 208, 621–624. [Google Scholar] [CrossRef]
- Terradas, M.; Munoz-Torres, P.M.; Belhadj, S.; Aiza, G.; Navarro, M.; Brunet, J.; Capellá, G.; Valle, L. Contribution to colonic polyposis of recently proposed predisposing genes and assessment of the prevalence of NTHL1- and MSH3-associated polyposes. Hum. Mutat. 2019, 40, 1910–1923. [Google Scholar] [CrossRef]
- Ciavarella, M.; Miccoli, S.; Prossomariti, A.; Pippucci, T.; Bonora, E.; Buscherini, F.; Palombo, F.; Zuntini, R.; Balbi, T.; Ceccarelli, C.; et al. Somatic APC mosaicism and oligogenic inheritance in genetically unsolved colorectal adenomatous polyposis patients. Eur. J. Hum. Genet. 2018, 26, 387–395. [Google Scholar] [CrossRef]
- Garre, P.; Martin, L.; Sanz, J.; Romero, A.; Tosar, A.; Bando, I.; Llovet, P.; Diaque, P.; Garcia-Paredes, B.; Diaz-Rubio, E.; et al. BRCA2 gene: A candidate for clinical testing in familial colorectal cancer type X. Clin. Genet. 2015, 87, 582–587. [Google Scholar] [CrossRef]
- Esteban-Jurado, C.; Franch-Exposito, S.; Munoz, J.; Ocana, T.; Carballal, S.; Lopez-Ceron, M.; Cuatrecasas, M.; Vila-Casadesus, M.; Lozano, J.J.; Serra, E.; et al. The Fanconi anemia DNA damage repair pathway in the spotlight for germline predisposition to colorectal cancer. Eur. J. Hum. Genet. 2016, 24, 1501–1505. [Google Scholar] [CrossRef]
- Calvete, O.; Martinez, P.; Garcia-Pavia, P.; Benitez-Buelga, C.; Paumard-Hernández, B.; Fernandez, V.; Dominguez, F.; Salas, C.; Romero-Laorden, N.; Garcia-Donas, J.; et al. A mutation in the POT1 gene is responsible for cardiac angiosarcoma in TP53-negative Li-Fraumeni-like families. Nat. Commun. 2015, 6, 8383. [Google Scholar] [CrossRef] [Green Version]
- Calvete, O.; Garcia-Pavia, P.; Domínguez, F.; Bougeard, G.; Kunze, K.; Braeuninger, A.; Teule, A.; Lasa, A.; Ramón, Y.; Cajal, T.; et al. The wide spectrum of POT1 gene variants correlates with multiple cancer types. Eur. J. Hum. Genet. 2017, 25, 1278–1281. [Google Scholar] [CrossRef] [Green Version]
- Bainbridge, M.N.; Armstrong, G.N.; Gramatges, M.M.; Bertuch, A.A.; Jhangiani, S.N.; Doddapaneni, H.; Lewis, L.; Tombrello, J.; Tsavachidis, S.; Liu, Y.; et al. Germline mutations in shelterin complex genes are associated with familial glioma. J. Natl. Cancer Inst. 2015, 107, 384. [Google Scholar] [CrossRef]
- Müller, C.; Krunic, M.; Wendt, J.; von Haeseler, A.; Okamoto, I. Germline Variants in the POT1-Gene in High-Risk Melanoma Patients in Austria. G3 (Bethesda) 2018, 8, 1475–1480. [Google Scholar] [CrossRef] [Green Version]
- McMaster, M.L.; Sun, C.; Landi, M.T.; Savage, S.A.; Rotunno, M.; Yang, X.R.; Jones, K.; Vogt, A.; Hutchinson, A.; Zhu, B.; et al. Germline mutations in Protection of Telomeres 1 in two families with Hodgkin lymphoma. Br. J. Haematol. 2018, 181, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Speedy, H.E.; Kinnersley, B.; Chubb, D.; Broderick, P.; Law, P.J.; Litchfield, K.; Jayne, S.; Dyer, M.J.S.; Dearden, C.; Follows, G.A.; et al. Germ line mutations in shelterin complex genes are associated with familial chronic lymphocytic leukemia. Blood 2016, 128, 2319–2326. [Google Scholar] [CrossRef] [Green Version]
- De Voer, R.M.; Geurts van Kessel, A.; Weren, R.D.; Ligtenberg, M.J.; Smeets, D.; Fu, L.; Vreede, L.; Kamping, E.J.; Verwiel, E.T.; Hahn, M.M.; et al. Germline mutations in the spindle assembly checkpoint genes BUB1 and BUB3 are risk factors for colorectal cancer. Gastroenterology 2013, 145, 544–547. [Google Scholar] [CrossRef]
- Mur, P.; De Voer, R.M.; Olivera-Salguero, R.; Rodriguez-Perales, S.; Pons, T.; Setien, F.; Aiza, G.; Valdes-Mas, R.; Bertini, A.; Pineda, M.; et al. Germline mutations in the spindle assembly checkpoint genes BUB1 and BUB3 are infrequent in familial colorectal cancer and polyposis. Mol. Cancer 2018, 17, 23. [Google Scholar] [CrossRef]
- Rio Frio, T.; Lavoie, J.; Hamel, N.; Geyer, F.C.; Kushner, Y.B.; Novak, D.J.; Wark, L.; Capelli, C.; Reis-Filho, J.S.; Mai, S.; et al. Homozygous BUB1B mutation and susceptibility to gastrointestinal neoplasia. N. Engl. J. Med. 2010, 363, 2628–2637. [Google Scholar] [CrossRef] [Green Version]
- DeRycke, M.S.; Gunawardena, S.R.; Middha, S.; Asmann, Y.W.; Schaid, D.J.; McDonnell, S.K.; Riska, S.M.; Eckloff, B.W.; Cunningham, J.M.; Fridley, B.L.; et al. Identification of novel variants in colorectal cancer families by high-throughput exome sequencing. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1239–1251. [Google Scholar] [CrossRef] [Green Version]
- Hahn, M.M.; Vreede, L.; Bemelmans, S.A.; van der Looij, E.; van Kessel, A.G.; Schackert, H.K.; Ligtenberg, M.J.; Hoogerbrugge, N.; Kuiper, R.P.; de Voer, R.M. Prevalence of germline mutations in the spindle assembly checkpoint gene BUB1B in individuals with early-onset colorectal cancer. Genes Chromosomes Cancer 2016, 55, 855–863. [Google Scholar] [CrossRef]
- Cicek, M.S.; Cunningham, J.M.; Fridley, B.L.; Serie, D.J.; Bamlet, W.R.; Diergaarde, B.; Haile, R.W.; Le Marchand, L.; Krontiris, T.G.; Younghusband, H.B.; et al. Colorectal cancer linkage on chromosomes 4q21, 8q13, 12q24, and 15q22. PLoS ONE 2012, 7, e38175. [Google Scholar] [CrossRef]
- Tanskanen, T.; Gylfe, A.E.; Katainen, R.; Taipale, M.; Renkonen-Sinisalo, L.; Jarvinen, H.; Mecklin, J.P.; Bohm, J.; Kilpivaara, O.; Pitkanen, E.; et al. Systematic search for rare variants in Finnish early-onset colorectal cancer patients. Cancer Genet. 2015, 208, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Spier, I.; Holzapfel, S.; Altmuller, J.; Zhao, B.; Horpaopan, S.; Vogt, S.; Chen, S.; Morak, M.; Raeder, S.; Kayser, K.; et al. Frequency and phenotypic spectrum of germline mutations in POLE and seven other polymerase genes in 266 patients with colorectal adenomas and carcinomas. Int. J. Cancer 2015, 137, 320–331. [Google Scholar] [CrossRef]
- Rogers, R.F.; Walton, M.I.; Cherry, D.L.; Collins, I.; Clarke, P.A.; Garrett, M.D.; Workman, P. CHK1 Inhibition Is Synthetically Lethal with Loss of B-Family DNA Polymerase Function in Human Lung and Colorectal Cancer Cells. Cancer Res. 2020, 80, 1735–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gylfe, A.E.; Katainen, R.; Kondelin, J.; Tanskanen, T.; Cajuso, T.; Hanninen, U.; Taipale, J.; Taipale, M.; Renkonen-Sinisalo, L.; Jarvinen, H.; et al. Eleven candidate susceptibility genes for common familial colorectal cancer. PLoS Genet. 2013, 9, e1003876. [Google Scholar] [CrossRef]
- Valle, L.; Vilar, E.; Tavtigian, S.V.; Stoffel, E.M. Genetic predisposition to colorectal cancer: Syndromes, genes, classification of genetic variants and implications for precision medicine. J. Pathol. 2019, 247, 574–588. [Google Scholar] [CrossRef]
- Martin-Morales, L.; Feldman, M.; Vershinin, Z.; Garre, P.; Caldes, T.; Levy, D. SETD6 dominant negative mutation in familial colorectal cancer type X. Hum. Mol. Genet. 2017, 26, 4481–4493. [Google Scholar] [CrossRef]
- Bonjoch, L.; Franch-Expósito, S.; Garre, P.; Belhadj, S.; Muñoz, J.; Arnau-Collell, C.; Díaz-Gay, M.; Gratacós-Mulleras, A.; Raimondi, G.; Esteban-Jurado, C.; et al. GERMLINE MUTATIONS IN FAF1 ARE ASSOCIATED WITH HEREDITARY COLORECTAL CANCER. Gastroenterology 2020, S0016-5085(20)30336-X. [Google Scholar] [CrossRef]
- Wei, C.; Peng, B.; Han, Y.; Chen, W.V.; Rother, J.; Tomlinson, G.E.; Boland, C.R.; Chaussabel, D.; Frazier, M.L.; Amos, C.I. Mutations of HNRNPA0 and WIF1 predispose members of a large family to multiple cancers. Fam. Cancer 2015, 14, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Coissieux, M.M.; Tomsic, J.; Castets, M.; Hampel, H.; Tuupanen, S.; Andrieu, N.; Comeras, I.; Drouet, Y.; Lasset, C.; Liyanarachchi, S.; et al. Variants in the netrin-1 receptor UNC5C prevent apoptosis and increase risk of familial colorectal cancer. Gastroenterology 2011, 141, 2039–2046. [Google Scholar] [CrossRef] [Green Version]
- Grady, W.M. Making the case for DCC and UNC5C as tumor-suppressor genes in the colon. Gastroenterology 2007, 133, 2045–2049. [Google Scholar] [CrossRef]
- Mazelin, L.; Bernet, A.; Bonod-Bidaud, C.; Pays, L.; Arnaud, S.; Gespach, C.; Bredesen, D.E.; Scoazec, J.Y.; Mehlen, P. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature 2004, 431, 80–84. [Google Scholar] [CrossRef]
- Küry, S.; Garrec, C.; Airaud, F.; Breheret, F.; Guibert, V.; Frenard, C.; Jiao, S.; Bonneau, D.; Berthet, P.; Bossard, C.; et al. Evaluation of the colorectal cancer risk conferred by rare UNC5C alleles. World J. Gastroenterol. 2014, 20, 204–213. [Google Scholar] [CrossRef]
- Mur, P.; Elena, S.C.; Ausso, S.; Aiza, G.; Rafael, V.M.; Pineda, M.; Navarro, M.; Brunet, J.; Urioste, M.; Lazaro, C.; et al. Scarce evidence of the causal role of germline mutations in UNC5C in hereditary colorectal cancer and polyposis. Sci. Rep. 2016, 6, 20697. [Google Scholar] [CrossRef]
- Schulz, E.; Klampfl, P.; Holzapfel, S.; Janecke, A.R.; Ulz, P.; Renner, W.; Kashofer, K.; Nojima, S.; Leitner, A.; Zebisch, A.; et al. Germline variants in the SEMA4A gene predispose to familial colorectal cancer type X. Nat. Commun. 2004, 5, 5191. [Google Scholar] [CrossRef] [Green Version]
- Kinnersley, B.; Chubb, D.; Dobbins, S.E.; Frampton, M.; Buch, S.; Timofeeva, M.N.; Castellvi-Bel, S.; Farrington, S.M.; Forsti, A.; Hampe, J.; et al. Correspondence: SEMA4A variation and risk of colorectal cancer. Nat. Commun. 2016, 7, 10611. [Google Scholar] [CrossRef] [Green Version]
- Vogelaar, I.P.; van der Post, R.S.; van de Vosse, E.; van Krieken, J.H.; Hoogerbrugge, N.; Ligtenberg, M.J.; Gómez García, E. Gastric cancer in three relatives of a patient with a biallelic IL12RB1 mutation. Fam. Cancer 2015, 14, 89–94. [Google Scholar] [CrossRef]
- Guo, J.M.; Zhang, Y.; Cheng, L.; Iwasaki, H.; Wang, H.; Kubota, T.; Tachibana, K.; Narimatsu, H. Molecular cloning and characterization of a novel member of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family, pp-GalNAc-T12. FEBS Lett. 2002, 524, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.M.; Chen, H.L.; Wang, G.M.; Zhang, Y.K.; Narimatsu, H. Expression of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase-12 in gastric and colonic cancer cell lines and in human colorectal cancer. Oncology 2004, 67, 271–276. [Google Scholar] [CrossRef]
- Wiesner, G.L.; Daley, D.; Lewis, S.; Ticknor, C.; Platzer, P.; Lutterbaugh, J.; MacMillen, M.; Baliner, B.; Willis, J.; Elston, R.C.; et al. A subset of familial colorectal neoplasia kindreds linked to chromosome 9q22.2-31.2. Proc. Natl. Acad. Sci. USA 2003, 100, 12961–12965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoglund, J.; Djureinovic, T.; Zhou, X.L.; Vandrovcova, J.; Renkonen, E.; Iselius, L.; Bisgaard, M.L.; Peltomaki, P.; Lindblom, A. Linkage analysis in a large Swedish family supports the presence of a susceptibility locus for adenoma and colorectal cancer on chromosome 9q22.32-31.1. J. Med. Genet. 2006, 43, e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, Z.E.; Carvajal-Carmona, L.G.; Barclay, E.; Gorman, M.; Martin, L.; Wood, W.; Rowan, A.; Donohue, C.; Spain, S.; Jaeger, E.; et al. Evidence of linkage to chromosome 9q22.33 in colorectal cancer kindreds from the United Kingdom. Cancer Res. 2006, 66, 5003–5006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray-McGuire, C.; Guda, K.; Adrianto, I.; Lin, C.P.; Natale, L.; Potter, J.D.; Newcomb, P.; Poole, E.M.; Ulrich, C.M.; Lindor, N.; et al. Confirmation of linkage to and localization of familial colon cancer risk haplotype on chromosome 9q22. Cancer Res. 2010, 70, 5409–5418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guda, K.; Moinova, H.; He, J.; Jamison, O.; Ravi, L.; Natale, L.; Lutterbaugh, J.; Lawrence, E.; Lewis, S.; Willson, J.K.; et al. Inactivating germ-line and somatic mutations in polypeptide N-acetylgalactosaminyltransferase 12 in human colon cancers. Proc. Natl. Acad. Sci. USA. 2009, 106, 12921–12925. [Google Scholar] [CrossRef] [Green Version]
- Clarke, E.; Green, R.C.; Green, J.S.; Mahoney, K.; Parfrey, P.S.; Younghusband, H.B.; Woods, M.O. Inherited deleterious variants in GALNT12 are associated with CRC susceptibility. Hum. Mutat. 2012, 33, 1056–1058. [Google Scholar] [CrossRef]
- Segui, N.; Pineda, M.; Navarro, M.; Lazaro, C.; Brunet, J.; Infante, M.; Duran, M.; Soto, J.L.; Blanco, I.; Capella, G.; et al. GALNT12 is not a major contributor of familial colorectal cancer type X. Hum. Mutat. 2014, 35, 50–52. [Google Scholar] [CrossRef]
- Venkatachalam, R.; Ligtenberg, M.J.; Hoogerbrugge, N.; Schackert, H.K.; Gorgens, H.; Hahn, M.M.; Kamping, E.J.; Vreede, L.; Hoenselaar, E.; van der Looij, E.; et al. Germline epigenetic silencing of the tumor suppressor gene PTPRJ in early-onset familial colorectal cancer. Gastroenterology 2010, 139, 2221–2224. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, R.; Verwiel, E.T.; Kamping, E.J.; Hoenselaar, E.; Gorgens, H.; Schackert, H.K.; van Krieken, J.H.; Ligtenberg, M.J.; Hoogerbrugge, N.; van Kessel, A.G.; et al. Identification of candidate predisposing copy number variants in familial and early-onset colorectal cancer patients. Int. J. Cancer 2011, 129, 1635–1642. [Google Scholar] [CrossRef]
- Weren, R.D.; Venkatachalam, R.; Cazier, J.B.; Farin, H.F.; Kets, C.M.; de Voer, R.M.; Vreede, L.; Verwiel, E.T.; van Asseldonk, M.; Kamping, E.J.; et al. Germline deletions in the tumour suppressor gene FOCAD are associated with polyposis and colorectal cancer development. J. Pathol. 2015, 236, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Pearlman, R.; Frankel, W.L.; Swanson, B.; Zhao, W.; Yilmaz, A.; Miller, K.; Bacher, J.; Bigley, C.; Nelsen, L.; Goodfellow, P.J.; et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol. 2017, 3, 464–471. [Google Scholar] [CrossRef]
- Kirchhoff, T.; Satagopan, J.M.; Kauff, N.D.; Huang, H.; Kolachana, P.; Palmer, C.; Rapaport, H.; Nafa, K.; Ellis, N.A.; Offit, K. Frequency of BRCA1 and BRCA2 mutations in unselected Ashkenazi Jewish patients with colorectal cancer. J. Natl. Cancer Inst. 2004, 96, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Niell, B.L.; Rennert, G.; Bonner, J.D.; Almog, R.; Tomsho, L.P.; Gruber, S.B. BRCA1 and BRCA2 founder mutations and the risk of colorectal cancer. J. Natl. Cancer Inst. 2004, 96, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Van Asperen, C.J.; Brohet, R.M.; Meijers-Heijboer, E.J.; Hoogerbrugge, N.; Verhoef, S.; Vasen, H.F.; Ausems, M.G.; Menko, F.H.; Gomez Garcia, E.B.; Klijn, J.G.; et al. Cancer risks in BRCA2 families: Estimates for sites other than breast and ovary. J. Med. Genet. 2005, 42, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Kadouri, L.; Hubert, A.; Rotenberg, Y.; Hamburger, T.; Sagi, M.; Nechushtan, C.; Abeliovich, D.; Peretz, T. Cancer risks in carriers of the BRCA1/2 Ashkenazi founder mutations. J. Med. Genet. 2007, 44, 467–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phelan, C.M.; Iqbal, J.; Lynch, H.T.; Lubinski, J.; Gronwald, J.; Moller, P.; Ghadirian, P.; Foulkes, W.D.; Armel, S.; Eisen, A.; et al. Incidence of colorectal cancer in BRCA1 and BRCA2 mutation carriers: Results from a follow-up study. Br. J. Cancer 2014, 110, 530–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feliubadaló, L.; López-Fernández, A.; Pineda, M.; Díez, O.; Del Valle, J.; Gutiérrez-Enríquez, S.; Teulé, A.; González, S.; Stjepanovic, N.; Salinas, M.; et al. Opportunistic testing of BRCA1, BRCA2 and mismatch repair genes improves the yield of phenotype driven hereditary cancer gene panels. Int. J. Cancer 2019, 145, 2682–2691. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, C.M.; Creavin, B.; O’Connell, E.P.; Kelly, L.; O’Sullivan, M.J.; Corrigan, M.A.; Redmond, H.P. Risk of colorectal cancer associated with BRCA1 and/or BRCA2 mutation carriers: Systematic review and meta-analysis. Br. J. Surg. 2020. [Google Scholar] [CrossRef]
- Dobbins, S.E.; Broderick, P.; Chubb, D.; Kinnersley, B.; Sherborne, A.L.; Houlston, R.S. Undefined familial colorectal cancer and the role of pleiotropism in cancer susceptibility genes. Fam. Cancer 2016, 15, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Oh, M.; McBride, A.; Yun, S.; Bhattacharjee, S.; Slack, M.; Martin, J.R.; Jeter, J.; Abraham, I. BRCA1 and BRCA2 Gene Mutations and Colorectal Cancer Risk: Systematic Review and Meta-analysis. J. Natl. Cancer Inst. 2018, 110, 1178–1189. [Google Scholar] [CrossRef] [Green Version]
- Yurgelun, M.B.; Masciari, S.; Joshi, V.A.; Mercado, R.C.; Lindor, N.M.; Gallinger, S.; Hopper, J.L.; Jenkins, M.A.; Buchanan, D.D.; Newcomb, P.A.; et al. Germline TP53 Mutations in Patients With Early-Onset Colorectal Cancer in the Colon Cancer Family Registry. JAMA Oncol. 2015, 1, 214–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, M.F.; Johansen, J.; Sylvander, A.E.; Bjornevoll, I.; Talseth-Palmer, B.A.; Lavik, L.A.S.; Xavier, A.; Engebretsen, L.F.; Scott, R.J.; Drablos, F.; et al. Use of multigene-panel identifies pathogenic variants in several CRC-predisposing genes in patients previously tested for Lynch Syndrome. Clin. Genet. 2017, 92, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.M.; Koeppe, E.; Everett, J.; Ulintz, P.; Kiel, M.; Osborne, J.; Williams, L.; Hanson, K.; Gruber, S.B.; Rozek, L.S. Germline Genetic Features of Young Individuals With Colorectal Cancer. Gastroenterology 2018, 154, 897–905–e1. [Google Scholar] [CrossRef]
- Khan, S.A.; Idrees, K.; Forslund, A.; Zeng, Z.; Rosenberg, S.; Pincas, H.; Barany, F.; Offit, K.; Laquaglia, M.P.; Paty, P.B. Genetic variants in germline TP53 and MDM2 SNP309 are not associated with early onset colorectal cancer. J. Surg. Oncol. 2008, 97, 621–625. [Google Scholar] [CrossRef] [Green Version]
- Kratz, C.P.; Achatz, M.I.; Brugières, L.; Frebourg, T.; Garber, J.E.; Greer, M.C.; Hansford, J.R.; Janeway, K.A.; Kohlmann, W.K.; McGee, R.; et al. Cancer Screening Recommendations for Individuals with Li-Fraumeni Syndrome. Clin. Cancer Res. 2017, 23, e38–e45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genetic/Familial High-Risk Assessment: Breast and Ovarian; Li-Fraumeni Syndrome. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf (accessed on 29 May 2020).
- Wang, L.; Baudhuin, L.M.; Boardman, L.A.; Steenblock, K.J.; Petersen, G.M.; Halling, K.C.; French, A.J.; Johnson, R.A.; Burgart, L.J.; Rabe, K.; et al. MYH mutations in patients with attenuated and classic polyposis and with young-onset colorectal cancer without polyps. Gastroenterology 2004, 127, 9–16. [Google Scholar] [CrossRef]
- Knopperts, A.P.; Nielsen, M.; Niessen, R.C.; Tops, C.M.; Jorritsma, B.; Varkevisser, J.; Wijnen, J.; Siezen, C.L.; Heine-Broring, R.C.; van Kranen, H.J.; et al. Contribution of bi-allelic germline MUTYH mutations to early-onset and familial colorectal cancer and to low number of adenomatous polyps: Case-series and literature review. Fam. Cancer 2013, 12, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Castillejo, A.; Vargas, G.; Castillejo, M.I.; Navarro, M.; Barbera, V.M.; Gonzalez, S.; Hernandez-Illan, E.; Brunet, J.; Ramon y Cajal, T.; Balmana, J.; et al. Prevalence of germline MUTYH mutations among Lynch-like syndrome patients. Eur. J. Cancer 2014, 50, 2241–2250. [Google Scholar] [CrossRef]
- Segui, N.; Navarro, M.; Pineda, M.; Koeger, N.; Bellido, F.; Gonzalez, S.; Campos, O.; Iglesias, S.; Valdes-Mas, R.; Lopez-Doriga, A.; et al. Exome sequencing identifies MUTYH mutations in a family with colorectal cancer and an atypical phenotype. Gut 2015, 64, 355–356. [Google Scholar] [CrossRef]
- Bellido, F.; Pineda, M.; Aiza, G.; Valdés-Mas, R.; Navarro, M.; Puente, D.A.; Pons, T.; González, S.; Iglesias, S.; Darder, E.; et al. POLE and POLD1 mutations in 529 kindred with familial colorectal cancer and/or polyposis: Review of reported cases and recommendations for genetic testing and surveillance. Genet. Med. 2016, 18, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Ngeow, J.; Heald, B.; Rybicki, L.A.; Orloff, M.S.; Chen, J.L.; Liu, X.; Yerian, L.; Willis, J.; Lehtonen, H.J.; Lehtonen, R.; et al. Prevalence of germline PTEN, BMPR1A, SMAD4, STK11, and ENG mutations in patients with moderate-load colorectal polyps. Gastroenterology 2013, 144, 1402–1409.e14095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieminen, T.T.; Abdel-Rahman, W.M.; Ristimaki, A.; Lappalainen, M.; Lahermo, P.; Mecklin, J.P.; Jarvinen, H.J.; Peltomaki, P. BMPR1A mutations in hereditary nonpolyposis colorectal cancer without mismatch repair deficiency. Gastroenterology 2011, 141, e23–e26. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rozadilla, C.; Brea-Fernández, A.; Bessa, X.; Alvarez-Urturi, C.; Abulí, A.; Clofent, J.; Payá, A.; Jover, R.; Xicola, R.; Llor, X.; et al. BMPR1A mutations in early-onset colorectal cancer with mismatch repair proficiency. Clin. Genet. 2013, 84, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Valle, L.; de Voer, R.M.; Goldberg, Y.; Sjursen, W.; Försti, A.; Ruiz-Ponte, C.; Caldés, T.; Garré, P.; Olsen, M.F.; Nordling, M.; et al. Update on genetic predisposition to colorectal cancer and polyposis. Mol. Asp. Med. 2019, 69, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Archambault, A.N.; Su, Y.R.; Jeon, J.; Thomas, M.; Lin, Y.; Conti, D.V.; Win, A.K.; Sakoda, L.C.; Lansdorp-Vogelaar, I.; Peterse, E.F.P.; et al. Cumulative Burden of Colorectal Cancer-Associated Genetic Variants Is More Strongly Associated With Early-Onset vs Late-Onset Cancer. Gastroenterology 2020, 158, 1274–1286.e12. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terradas, M.; Capellá, G.; Valle, L. Dominantly Inherited Hereditary Nonpolyposis Colorectal Cancer Not Caused by MMR Genes. J. Clin. Med. 2020, 9, 1954. https://doi.org/10.3390/jcm9061954
Terradas M, Capellá G, Valle L. Dominantly Inherited Hereditary Nonpolyposis Colorectal Cancer Not Caused by MMR Genes. Journal of Clinical Medicine. 2020; 9(6):1954. https://doi.org/10.3390/jcm9061954
Chicago/Turabian StyleTerradas, Mariona, Gabriel Capellá, and Laura Valle. 2020. "Dominantly Inherited Hereditary Nonpolyposis Colorectal Cancer Not Caused by MMR Genes" Journal of Clinical Medicine 9, no. 6: 1954. https://doi.org/10.3390/jcm9061954





