The Impact of Marijuana on the Cardiovascular System: A Review of the Most Common Cardiovascular Events Associated with Marijuana Use
Abstract
1. Introduction
2. Cannabis
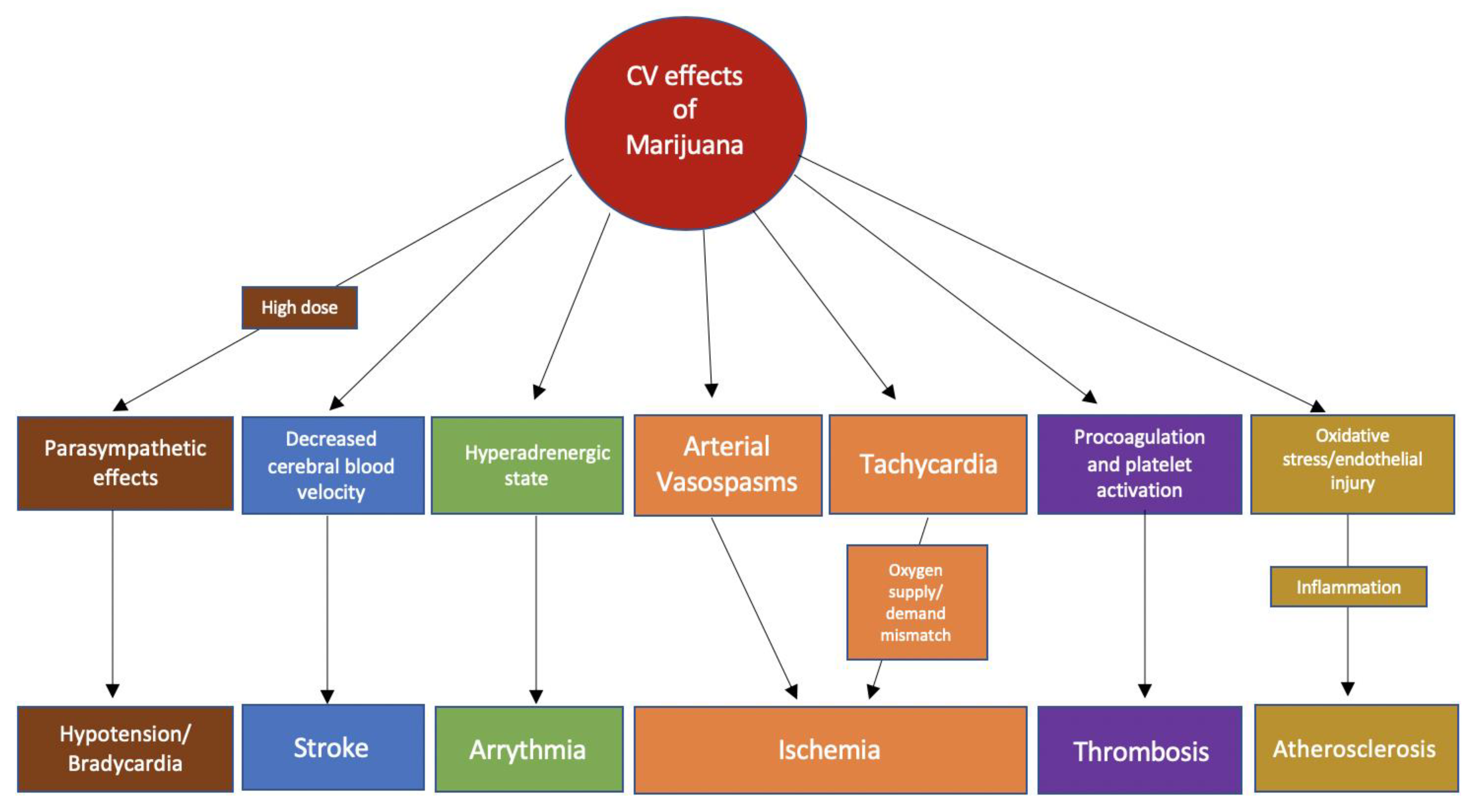
3. Physiological Effects of Marijuana
3.1. Molecular Mechanisms of G-Protein Coupled Cannabinoid Receptors
3.2. Effects of Marijuana on the Autonomic Nervous System
3.3. Effects of Marijuana on Myocardial Oxygen Demand
3.4. Effect of Marijuana on Thrombosis
3.5. Effect of Marijuana on the Inflammatory and Atherosclerotic Pathways
3.6. Effect of Marijuana on Vascular Tissue
4. Pharmacokinetics
5. Tolerance
6. Myocardial Infarction
7. Cardiac Arrythmias
8. Heart Failure and Cardiomyopathy
9. Cannabis Arteritis
10. Discussion
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations Office on Drugs and Crime (UNODC). World Drug Report 2019 (United Nations Publication, Sales No. E.19. X. 8); Division for Policy Analysis and Public Affairs, United Nations Office on Drugs and Crime: Vienna, Austria, 2019. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). Results from the 2018 National Survey on Drug Use and Health: Detailed Tables. Available online: https://www.samhsa.gov/data/report/2018-nsduh-detailed-tables (accessed on 14 December 2019).
- National Institute on Drug Abuse (NIDA). Available online: https://www.drugabuse.gov/drugs-abuse/marijuana (accessed on 11 February 2020).
- Azofeifa, A.; Mattson, M.E.; Schauer, G.; McAfee, T.; Grant, A.; Lyerla, R. National estimates of marijuana use and related indicators—National Survey on Drug Use and Health, United States, 2002–2014. MMWR Surveill. Summ. 2016, 65, 1–25. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Drug Abuse (NIDA). Vaping of Marijuana on the Rise Among Teens. Available online: https://www.drugabuse.gov/news-events/news-releases/2019/12/vaping-marijuana-rise-among-teens. (accessed on 11 February 2020).
- Singh, A.; Saluja, S.; Kumar, A.; Agrawal, S. Cardiovascular complications of marijuana and related substances: A review. Cardiol. Ther. 2018, 7, 45–59. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Oks, M.; Esposito, M.; Steinberg, H.; Makaryus, M. “Tree-in-Bloom”: Severe acute lung injury induced by vaping cannabis oil. Ann. ATS 2017, 14, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Hasin, D.S.; Saha, T.D.; Kerridge, B.T.; Goldstein, R.B.; Chou, S.P.; Zhang, H.; Jung, J.; Pickering, R.P.; Ruan, W.J.; Smith, S.M.; et al. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 2015, 72, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Kalla, A.; Krishnamoorthy, P.M.; Gopalakrishnan, A.; Figueredo, V.M. Cannabis use predicts risks of heart failure and cerebrovascular accidents: Results from the National Inpatient Sample. J. Cardiovasc. Med. 2018, 19, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Jouanjus, E.; Lapeyre-Mestre, M.; Micallef, J. Cannabis use: Signal of increasing risk of serious cardiovascular disorders. J. Am. Heart Assoc. 2014, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abouk, R.; Adams, S. Examining the relationship between medical cannabis laws and cardiovascular deaths in the US. Int. J. Drug Policy 2018, 53, 1–7. [Google Scholar] [CrossRef]
- Thomas, G.; Kloner, R.A.; Rezkalla, S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: What cardiologists need to know. Am. J. Cardiol. 2014, 113, 187–190. [Google Scholar] [CrossRef]
- Ghosh, M.; Naderi, S. Cannabis and cardiovascular disease. Curr. Atheroscler. Rep. 2019, 21, 1–6. [Google Scholar] [CrossRef]
- National Institute on Drug Abuse (NIDA). FDA Approves First Drug Derived from Marijuana. Available online: https://www.drugabuse.gov/about-nida/noras-blog/2018/07/fda-approves-first-drug-derived-marijuana (accessed on 11 February 2020).
- Pacher, P.; Steffens, S.; Haskó, G.; Schindler, T.H.; Kunos, G. Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly. Nat. Rev. Cardiol. 2017, 15, 151–166. [Google Scholar] [CrossRef]
- Richter, J.S.; Quenardelle, V.; Rouyer, O.; Raul, J.S.; Beaujeux, R.; Gény, B.; Wolff, V. A systematic review of the complex effects of cannabinoids on cerebral and peripheral circulation in animal models. Front. Physiol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid receptors: Where they are and what they do. J. Neuroendocrinol. 2008, 20 (Suppl. 1), 10–14. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Bondarenko, A.I. Cannabinoids and cardiovascular system. In Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2019; Volume 1162, pp. 63–87. [Google Scholar] [CrossRef]
- DeFilippis, E.M.; Bajaj, N.S.; Singh, A.; Malloy, R.; Givertz, M.M.; Blankstein, R.; Bhatt, D.L.; Vaduganathan, M. Marijuana use in patients with cardiovascular disease: JACC review topic of the week. J. Am. Coll. Cardiol. 2020, 75, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Baconsfirld, P.; Ginsburg, J.; Rainsbury, R. Marihuana smoking cardiovascular effects in men and possible mechanisms. N. Engl. J. Med. 1972, 287, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Gorelick, D.A.; Heishman, S.J.; Preston, K.L.; Nelson, R.A.; Moolchan, E.T.; Frank, R.A.; Dsouza, D.C.; Kosten, T.R. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch. Gen. Psychiatry 2001, 58, 322–328. [Google Scholar] [CrossRef]
- Kanakis, C.; Rosen, K.M. The cardiovascular effects of marihuana in man. Chest 1977, 72, 2–3. [Google Scholar] [CrossRef]
- Sidney, S. Cardiovascular consequences of marijuana use. J. Clincal Pharmacol. 2002, 42, 64–70. [Google Scholar] [CrossRef]
- Franz, C.A.; Frishman, W.H. Marijuana use and cardiovascular disease. Cardiol. Rev. 2016, 24, 158–162. [Google Scholar] [CrossRef]
- Niederhoffer, N.; Schmid, K.; Szabo, B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn. Schmiedebergs. Arch. Pharmacol. 2003, 367, 434–443. [Google Scholar] [CrossRef]
- Fisher, B.A.C.; Ghuran, A.; Vadamalai, V.; Antonios, T.F. Cardiovascular complications induced by cannabis smoking: A case report and review of the literature. Emerg. Med. J. 2005, 22, 679–680. [Google Scholar] [CrossRef] [PubMed]
- Renault, P.F.; Schuster, C.R.; Heinrich, R.; Freeman, D.X. Marihuana: Standardized Smoke Administration and Dose Effect Curves on Heart Rate in Humans. Am. Assoc. Adv. Sci. 1971, 174, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.J.; Wilson, W.H.; Humphreys, D.; Lowe, J.V.; Wiethe, K.E. middle cerebral artery velocity during upright posture after marijuana smoking. Acta Psychiatr. Scand. 1992, 86, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.T. Cardiovascular system effects of marijuana. J. Clin. Pharmacol. 2002, 42, 58–63. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Levisman, J.A.; Abbasi, A.S.; Shapiro, B.J.; Ellis, N.M. Short term effects of smoked marihuana on left ventricular function in man. Chest 1977, 72, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Rosenburg, J.; Rogers, W.; Bachman, J.; Jones, R. Crdiovascular effects of intravenous Delta-9-Tetrahydrocannabinol: Autonomic nervous mechanisms. Clin. Pharmacol. Ther. 1979, 25, 440–446. [Google Scholar] [CrossRef]
- Aronow, W.; Cassidy, J. Effects of smoking marihuana and of a high nicotine cigarette on Angina Pectoris. Clin. Pharmacol. Ther. 1975, 17, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.; Lief, P.; Kulp, R.; Smith, T. Combination of Delta 9 Tetrahydrocannabinol with oxymorphone or pentobarbitol. Anesthesiology 1973, 42, 674–684. [Google Scholar] [CrossRef]
- Aronow, W.; Cassidy, J. Effect of marihuana and placebo-marihuana smoking on Angina Pectoris. N. Engl. J. Med. 1974, 291, 65–66. [Google Scholar] [CrossRef]
- Bonz, A.; Laser, M.; Küllmer, S.; Kniesch, S.; Babin-Ebell, J.; Popp, V.; Ertl, G.; Wagner, J.A. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J. Cardiovasc. Pharmacol. 2003, 41, 657–664. [Google Scholar] [CrossRef]
- Marchetti, D.; Spagnolo, A.; De Matteis, V.; Filograna, L.; De Giovanni, N. Coronary thrombosis and marijuana smoking: A case report and narrative review of the literature. Drug Test. Anal. 2015, 8, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Dahdouh, Z.; Roule, V.; Lognoné, T.; Sabatier, R.; Grollier, G. Cannabis and coronary thrombosis: What is the role of platelets? Platelets 2012, 23, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Casier, I.; Vanduynhoven, P.; Haine, S.; Vrints, C.; Jorens, P.G. Is recent cannabis use associated with acute coronary syndromes? An illustrative case series. Acta Cardiol. 2014, 69, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Hartung, B.; Kauferstein, S.; Ritz-Timme, S.; Daldrup, T. Sudden unexpected death under acute influence of cannabis. Forensic Sci. Int. 2014, 237, 11–14. [Google Scholar] [CrossRef]
- Patel, R.; Kamil, S.; Bachu, R.; Adikey, A.; Ravat, V.; Kaur, M.; Tankersley, W.E.; Goyal, H. Marijuana use and acute myocardial infarction: A systemic review of published cases in the literature. Trends Cardiovasc. Med. 2019, 30, 298–307. [Google Scholar] [CrossRef]
- Deusch, E.; Kress, H.G.; Kraft, B.; Kozek-Langenecker, S.A. The procoagulatory effects of Delta-9-Tetrahydrocannabinol in human platelets. Anesth. Analg. 2004, 99, 1127–1130. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Haskó, G.; Liaudet, L.; MacKie, K.; Pacher, P. Cannabinoid-1 receptor activation induces reactive oxygen species—Dependent and—Independent mitogen—Activated protein kinase activation and cell death in human coronary artery endothelial cells. Br. J. Pharmacol. 2010, 160, 688–700. [Google Scholar] [CrossRef]
- Han, K.H.; Lim, S.; Ryu, J.; Lee, C.W.; Kim, Y.; Kang, J.H.; Kang, S.S.; Ahn, Y.K.; Park, C.S.; Kim, J.J. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 2009, 84, 378–386. [Google Scholar] [CrossRef]
- Hammel, D.; Zhang, L.; Ma, F.; Abshire, S. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur. J. Pain 2016, 20, 936–948. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Lanuti, M.; Catanzaro, G.; Fezza, F.; Rapino, C.; Maccarrone, M. Detailed characterization of the endocannabinoid system in human macrophages and foam cells, and anti-inflammatory role of type-2 cannabinoid receptor. Atherosclerosis 2014, 233, 55–63. [Google Scholar] [CrossRef]
- Dol-Gleizes, F.; Paumelle, R.; Visentin, V.; Marés, A.M.; Desitter, P.; Hennuyer, N.; Gilde, A.; Staels, B.; Schaeffer, P.; Bono, F. Rimonabant, a selective cannabinoid CB1 receptor antagonist, inhibits atherosclerosis in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Sugamura, K.; Sugiyama, S.; Nozaki, T.; Matsuzawa, Y.; Izumiya, Y.; Miyata, K.; Nakayama, M.; Kaikita, K.; Obata, T.; Takeya, M.; et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation 2009, 119, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Steffens, S.; Mach, F. Towards a therapeutic use of selective CB2 cannabinoid receptor ligands for atherosclerosis. Future Cardiol. 2006, 2, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Steffens, S.; Veillard, N.R.; Arnaud, C.; Pelli, G.; Burger, F.; Staub, C.; Zimmer, A.; Frossard, J.L.; Mach, F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 2005, 434, 782–786. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Kendall, D.A.; Randall, M.D. Further characterization of the time-dependent vascular effects of Delta9-Tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 2006, 317, 428–438. [Google Scholar] [CrossRef]
- Huestis, M.A. Humman cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Murthy, P.; Bharath, M.M.S. Chemistry, metabolism, and toxicology of cannabis: Clinical implications. Iran. J. Psychiatry 2012, 7, 149–156. [Google Scholar]
- Draz, E.I.; Oreby, M.M.; Elsheikh, E.A.; Khedr, L.A.; Atlam, S.A. Marijuana use in acute coronary syndromes. Am. J. Drug Alcohol Abuse 2017, 43, 576–582. [Google Scholar] [CrossRef]
- Hodcroft, C.J.; Rossiter, M.C.; Buch, A.N. Cannabis-associated myocardial infarction in a young man with normal coronary arteries. J. Emerg. Med. 2014, 47, 277–281. [Google Scholar] [CrossRef]
- Clark, B.C.; Georgekutty, J.; Berul, C.I. Myocardial ischemia secondary to synthetic cannabinoid (K2) use in pediatric patients. J. Pediatr. 2015, 167, 757–761. [Google Scholar] [CrossRef]
- Desai, R.; Patel, U.; Sharma, S.; Amin, P.; Bhuva, R.; Patel, M.S.; Sharma, N.; Shah, M.; Patel, S.; Savani, S.; et al. Recreational marijuana use and acute myocardial infarction: Insights from nationwide inpatient sample in the United States. Cureus 2017, 9, e1816. [Google Scholar] [CrossRef] [PubMed]
- Ramphul, K.; Mejias, S.G.; Joynauth, J. Cocaine, amphetamine, and cannabis use increases the risk of acute myocardial infarction in teenagers. Am. J. Cardiol. 2019, 123, 354. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, E.M.; Singh, A.; Divakaran, S.; Gupta, A.; Collins, B.L.; Biery, D.; Qamar, A.; Fatima, A.; Ramsis, M.; Pipilas, D.; et al. Cocaine and marijuana use among young adults with myocardial infarction. J. Am. Coll. Cardiol. 2018, 71, 2540–2551. [Google Scholar] [CrossRef] [PubMed]
- Thankavel, P.; Mir, A.; Ramaciotti, C. Elevated troponin levels in previously healthy children: Value of diagnostic modalities and the importance of a drug screen. Cardiol. Young 2014, 24, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.; Stevenson, R.S. Marijuana lollipop-induced myocardial infarction. Can. J. Cardiol. 2019, 35, 229.e1–229.e3. [Google Scholar] [CrossRef]
- Nawrot, T.S.; Perez, L.; Künzli, N.; Munters, E.; Nemery, B. Public health importance of triggers of myocardial infarction: A comparative risk assessment. Lancet 2011, 377, 732–740. [Google Scholar] [CrossRef]
- Jouanjus, E.; Raymond, V.; Lapeyre-Mestre, M.; Wolff, V. What Is the current knowledge about the cardiovascular risk for users of cannabis-based products? A systematic review. Curr. Atheroscler. Rep. 2017, 19, 26. [Google Scholar] [CrossRef]
- Lorenz, D.R.; Dutta, A.; Mukerji, S.S.; Holman, A.; Uno, H.; Gabuzda, D. Marijuana use impacts midlife cardiovascular events in HIV-infected men. Clin. Infect. Dis. 2017, 65, 626–635. [Google Scholar] [CrossRef]
- Mittleman, M.A.; Lewis, R.A.; Maclure, M.; Sherwood, J.B.; Muller, J.E. Triggering myocardial infarction by marijuana. Circulation 2001, 103, 2805–2809. [Google Scholar] [CrossRef]
- Stanley, C.; O’Sullivan, S.E. Vascular targets for cannabinoids: Animal and human studies. Br. J. Pharmacol. 2014, 171, 1361–1378. [Google Scholar] [CrossRef]
- Richards, J.R.; Bing, M.L.; Moulin, A.K.; Elder, J.W.; Robert, T.; Summers, P.J.; Laurin, E.G.; Richards, J.R.; Bing, M.L.; Moulin, A.K.; et al. Cannabis use and acute coronary syndrome. Clin. Toxicol. 2019, 3650, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Artiles, A.; Awan, A.; Karl, M.; Santini, A. Cardiovascular effects of cannabis (marijuana): A timely update. Wiley 2019, 33, 1592–1594. [Google Scholar] [CrossRef]
- Wengrofsky, P.; Mubarak, G.; Shim, A.; Kariyanna, P.; Budzidkowski, A.; Schwartz, J.; Mcfarlane, S.I. Recurrent STEMI precipitated by marijuana use: Case report and literature review. Am. J. Med. Case Rep. 2018, 6, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Mukamal, K.; Maclure, M.; Muller, J.E.; Mittleman, M.A. An exploratory proscpective study of murjuana use and mortality following acute myocardial infarction. Am. Heart J. 2008, 155, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Frost, L.; Mostofsky, E.; Rosenbloom, J.I. Marijuana use and long term mortality among survivors of acute myocardial infarction. Am. Heart J. 2013, 165, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.P.; Auer, R.; Bancks, M.P.; Goff, D.C., Jr.; Lewis, C.E.; Pletcher, M.J.; Rana, J.S.; Shikany, J.M.; Sidney, S. Cumulative lifetime marijuana use and incident cardiovascular disease in Middle Age: The Coronary Artery Risk Development in Young Adults (CARDIA) study. AJPH Res. 2017, 107, 601–606. [Google Scholar] [CrossRef]
- Petronis, K.R.; Anthony, J.C. An epidemiologic investigation of marijuana- and cocaine-related palpitations. Drug Alcohol Depend. 1989, 23, 219–226. [Google Scholar] [CrossRef]
- Desai, R.; Fong, H.K.; Shah, K.; Kaur, V.P.; Savani, S. Rising trends in hospitalizations for cardiovascular events among young cannabis users (18–39 years) wyithout other substance abuse. Medicina (B. Aires) 2019, 55, 1–6. [Google Scholar]
- Korantzopoulos, P.; Liu, T.; Papaioannides, D.; Li, G.; Goudevenos, J.A. Atrial fibrillation and marijuana smoking. Int. J. Clin. Pract. 2008, 62, 308–313. [Google Scholar] [CrossRef]
- Kariyanna, P.T.; Wengrofsky, P.; Jayarangaiah, A.; Haseeb, S. Marijuana and cardiac arrhythmias: A scoping study. Int. J. Clin. Res. Trials 2019, 4, 1–11. [Google Scholar] [CrossRef]
- Miller, R.H.; Dhingra, R.C.; Kanakis, C.; Amat-y-Leon, F.; Rosen, K.M. The electrophysiological effects of Delta-9-Tetrahydrocannabinol (cannabis) on cardiac conduction in man. Am. Heart J. 1977, 94, 740–747. [Google Scholar] [CrossRef]
- Khouzam, R.N.; Kabra, R.; Soufi, M.K. Marijuana, bigeminal premature ventricular contractions and sluggish coronary flow: Are they related? J. Cardiol. Cases 2013, 8, 121–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bachs, L.; Mørland, H. Acute cardiovascular fatalities following cannabis use. Forensic Sci. Int. 2001, 124, 200–203. [Google Scholar] [CrossRef]
- Rezkalla, S.H.; Sharma, P.; Kloner, R.A. Coronary no-flow and ventricular tachycardia associated with habitual marijuana use. Ann. Emerg. Med. 2003, 42, 365–369. [Google Scholar] [CrossRef]
- Singh, A.; Argwal, S.; Manda, Y.; Nanda, S.; Shirani, J. Marijuana (cannabis) use is an independent predictor of stress cardiomyopathy in younger men. Circulation 2016, 134, A14100. [Google Scholar]
- Nogi, M.; Fergusson, D.; Chiaco, J.M.C. Mid-ventricular variant takotsubo cardiomyopathy associated with cannabinoid hyperemesis syndrome: A case report. Hawaii J. Med. Public Health 2014, 73, 115–118. [Google Scholar] [PubMed]
- Del Buono, M.G.; O’Quinn, M.P.; Garcia, P.; Gerszten, E.; Roberts, C.; Moeller, F.G.; Abbate, A. Cardiac arrest due to ventricular fibrillation in a 23-year-old woman with broken heart syndrome. Cardiovasc. Pathol. 2017, 30, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Nahas, G.G. Cannabis Arteritis. N. Engl. J. Med. 1971, 284, 113. [Google Scholar] [CrossRef]
- Desbois, A.C.; Cacoub, P. Cannabis-associated arterial disease. Ann. Vasc. Surg. 2013, 27, 996–1005. [Google Scholar] [CrossRef]
- Santos, R.P.; Resende, C.I.P.; Vieira, A.P.; Brito, C. Cannabis arteritis: Ever more important to consider. BMJ Case Rep. 2017, 10–13. [Google Scholar] [CrossRef]
- Disdier, P.; Granel, B.; Serratrice, J.; Constans, J.; Michon-Pasturel, U.; Hachulla, E.; Conri, C.; Devulder, B.; Swiader, L.; Piquet, P.; et al. Cannabis arteritis revisited—Ten new case reports. Angiology 2001, 52, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Combemale, P.; Consort, T.; Denis-Thelis, L.; Estival, J.L.; Dupin, M.; Kanitakis, J. Cannabis arteritis. Br. J. Dermatol. 2005, 152, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, I.; Garsaud, A.M.; Saint-Cyr, I.; Quitman, O.; Sanchez, B.; Quist, D. Cannabis arteritis: A new case report and a review of literature. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, V.N.; Menezes, A.R.; Deschutter, A.; Lavie, C.J. The cardiovascular effects of marijuana: Are the potential adverse effects worth the high? Sci. Med. 2019, 116, 146–153. [Google Scholar]
- Stith, S.S.; Vigil, J.M. Federal barriers to cannabis research. Science 2016, 352, 1182. [Google Scholar] [CrossRef] [PubMed]
- Choo, E.K.; Emery, S.L. Clearing the haze: The complexities and challenges of research on state marijuana laws. Ann. N. Y. Acad. Sci. 2017, 1394, 55–73. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latif, Z.; Garg, N. The Impact of Marijuana on the Cardiovascular System: A Review of the Most Common Cardiovascular Events Associated with Marijuana Use. J. Clin. Med. 2020, 9, 1925. https://doi.org/10.3390/jcm9061925
Latif Z, Garg N. The Impact of Marijuana on the Cardiovascular System: A Review of the Most Common Cardiovascular Events Associated with Marijuana Use. Journal of Clinical Medicine. 2020; 9(6):1925. https://doi.org/10.3390/jcm9061925
Chicago/Turabian StyleLatif, Zara, and Nadish Garg. 2020. "The Impact of Marijuana on the Cardiovascular System: A Review of the Most Common Cardiovascular Events Associated with Marijuana Use" Journal of Clinical Medicine 9, no. 6: 1925. https://doi.org/10.3390/jcm9061925
APA StyleLatif, Z., & Garg, N. (2020). The Impact of Marijuana on the Cardiovascular System: A Review of the Most Common Cardiovascular Events Associated with Marijuana Use. Journal of Clinical Medicine, 9(6), 1925. https://doi.org/10.3390/jcm9061925




