Serum Metabolic Signatures of Chronic Limb-Threatening Ischemia in Patients with Peripheral Artery Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort and Design
2.2. Baseline Patient Clinical Assessments
2.3. Chemicals and Reagents
2.4. Serum Creatinine Measurement by Jaffé Method
2.5. Serum Sample Collection and Preparation
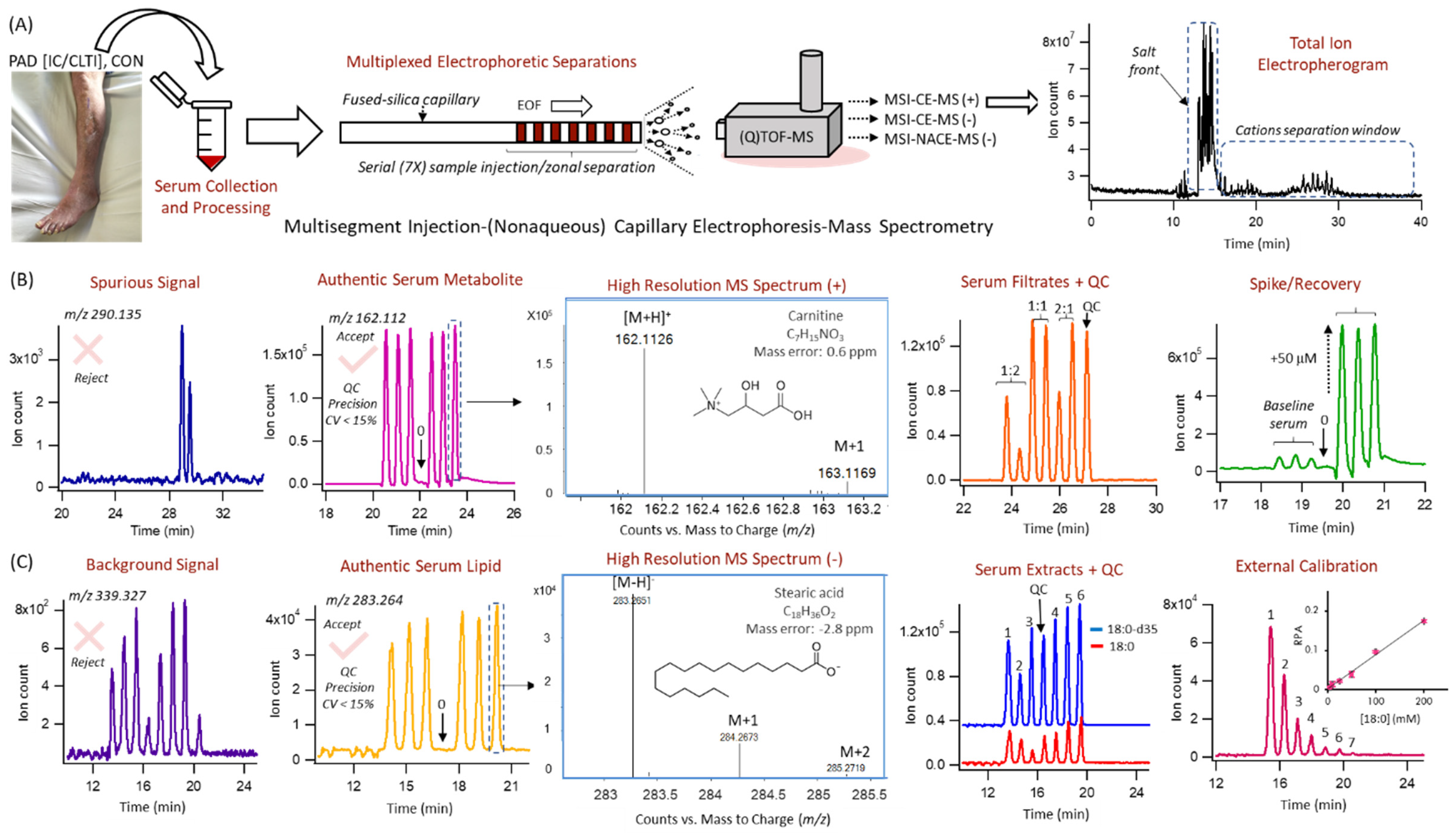
2.6. Hydrophilic Metabolome Profiling by MSI–CE–MS
2.7. Lipophilic Metabolome Profiling by MSI–NACE–MS
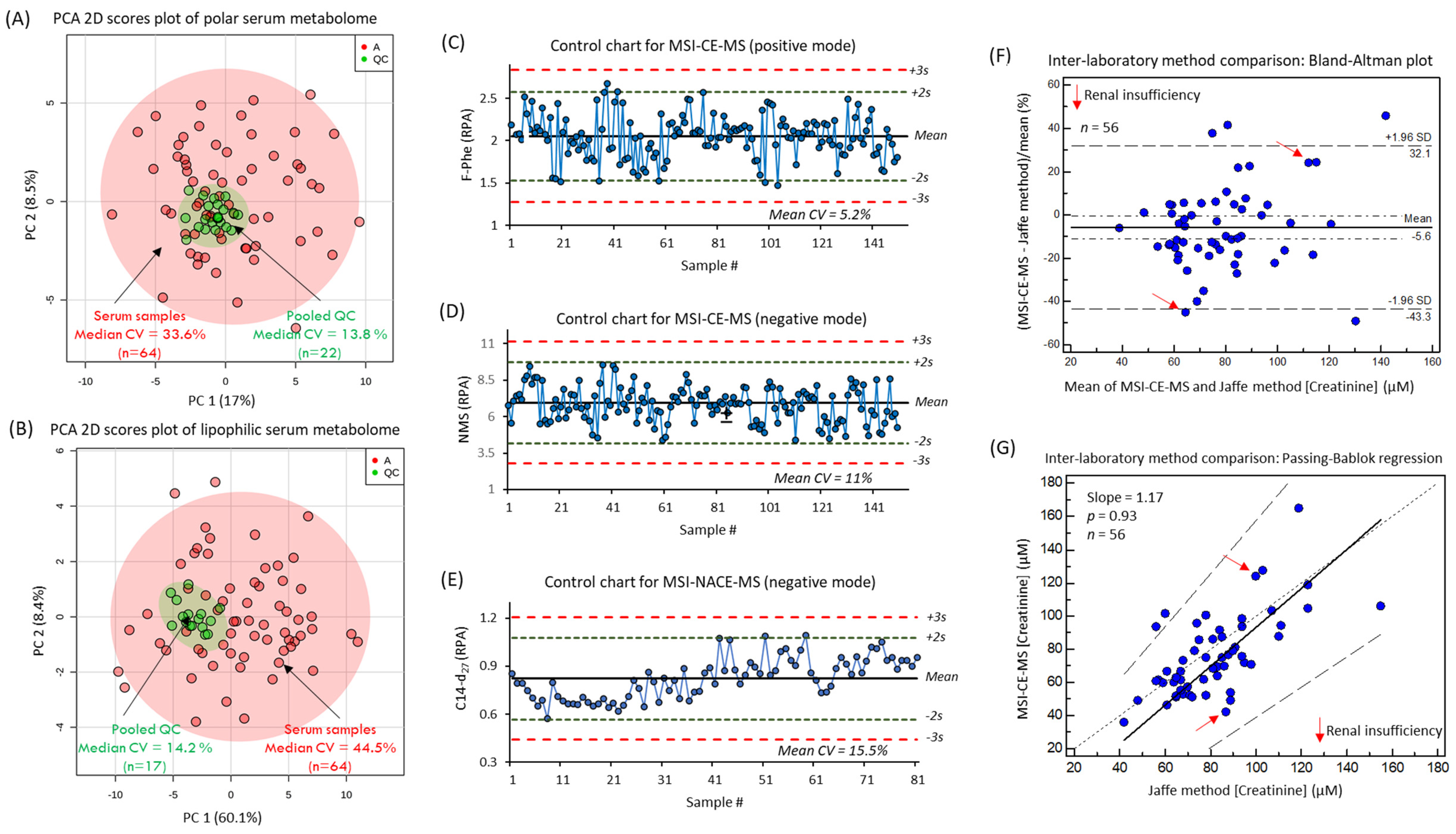
2.8. Data Processing and Statistical Analyses
3. Results
3.1. Cohort Demographics and Clinical Characteristics
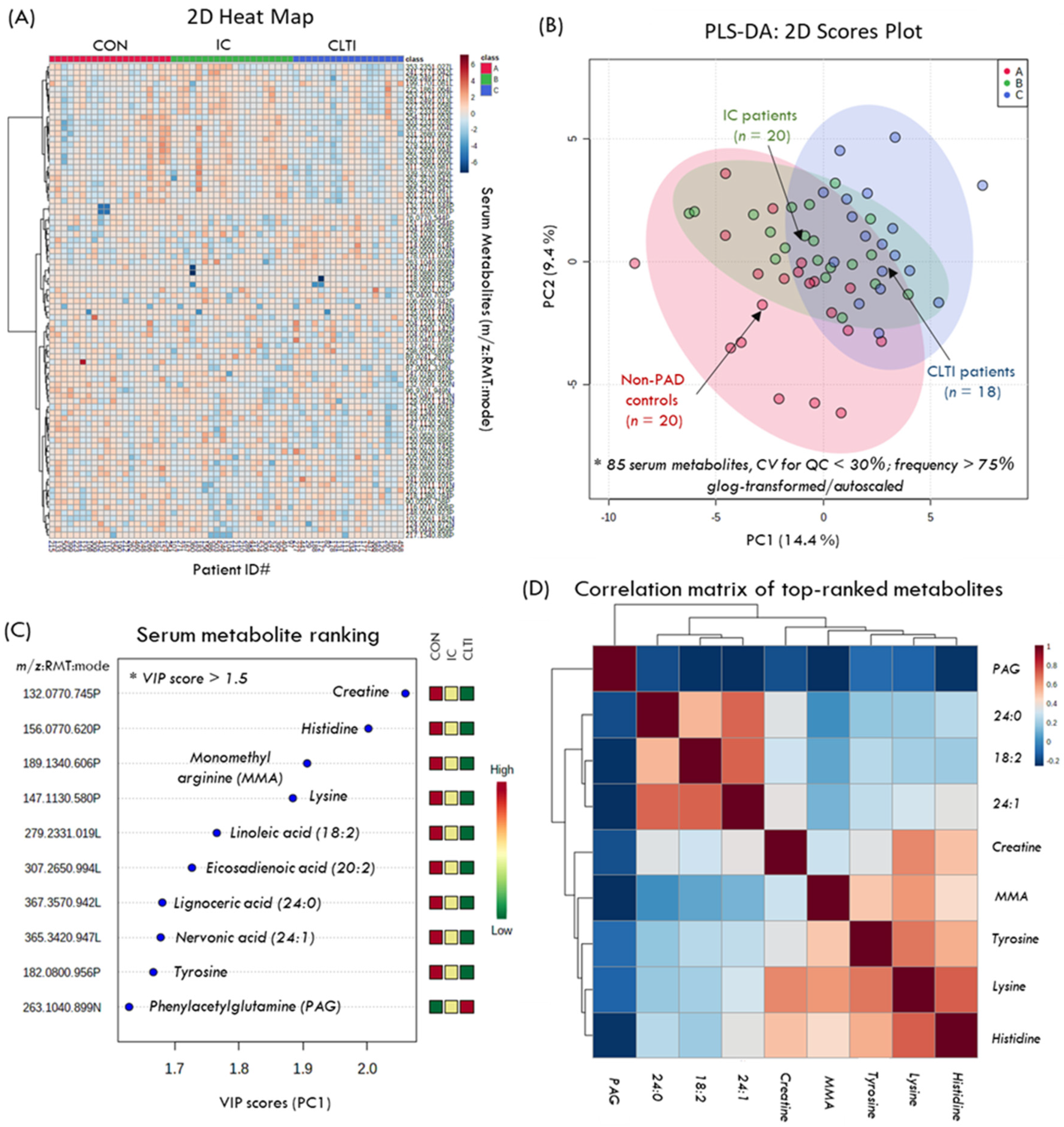
3.2. The Serum Metabolome of PAD Patients
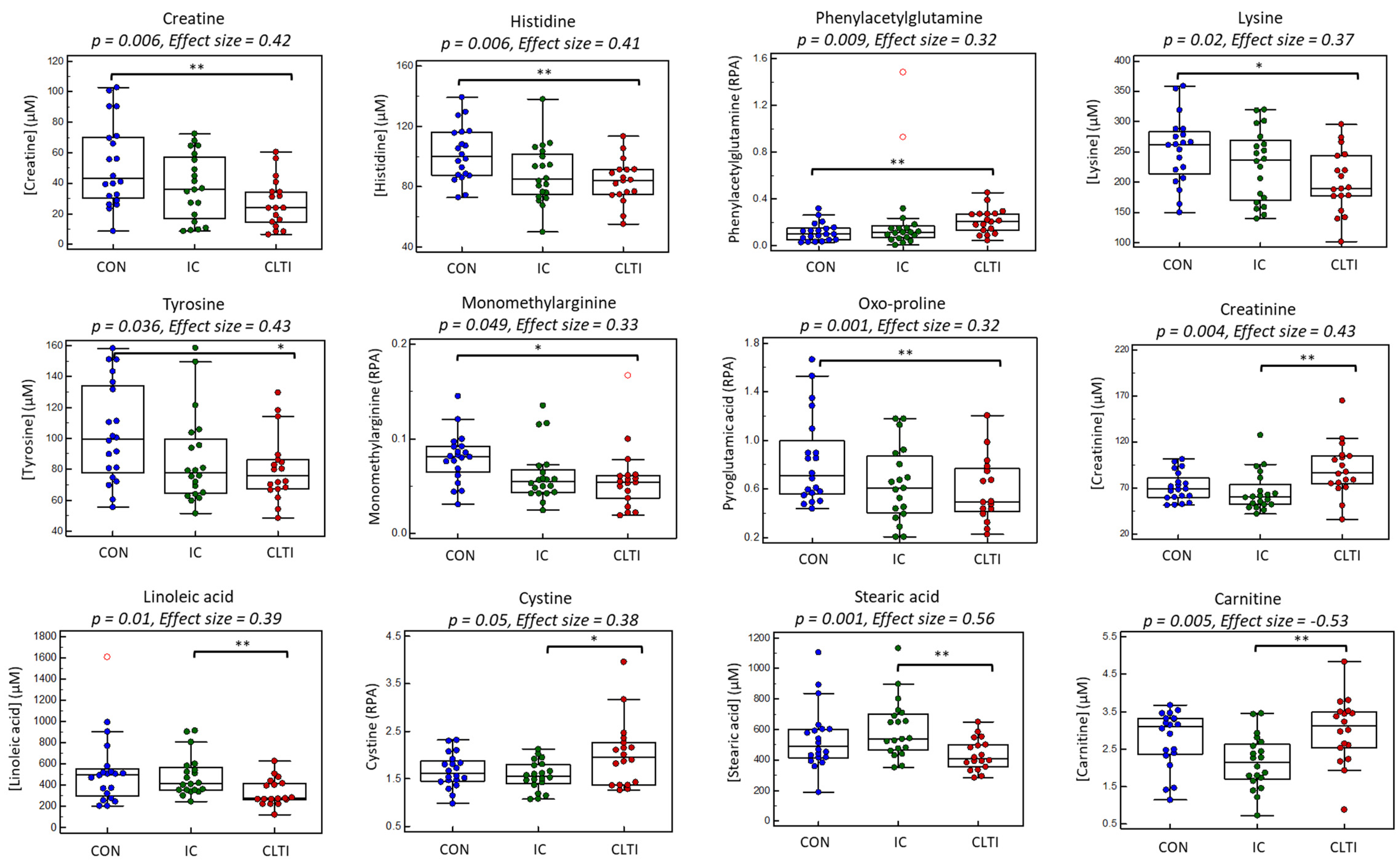
3.3. Differentiating Serum Metabolites of PAD Progression
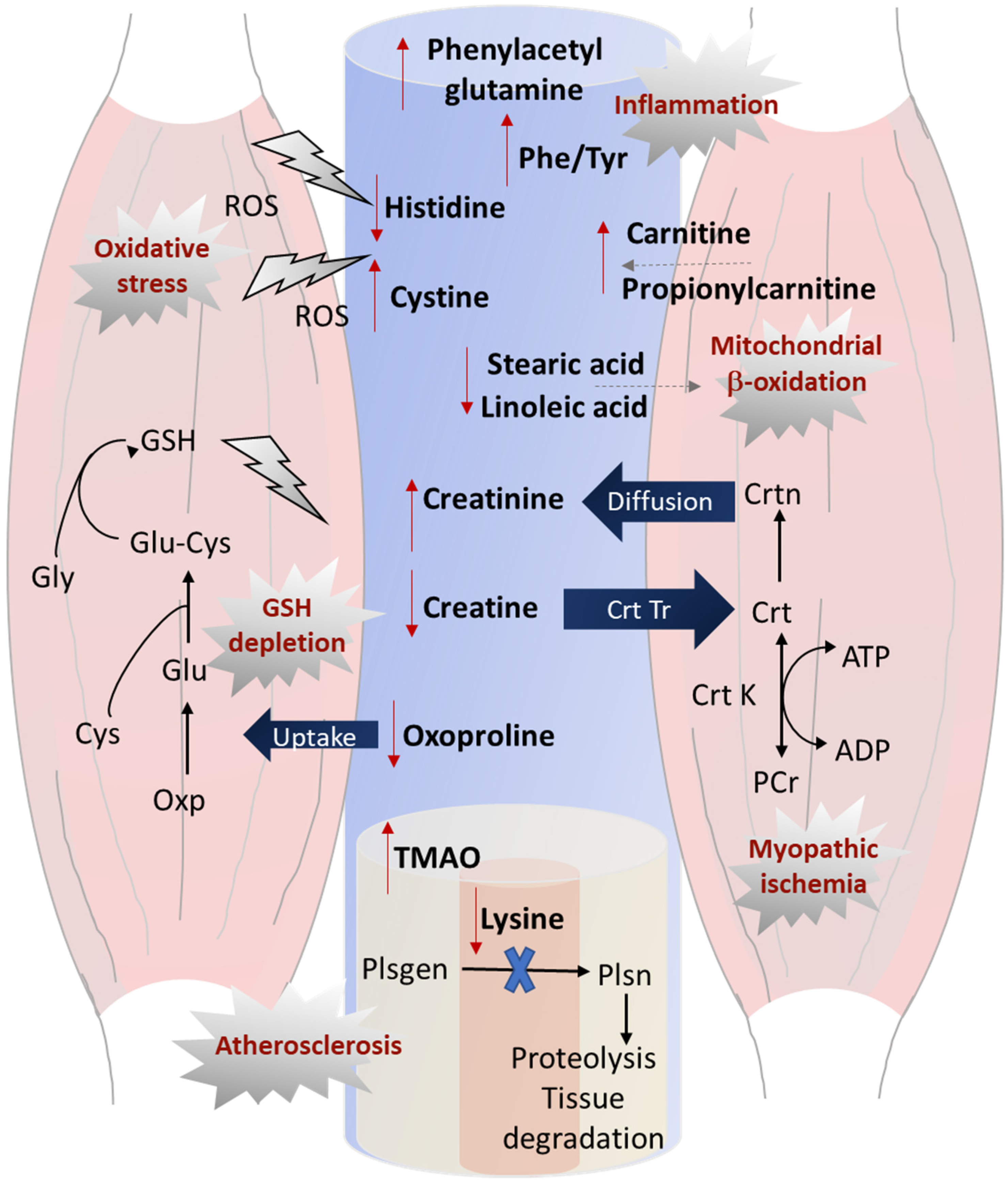
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hirsch, A.T.; Criqui, M.H.; Treat-Jacobson, D.; Regensteiner, J.G.; Creager, M.A.; Olin, J.W.; Krook, S.H.; Hunninghake, D.B.; Comerota, A.J.; Walsh, M.E.; et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001, 286, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, 3S–125S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dormandy, J.; Belcher, G.; Broos, P.; Eikelboom, B.; Laszlo, G.; Konrad, P.; Moggi, L.; Mueller, U. Prospective study of 713 below–knee amputations for ischaemia and the effect of a prostacyclin analogue on healing. Br. J. Surg. 1994, 81, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Varu, V.N.; Hogg, M.E.; Kibbe, M.R. Critical limb ischemia. J. Vasc. Surg. 2010, 51, 230–241. [Google Scholar] [CrossRef] [Green Version]
- Almasri, J.; Adusumalli, J.; Asi, N.; Lakis, S.; Alsawas, M.; Prokop, L.J.; Bradbury, A.; Kolh, P.; Conte, M.S.; Murad, M.H. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, 126S–136S. [Google Scholar] [CrossRef] [Green Version]
- Cooke, J.P.; Wilson, A.M. Biomarkers of peripheral arterial disease. J Am. Coll. Cardiol. 2010, 55, 2017–2023. [Google Scholar] [CrossRef] [Green Version]
- Hyun, S.; Forbang, N.I.; Allison, M.A.; Denenberg, J.O.; Criqui, M.H.; Ix, J.H. Ankle-brachial index, toe-brachial index, and cardiovascular mortality in persons with and without diabetes mellitus. J. Vasc. Surg. 2014, 60, 390–395. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care 2003, 26, 3333–3341. [Google Scholar] [CrossRef] [Green Version]
- Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 177–183. [CrossRef] [Green Version]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.-F.; Shah, S.H.; Newgard, C.B. Cardiovascular metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Canela, M.; Toledo, E.; Clish, C.B.; Hruby, A.; Liang, L.; Salas-Salvadó, J.; Razquin, C.; Corella, D.; Estruch, R.; Ros, E.; et al. Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED Trial. Clin. Chem. 2016, 62, 582–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.H.; Sun, J.-L.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; Pieper, K.S.; Haynes, C.; Hauser, E.R.; Kraus, W.E.; Granger, C.B.; et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am. Heart J. 2012, 163, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; McDermott, M.M.; Liu, K.; Kuo, C.-H.; Wang, S.-Y.; Tao, H.; Tseng, Y.J. Plasma metabolomic profiles predict near-term death among individuals with lower extremity peripheral arterial disease. J. Vasc. Surg. 2013, 58, 989–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagura, M.; Kals, J.; Kilk, K.; Serg, M.; Kampus, P.; Eha, J.; Soomets, U.; Zilmer, M. Metabolomic signature of arterial stiffness in male patients with peripheral arterial disease. Hypertens. Res. 2015, 38, 840–846. [Google Scholar] [CrossRef]
- Ismaeel, A.; Franco, M.E.; Lavado, R.; Papoutsi, E.; Casale, G.P.; Fuglestad, M.; Mietus, C.J.; Haynatzki, G.R.; Smith, R.S.; Bohannon, W.T.; et al. Altered metabolomic profile in patients with peripheral artery disease. J. Clin. Med. 2019, 8, 1463. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Aguilera, A.; Fernández-Arroyo, S.; Cabre, N.; Luciano-Mateo, F.; Baiges-Gaya, G.; Fibla, M.; Martín-Paredero, V.; Menendez, J.A.; Camps, J.; Joven, J. Plasma energy-balance metabolites discriminate asymptomatic patients with peripheral artery disease. Mediat. Inflamm. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, R.B.; Baker, J.D.; Ernst, C.; Johnston, K.W.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538. [Google Scholar] [CrossRef] [Green Version]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2018, 72, e91–e220. [Google Scholar]
- Ou, M.; Song, Y.; Li, S.; Liu, G.; Jia, J.; Zhang, M.; Zhang, H.; Yu, C. LC-MS/MS method for serum creatinine: Comparison with enzymatic method and Jaffé method. PLoS ONE 2015, 10, e0133912. [Google Scholar] [CrossRef] [Green Version]
- Wild, J.; Shanmuganathan, M.; Hayashi, M.; Potter, M.; Britz-McKibbin, P. Metabolomics for improved treatment monitoring of phenylketonuria: Urinary biomarkers for non-invasive assessment of dietary adherence and nutritional deficiencies. Analyst 2019, 144, 6595–6608. [Google Scholar] [CrossRef] [PubMed]
- Saoi, M.; Li, A.; McGlory, C.; Stokes, T.; von Allmen, M.T.; Phillips, S.M.; Britz-McKibbin, P. Metabolic perturbations from step reduction in older persons at risk for sarcopenia: Plasma biomarkers of abrupt changes in physical activity. Metabolites 2019, 9, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azab, S.; Ly, R.; Britz-McKibbin, P. Robust method for high-throughput screening of fatty acids by multisegment injection-nonaqueous capillary electrophoresis–mass spectrometry with stringent quality control. Anal. Chem. 2019, 91, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Azab, S.M.; de Souza, R.J.; Teo, K.K.; Anand, S.S.; Williams, N.C.; Holzschuher, J.; McGlory, C.; Phillips, S.M.; Britz-McKibbin, P. Serum non-esterified fatty acids have utility as dietary biomarkers of fat intake from fish, fish oil and dairy in women. J. Lipid Res. 2020, 61, 933–944. [Google Scholar] [CrossRef] [Green Version]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Kuehnbaum, N.L.; Kormendi, A.; Britz-McKibbin, P. Multisegment injection-capillary electrophoresis-mass spectrometry: A high-throughput platform for metabolomics with high data fidelity. Anal. Chem. 2013, 85, 10664–10669. [Google Scholar] [CrossRef]
- Nori de Macedo, A.; Mathiaparanam, S.; Brick, L.; Keenan, K.; Gonska, T.; Pedder, L.; Hill, S.; Britz-McKibbin, P. The sweat metabolome of screen-positive cystic fibrosis infants: Revealing mechanisms beyond impaired chloride transport. ACS Cent. Sci. 2017, 3, 904–913. [Google Scholar] [CrossRef]
- Wellington, N.; Shanmuganathan, M.; de Souza, R.J.; Zulyniak, M.A.; Azab, S.; Bloomfield, J.; Mell, A.; Ly, R.; Desai, D.; Anand, S.S.; et al. Metabolic trajectories following contrasting Prudent and Western diets from food provisions: Robust biomarkers of short-term changes in habitual diet. Nutrients 2019, 11, 2407. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Ly, R.; Gill, B.; Zhu, Y.; Moran-Mirabal, J.; Britz-McKibbin, P. Robust and high-throughput method for anionic metabolite profiling: Preventing polyimide aminolysis and capillary breakages under alkaline conditions in capillary electrophoresis-mass spectrometry. Anal. Chem. 2016, 88, 10710–10719. [Google Scholar] [CrossRef]
- DiBattista, A.; Rampersaud, D.; Lee, H.; Kim, M.; Britz-McKibbin, P. High throughput screening method for systematic surveillance of drugs of abuse by multisegment injection–capillary electrophoresis–mass spectrometry. Anal. Chem. 2017, 89, 11853–11861. [Google Scholar] [CrossRef]
- Dunn, W.B.; Erban, A.; Weber, R.J.M.; Creek, D.J.; Brown, M.; Breitling, R.; Hankemeier, T.; Goodacre, R.; Neumann, S.; Kopka, J.; et al. Mass appeal: Metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics 2013, 9, 44–66. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Pinto-Sanchez, M.I.; Bercik, P.; Britz-McKibbin, P. Metabolomics reveals elevated urinary excretion of collagen degradation and epithelial cell turnover products in irritable bowel syndrome patients. Metabolomics 2019, 15, 82. [Google Scholar] [CrossRef]
- Mahieu, N.G.; Patti, G.J. Systems-level annotation of a metabolomics data set reduces 25,000 features to fewer than 1000 unique metabolites. Anal. Chem. 2017, 89, 10397–10406. [Google Scholar] [CrossRef] [PubMed]
- Swedko, P.J.; Clark, H.D.; Paramsothy, K.; Akbari, A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch. Intern. Med. 2003, 163, 356–360. [Google Scholar] [CrossRef]
- Gilstrap, L.G.; Wang, T.J. Biomarkers and cardiovascular risk assessment for primary prevention: An update. Clin. Chem. 2012, 58, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saoi, M.; Percival, M.; Nemr, C.; Li, A.; Gibala, M.; Britz-McKibbin, P. Characterization of the human skeletal muscle metabolome for elucidating the mechanisms of bicarbonate ingestion on strenuous interval exercise. Anal. Chem. 2019, 91, 4709–4718. [Google Scholar] [CrossRef]
- Wade, A.M.; Tucker, H.N. Antioxidant characteristics of L-histidine. J. Nutr. Biochem. 1998, 9, 308–315. [Google Scholar] [CrossRef]
- Tomé, D.; Bos, C. Lysine requirement through the human life cycle. J. Nutr. 2007, 137, 1642S–1645S. [Google Scholar] [CrossRef] [Green Version]
- Castellano, I.; Merlino, A. (Eds.) Gamma-glutamyl transpeptidases: Structure and function. In Gamma-Glutamyl Transpeptidases: Structure and Function; Springer Briefs in Biochemistry and Molecular Biology; Springer: Basel, Switzerland, 2013; pp. 1–57. ISBN 978-3-0348-0682-4. [Google Scholar]
- Steven, S.; Daiber, A.; Dopheide, J.F.; Münzel, T.; Espinola-Klein, C. Peripheral artery disease, redox signaling, oxidative stress—Basic and clinical aspects. Redox Biol. 2017, 12, 787–797. [Google Scholar] [CrossRef]
- Saoi, M.; Sasaki, K.; Sagawa, H.; Abe, K.; Kogiso, T.; Tokushige, K.; Hashimoto, E.; Ohashi, Y.; Britz-McKibbin, P. High throughput screening of serum γ-glutamyl dipeptides for risk assessment of nonalcoholic steatohepatitis with impaired glutathione salvage pathway. J. Proteome Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hajjari, J.; Tahhan, A.S.; Alkhoder, A.; Kaseer, B.; Naser, A.; Almuwaqqat, Z.; Gafeer, M.M.; Choudhary, F.; Patel, K.; Patel, A.; et al. Markers of oxidative stress and peripheral artery disease. J. Am. Coll. Cardiol. 2018, 71, A2076. [Google Scholar] [CrossRef]
- Poesen, R.; Claes, K.; Evenepoel, P.; de Loor, H.; Augustijns, P.; Kuypers, D.; Meijers, B. Microbiota-derived phenylacetylglutamine associates with overall mortality and cardiovascular disease in patients with CKD. J. Am. Soc. Nephrol. 2016, 27, 3479–3487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders Thomas, A.B. Omega-6 fatty acids and cardiovascular disease. Circulation 2019, 139, 2437–2439. [Google Scholar] [CrossRef]
- Matti, M.; Wu, H.Y.; Imamura, F.; Del Gobbo, L.C.; Fretts, A.; De Goede, J.; Shi, P.; Tintle, N.; Wennberg, M.; Aslibekyan, S.; et al. Biomarkers of dietary omega-6 fatty acids and incident cardiovascular disease and mortality. Circulation 2019, 139, 2422–2436. [Google Scholar]
- Grenon, S.M.; Hughes-Fulford, M.; Rapp, J.; Conte, M.S. Polyunsaturated fatty acids and peripheral artery disease. Vasc. Med. 2012, 17, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Canela, M.; Estruch, R.; Corella, D.; Salas-Salvadó, J.; Martínez-González, M.A. Association of Mediterranean diet with peripheral artery disease: The PREDIMED Randomized Trial. JAMA 2014, 311, 415. [Google Scholar] [CrossRef]
- Weitkunat, K.; Schumann, S.; Nickel, D.; Hornemann, S.; Petzke, K.J.; Schulze, M.B.; Pfeiffer, A.F.; Klaus, S. Odd-chain fatty acids as a biomarker for dietary fiber intake: A novel pathway for endogenous production from propionate. Am. Clin. Nutr. 2017, 105, 1544–1551. [Google Scholar] [CrossRef] [Green Version]
- Senyilmaz-Tiebe, D.; Pfaff, D.H.; Virtue, S.; Schwarz, K.V.; Fleming, T.; Altamura, S.; Muckenthaler, M.U.; Okun, J.G.; Vidal-Puig, A.; Nawroth, P.; et al. Dietary stearic acid regulates mitochondria in vivo in humans. Nat. Commun. 2018, 9, 3129. [Google Scholar] [CrossRef]
- Weiss, D.J.; Casale, G.P.; Koutakis, P.; Nella, A.A.; Swanson, S.A.; Zhu, Z.; Miserlis, D.; Johanning, J.M.; Pipinos, I.I. Oxidative damage and myofiber degeneration in the gastrocnemius of patients with peripheral arterial disease. J. Transl. Med. 2013, 11, 230. [Google Scholar] [CrossRef] [Green Version]
- Senthong, V.; Wang, Z.; Fan, Y.; Wu, Y.; Hazen, S.L.; Tang, W.H.W. Trimethylamine N-oxide and mortality risk in patients with peripheral artery disease. J. Am. Heart Assoc. 2016, 5, e004237. [Google Scholar] [CrossRef] [PubMed]
- Roncal, C.; Martínez-Aguilar, E.; Orbe, J.; Ravassa, S.; Fernandez-Montero, A.; Saenz-Pipaon, G.; Ugarte, A.; Estella-Hermoso de Mendoza, A.; Rodriguez, J.A.; Fernández-Alonso, S.; et al. Trimethylamine-n-oxide (TMAO) predicts cardiovascular mortality in peripheral artery disease. Sci. Rep. 2019, 9, 15580. [Google Scholar] [CrossRef] [PubMed]
- Mlekusch, W.; Exner, M.; Sabeti, S.; Amighi, J.; Schlager, O.; Wagner, O.; Minar, E.; Schillinger, M. Serum creatinine predicts mortality in patients with peripheral artery disease: Influence of diabetes and hypertension. Atherosclerosis 2004, 175, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Ormstad, H.; Verkerk, R.; Sandvik, L. Serum phenylalanine, tyrosine, and their ratio in acute ischemic stroke: On the trail of a biomarker? J. Mol. Neurosci. 2016, 58, 102–108. [Google Scholar] [CrossRef]
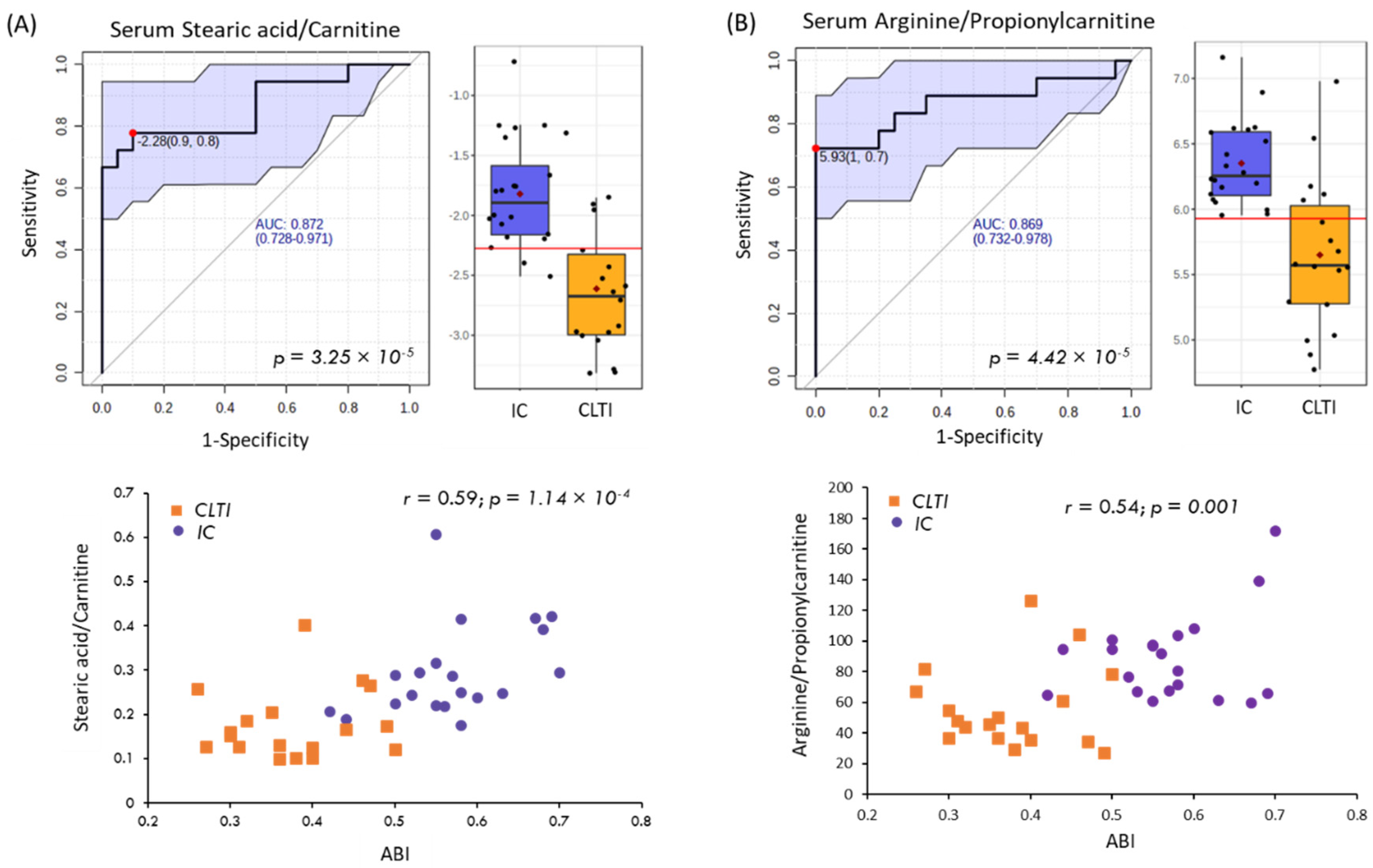
| Parameter | CON (n = 20) | IC (n = 20) | CLTI (n = 18) | p-Value |
|---|---|---|---|---|
| Rutherford stage | - | 1–3 (2.75 ± 0.4) | ≥4 (4.11 ± 0.3) | - |
| Walking distance (m) | >1000 | 530 | <160 | - |
| ABI | 1.08 ± 0.09 | 0.57 ± 0.08 | 0.38 ± 0.07 | 3.06 × 10−33; 2.36 × 10−9 |
| Age (years) | 62.6 ± 6.6 | 61.0 ± 7.4 | 65.2 ± 5.6 | 0.151; 0.055 |
| BMI (kg/m2) | 26.6 ± 2.5 | 24.3 ± 3.0 | 24.9 ± 3.6 | 0.061; 0.631 |
| HbA1c (%) | 5.75 ± 0.51 | 5.98 ± 0.50 | 5.58 ± 0.99 | 0.217; 0.124 |
| Leukocytes | 6.6 ± 2.2 | 7.8 ± 2.5 | 8.4 ± 3.4 | 0.156; 0.534 |
| Platelets | 251 ± 76 | 244 ± 64 | 209 ± 65 | 0.152; 0.118 |
| Males (%) | 50 (10/20) | 55 (11/20) | 72 (13/18) | 0.401; 0.224 |
| Smoking (%) | 55 (11/20) | 95 (19/20) | 94 (17/18) | 0.002; 0.730 |
| Diabetes mellitus (%) | 0 | 0 | 0 | - |
| Hypertension (%) | 40 (8/20) | 65 (13/20) | 72 (13/18) | 0.099; 0.450 |
| Hyperlipidemia (%) | 39 (7/20) | 85 (17/20) | 83 (15/18) | 0.001; 0.616 |
| Renal insufficiency (%) | 0 (0/20) | 5 (1/20) | 6 (1/18) | 0.76; 0.730 |
| Coronary artery disease (%) | 0 (0/20) | 40 (8/20) | 61 (11/18) | 0.001; 0.165 |
| Statin use (%) | 30 (6/20) | 80 (16/20) | 100 (18/18) | <0.001; 0.066 |
| Antiplatelet use (%) | 50 (10/20) | 100 (20/20) | 100 (18/18) | <0.001; - |
| Metabolite ID | m/z:RMT:mode | F-Value | p-Value Overall | p-Value Linear a | Effect Size b | p-Value Contrast 1 PAD:CON c | FC PAD:CON c | p-Value Contrast 2 CLTI:IC d | FC CLTI:IC d | r Correlation to ABI e | p-Value for r |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Creatine, HMDB000064 | 132.077:0.745:p | 6.02 * | 0.006 | 0.002 | 0.422 | 0.008 | 0.65 | 0.097 | 0.75 | 0.44 | 0.001 |
| Histidine, HMDB000117 | 156.077:0.620:p | 5.54 | 0.006 | 0.003 | 0.410 | 0.002 | 0.85 | 0.435 | 0.95 | 0.38 | 0.004 |
| Phenylacetylglutamine, HMDB0006344 | 263.104:0.899:n | 5.43 * | 0.009 | 0.017 | 0.319 | 0.030 | 1.89 | 0.880 | 0.94 | −0.30 | 0.020 f |
| Lysine, HMDB0000182 | 147.113:0.580:p | 4.23 | 0.020 | 0.005 | 0.365 | 0.014 | 0.85 | 0.137 | 0.88 | 0.35 | 0.007 f |
| Tyrosine, HMDB0000158 | 182.080:0.9564:p | 3.53 | 0.036 | 0.014 | 0.338 | 0.012 | 0.81 | 0.520 | 0.94 | 0.34 | 0.008 f |
| Monomethylarginine, HMDB0029416 | 189.134:0.606:p | 3.19 | 0.049 | 0.022 | 0.332 | 0.005 | 0.74 | 0.378 | 0.93 | 0.32 | 0.014 f |
| Oxo-proline, HMDB0000267 | 128.035:1.137:n | 2.89 | 0.054 | 0.028 | 0.316 | 0.021 | 0.69 | 0.638 | 0.86 | 0.34 | 0.013 |
| Creatinine | Jaffé method | 6.57 | 0.003 | 0.002 | 0.446 | 0.055 | 1.27 | 0.003 | 1.25 | −0.31 | 0.020 |
| Creatinine, HMDB0000562 | 114.066:0.614:p | 6.14 | 0.004 | 0.011 | 0.428 | 0.271 | 1.07 | 0.001 | 1.30 | −0.30 | 0.035 |
| Linoleic acid (18:2n-6), HMDB0000673 | 279.233:1.0189:l | 4.96 | 0.010 | 0.007 | 0.390 | 0.101 | 0.78 | 0.009 | 0.95 | 0.24 | 0.066 |
| Eicosadienoic acid (20:2), HMDB0005060 | 307.265:0.994:l | 4.30 | 0.018 | 0.010 | 0.368 | 0.089 | 0.79 | 0.019 | 1 | 0.25 | 0.061 |
| Nervonic acid (24:1), HMDB0002368 | 365.342:0.947:l | 3.96 | 0.025 | 0.012 | 0.356 | 0.095 | 0.86 | 0.026 | 0.75 | 0.23 | 0.085 |
| Phenylalanine/Tyrosine | - | 3.67 | 0.032 | 0.026 | 0.343 | 0.22 | 1.10 | 0.018 | 1.15 | −0.25 | 0.055 |
| Behenic acid (22:0), HMDB0000944 | 339.327:0.969:l | 3.49 | 0.038 | 0.026 | 0.336 | 0.187 | 0.91 | 0.024 | 0.75 | 0.22 | 0.105 |
| Lignoceric acid (23:0), HMDB0002003 | 367.358:0.942:l | 3.45 | 0.037 | 0.015 | 0.334 | 0.088 | 0.84 | 0.045 | 0.75 | 0.26 | 0.050 |
| Cystine, HMDB0000574 | 241.030:0.933:p | 3.15 * | 0.050 | 0.028 | 0.377 | 0.305 | 1.07 | 0.019 | 1.29 | −0.24 | 0.065 |
| Metabolite ID | m/z:RMT:mode | p-Value | FDR q-Value a | FC b (CLTI/IC) | r Correlation to ABI c | p-Value for r |
|---|---|---|---|---|---|---|
| Stearic acid (18:0), HMDB0000827 | 283.264:1.005:l | 0.001 | 0.014 | 0.72 | 0.51 | 0.001 |
| Linoleic acid (18:2n-6) HMDB0000673 | 279.233:1.019:l | 0.003 | 0.028 | 0.68 | 0.39 | 0.016 |
| Heptadecanoic acid (17:0), HMDB0002259 | 269.249:1.030:l | 0.003 | 0.029 | 0.72 | 0.43 | 0.007 |
| Palmitic acid (16:0), HMDB0000220 | 255.233:1.030:l | 0.004 | 0.030 | 0.73 | 0.37 | 0.024 |
| Creatinine, HMDB0000562 | 114.066:0.614:p | 0.004 | 0.031 | 1.30 | −0.45 | 0.004 |
| Carnitine, HMDB0000062 | 162.112:0.719:p | 0.005 | 0.031 | 1.28 | −0.48 | 0.002 |
| Oleic acid; 18:1n-9 HMDB0000207 | 281.249:1.013:l | 0.005 | 0.031 | 0.71 | −0.04 | 0.756 |
| Heptadecenoic acid (17:1n-9), HMDB0062437 | 267.233:1.026:l | 0.008 | 0.043 | 0.73 | −0.01 | 0.961 |
| Propionylcarnitine, HMDB0000824 | 218.138:0.784:p | 0.008 | 0.043 | 1.37 | 0.09 | 0.507 |
| Eicosadienoic acid (20:2n-6), HMDB0005060 | 307.265:0.994:l | 0.009 | 0.047 | 0.72 | 0.37 | 0.023 |
| Pentadecanoic acid (15:0), HMDB0000826 | 241.217:1.042:l | 0.010 | 0.047 | 0.66 | 0.33 | 0.044 |
| Cystine, HMDB0000574 | 241.0299:0.933:p | 0.014 | 0.061 | 1.29 | −0.48 | 0.002 |
| Arachidic acid (20:0n-3), HMDB0002212 | 311.296:0.981:l | 0.015 | 0.061 | 0.68 | 0.39 | 0.015 |
| Trimethylamine-N-oxide, HMDB0000925 | 76.077:0.544:p | 0.019 | 0.080 | 1.60 | −0.44 | 0.005 |
| Nervonic acid (24:1), HMDB0002368 | 365.342:0.947:l | 0.024 | 0.091 | 0.75 | 0.29 | 0.083 |
| Phe/Tyr ratio | - | 0.022 | 0.103 | 1.19 | −0.33 | 0.041 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azab, S.M.; Zamzam, A.; Syed, M.H.; Abdin, R.; Qadura, M.; Britz-McKibbin, P. Serum Metabolic Signatures of Chronic Limb-Threatening Ischemia in Patients with Peripheral Artery Disease. J. Clin. Med. 2020, 9, 1877. https://doi.org/10.3390/jcm9061877
Azab SM, Zamzam A, Syed MH, Abdin R, Qadura M, Britz-McKibbin P. Serum Metabolic Signatures of Chronic Limb-Threatening Ischemia in Patients with Peripheral Artery Disease. Journal of Clinical Medicine. 2020; 9(6):1877. https://doi.org/10.3390/jcm9061877
Chicago/Turabian StyleAzab, Sandi M., Abdelrahman Zamzam, Muzammil H. Syed, Rawand Abdin, Mohammad Qadura, and Philip Britz-McKibbin. 2020. "Serum Metabolic Signatures of Chronic Limb-Threatening Ischemia in Patients with Peripheral Artery Disease" Journal of Clinical Medicine 9, no. 6: 1877. https://doi.org/10.3390/jcm9061877
APA StyleAzab, S. M., Zamzam, A., Syed, M. H., Abdin, R., Qadura, M., & Britz-McKibbin, P. (2020). Serum Metabolic Signatures of Chronic Limb-Threatening Ischemia in Patients with Peripheral Artery Disease. Journal of Clinical Medicine, 9(6), 1877. https://doi.org/10.3390/jcm9061877









