Evaluation of Transcatheter Alcohol-Mediated Perivascular Renal Denervation to Treat Resistant Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
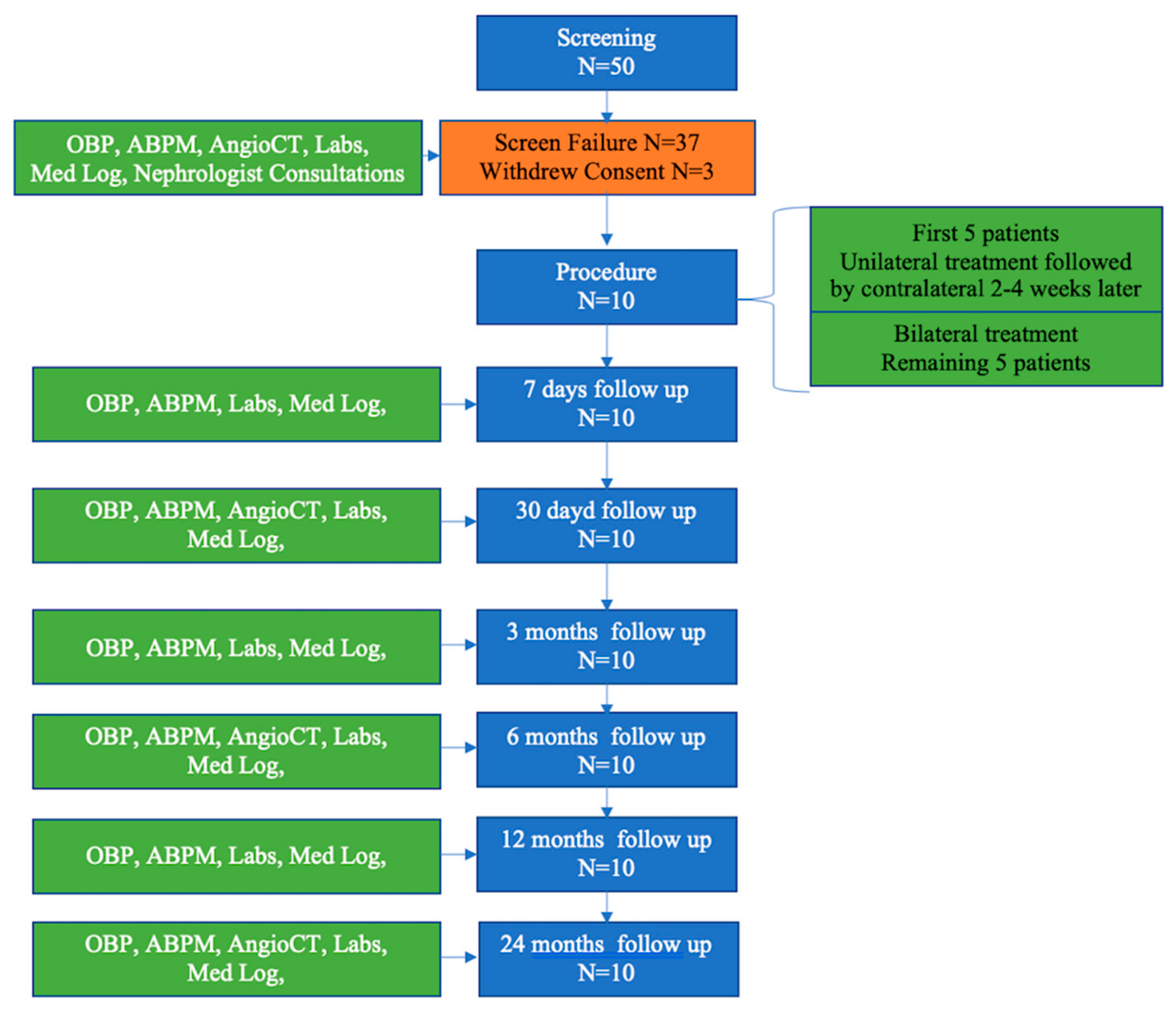
2.2. Study Design
2.3. Study Procedures
2.3.1. Baseline
2.3.2. Renal Denervation Procedure
2.3.3. Pre-Discharge
2.3.4. Follow-Up
2.4. Study Oversight
3. Statistical Analysis
4. Results
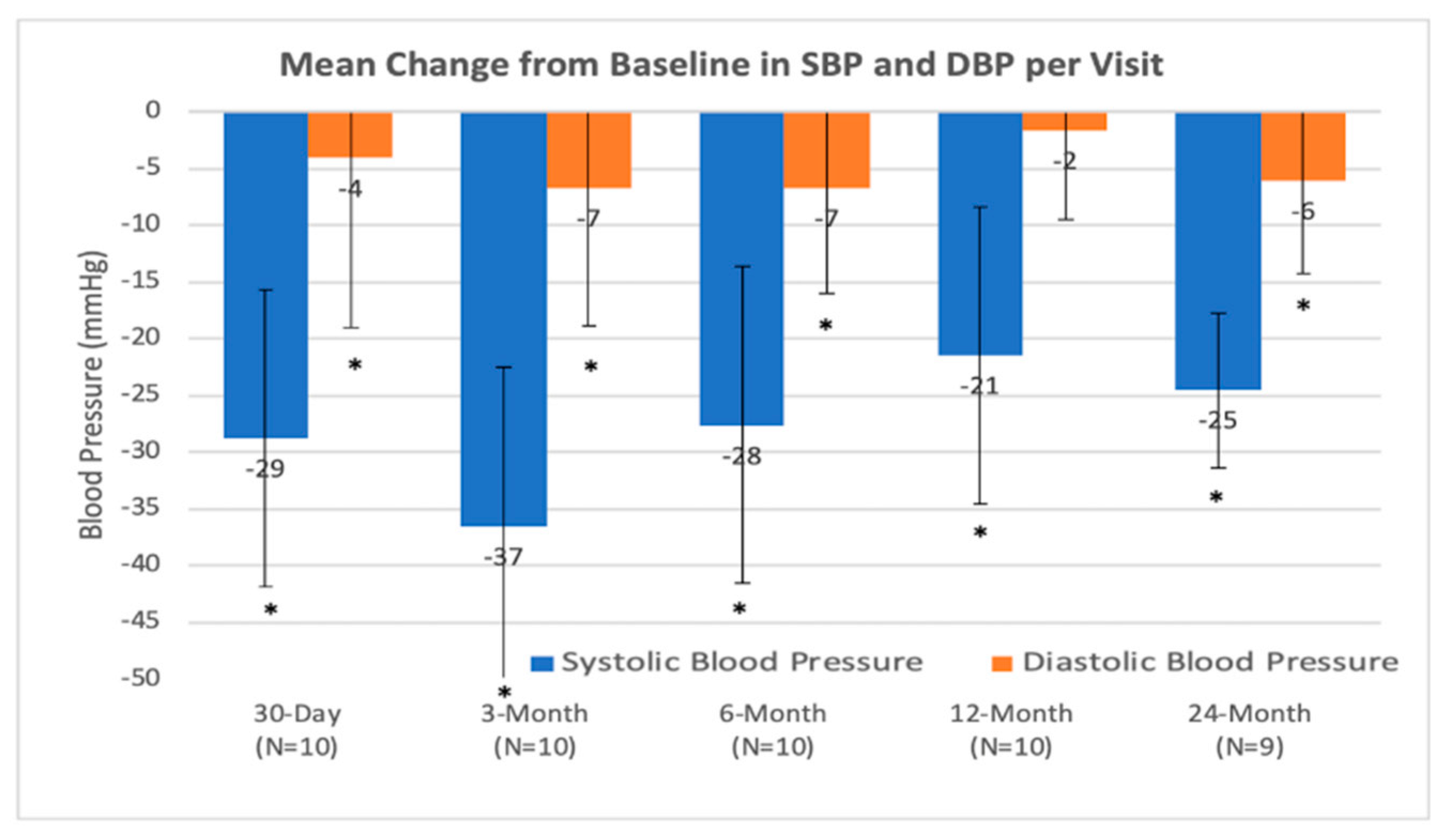
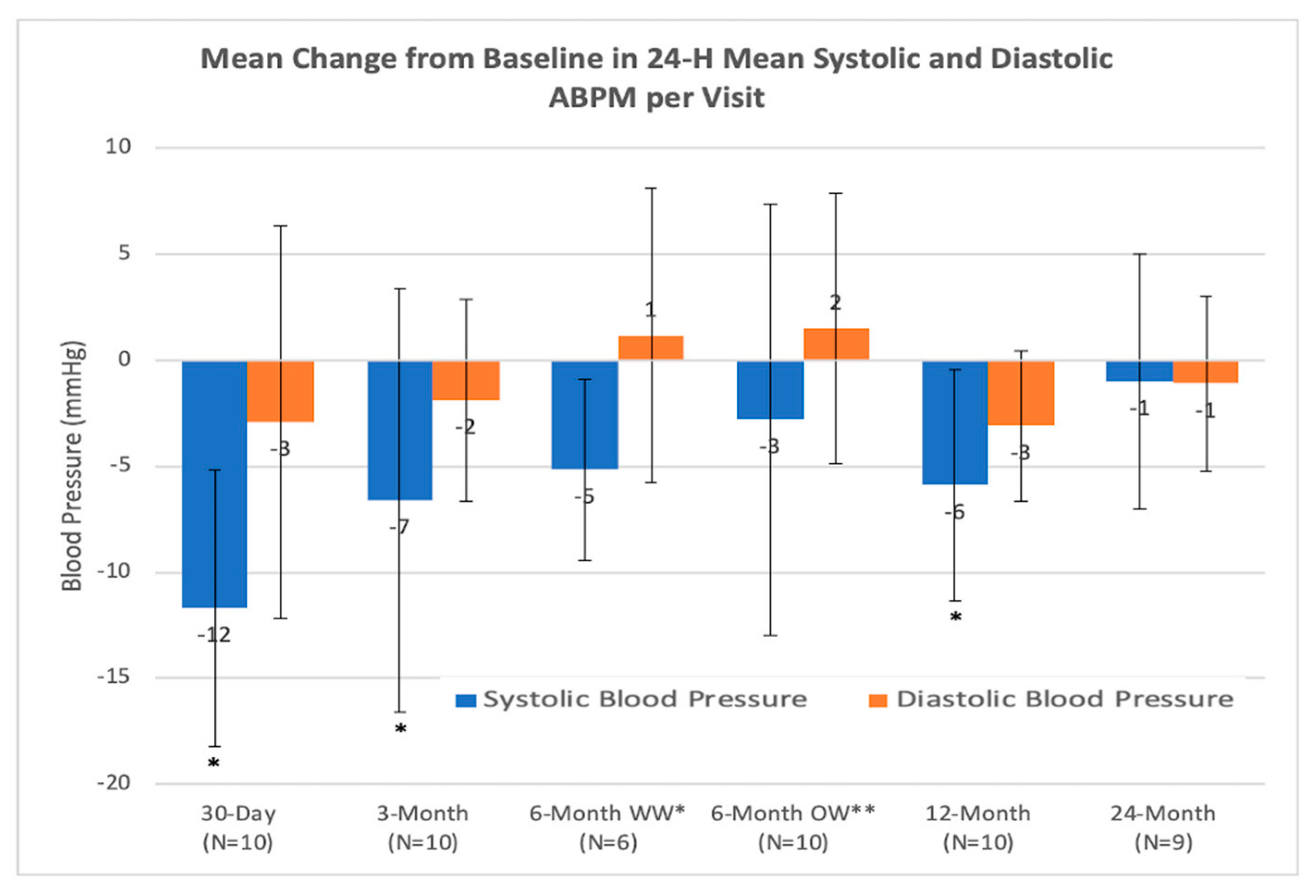
4.1. Safety Objectives
4.2. Efficiency Objectives
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolf-Maier, K.; Cooper, R.S.; Banegas, J.R.; Giampaoli, S.; Hense, H.W.; Joffres, M.; Kastarinen, M.; Poulter, N.; Primatesta, P.; Rodríguez-Artalejo, F.; et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA 2003, 289, 2363–2369. [Google Scholar] [CrossRef] [PubMed]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [PubMed]
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. Executive summary: Heart disease and stroke statistics—2010 update: A report from the american heart association. Circulation 2010, 121, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Georgianos, P.; Bakris, G.L. Resistant hypertension—Its identification and epidemiology. Nat. Rev. Nephrol. 2012, 9, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Bakris, G.L.; Nathan, S. Renal denervation and left ventricular mass regression: A benefit beyond blood pressure reduction? J. Am. Coll. Cardiol. 2013, 63, 1924–1925. [Google Scholar] [CrossRef]
- DiBona, G.F. Physiology in perspective: The wisdom of the body. Neural control of the kidney. Am. J. Physiol. Integr. Comp. Physiol. 2005, 289, R633–R641. [Google Scholar] [CrossRef]
- Böhm, M.; Linz, D.; Ukena, C.; Esler, M.; Mahfoud, F. Renal denervation for the treatment of cardiovascular high risk-hypertension or beyond? Circ. Res. 2014, 115, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, F.; Schlaich, M.P.; Böhm, M.; Esler, M.; Lüscher, T.F. Catheter-based renal denervation: The next chapter begins. Eur. Hear. J. 2018, 39, 4144–4149. [Google Scholar] [CrossRef]
- Townsend, R.R.; Mahfoud, F.; E Kandzari, D.; Kario, K.; Pocock, S.; A Weber, M.; Ewen, S.; Tsioufis, K.; Tousoulis, D.; Sharp, A.S.P.; et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): A randomised, sham-controlled, proof-of-concept trial. Lancet 2017, 390, 2160–2170. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Böhm, M.; Mahfoud, F.; Townsend, R.R.; A Weber, M.; Pocock, S.; Tsioufis, K.; Tousoulis, D.; Choi, J.W.; East, C.; et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018, 391, 2346–2355. [Google Scholar] [CrossRef]
- Azizi, M.; Schmieder, R.E.; Mahfoud, F.; Weber, M.A.; Daemen, J.; Davies, J.; Basile, J.; Kirtane, A.J.; Wang, Y.; Lobo, M.D.; et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): A multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018, 391, 2335–2345. [Google Scholar] [CrossRef]
- Fischell, T.A.; Ebner, A.; Gallo, S.; Ikeno, F.; Minarsch, L.; Vega, F.; Haratani, N.; Ghazarossian, V.E. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system. JACC Cardiovasc. Interv. 2016, 9, 589–598. [Google Scholar] [CrossRef]
- Fischell, T.A.; Vega, F.; Raju, N.; Johnson, E.T.; Kent, D.J.; Ragland, R.R.; Fischell, D.R.; Almany, S.L.; Ghazarossian, V.E. Ethanol-mediated perivascular renal sympathetic denervation: Preclinical validation of safety and efficacy in a porcine model. EuroIntervention 2013, 9, 140–147. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar]
- Krum, H.; Schlaich, M.P.; Sobotka, P.A.; Böhm, M.; Mahfoud, F.; Rocha-Singh, K.; Katholi, R.E.; Esler, M.D. Percutaneous renal denervation in patients with treatment-resistant hypertension: Final 3-year report of the Symplicity HTN-1 study. Lancet 2014, 383, 622–629. [Google Scholar] [CrossRef]
- Esler, M.; Bohm, M.; Sievert, H.; Rump, C.L.; Schmieder, R.E.; Krum, H.; Mahfoud, F.; Schlaich, M.P. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur. Heart J. 2014, 35, 1752–1759. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Kandzari, D.E.; O’Neill, W.W.; D’Agostino, R.; Flack, J.M.; Katzen, B.T.; Leon, M.B.; Liu, M.; Mauri, L.; Negoita, M.; et al. A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med. 2014, 370, 1393–1401. [Google Scholar] [CrossRef]
- Sakakura, K.; Ladich, E.; Cheng, Q.; Otsuka, F.; Yahagi, K.; Fowler, D.R.; Kolodgie, F.D.; Virmani, R.; Joner, M. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J. Am. Coll. Cardiol. 2014, 64, 635–643. [Google Scholar] [CrossRef]
- Mahfoud, F.; Tunev, S.; Ewen, S.; Cremers, B.; Ruwart, J.; Schulz-Jander, D.; Linz, D.; Davies, J.; Kandzari, D.E.; Whitbourn, R.; et al. Impact of lesion placement on efficacy and safety of catheter-based radiofrequency renal denervation. J. Am. Coll. Cardiol. 2015, 66, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria |
|---|
| Adult patient, aged 18–75, male or female |
| Patient has a clinic systolic blood pressure ≥ 160 mmHg (or ≥150 mmHg in type 2 diabetic patients) based on the average of 3 office/clinic measurements taken manually |
| Patient has a daytime mean systolic pressure ≥ 135 mmHg based on 24 h ambulatory blood pressure monitoring |
| Patient is receiving a stable medication regimen of at least 3 anti-hypertensive medications of different classes (for at least 4 weeks), one of which must be a diuretic, and the medication regimen is not expected to change for at least 1 month |
| Patient has an eGFR ≥ 45 mL/min, based on the Chronic Kidney Disease Epidemiology Colaboration (CKD-EPI) equation |
| Patient has optimal renal artery anatomy (no clear abnormalities) based on Investigator’s evaluation of computed tomography examination and/or angiogram including: Single artery of 5–7 mm in diameter (two arteries are acceptable if diameter of second artery is ≤2 mm) No aneurysms No excessive tortuosity |
| No previous stenting or balloon angioplasty of the renal arteries |
| Patient has provided written informed consent |
| Exclusion Criteria |
|---|
| Patient has known or suspected secondary hypertension |
| Patient has type 1 diabetes mellitus |
| Patient requires chronic oxygen support |
| Patient has primary or secondary pulmonary hypertension |
| Patient has a known bleeding diathesis or is receiving anticoagulant drugs during the 7 days prior to treatment |
| Patient has thrombocytopenia (platelet count <100,000 platelets/µL) |
| Patient is pregnant or nursing |
| Patient has significant imaging-assessed renovascular abnormalities including short length main renal artery and renal artery stenosis > 70% of the normal diameter segment |
| Patient has history of nephrectomy, kidney tumor or hydronephrosis |
| Patient is known to have a unilateral non-functioning kidney or unequal renal size (>2 cm difference in renal length between kidneys) |
| Patient has a renal transplant |
| Patient has a history of kidney stones |
| Patient has a history of heterogeneities in the kidney such as cysts or tumors |
| Patient has a history of pyelonephritis |
| Patient has a history of myocardial infarction, unstable angina pectoris, or cerebrovascular accident within the last six months |
| Patient has hemodynamically significant valvular heart disease |
| Patient has heart failure (NYHA III or IV) or had an ejection fraction ≤ 30% |
| Patient has a known allergy to contrast media |
| Patient has a life expectancy of <12 months |
| Patient is currently enrolled in other potentially confounding research, i.e., another therapeutic or interventional research trial. Patients enrolled in observational registries may still be eligible |
| Characteristic | All Treated Subjects n = 10 |
|---|---|
| Age, years (mean (min; max)) | 59.9 (46;67) |
| Male gender | 50% (5/10) |
| Renal Disease | 0% (0/10) |
| Chronic Kidney Disease | 10% (1/10) |
| Hypertension | 100% (10/10) |
| Diabetes (Type II) | 40% (4/10) |
| Arrhythmia | 0% (0/10) |
| Atrial Fibrillation/Atrial Flutter | 0% (0/10) |
| Prior Stroke or TIA | 10% (1/10) |
| Peripheral Vascular Occlusive Disease | 10% (1/10) |
| Hyperlipidemia/Dyslipidemia | 60% (6/10) |
| Angio-determined Coronary Artery Disease | 30% (3/10) |
| Prior Myocardial Infarction | 20% (2/10) |
| Previous History of Congestive Heart Failure | 0% (0/10) |
| Chronic Obstructive Pulmonary Disease | 0% (0/10) |
| History of Smoking | 30% (3/10) |
| Defined Daily Dose (DDD) of anti-hypertensive | 4.8 ± 1.3 |
| Thiazide Diuretic | 90% (9/10) |
| Loop Diuretics | 30% (3/10) |
| Beta-Blocker | 90% (9/10) |
| Alpha -1 Blocker | 50% (5/10) |
| Centrally Acting Alpha Agonist | 20% (2/10) |
| Direct Acting Vasodilator | 50% (5/10) |
| ACE Inhibitor | 70% (7/10) |
| Calcium Channel Blocker | 50% (5/10) |
| Angiotensin II Receptor Blocker | 50% (5/10) |
| Diuretic Aldosterone Receptor Blockers | 20% (2/10) |
| Parameters | Number of Procedures (n = 15 Procedures) (n = 20 Arteries) Mean ± SD (Min; Max) |
|---|---|
| Time from Insertion of Peregrine Catheter into Renal Artery to Removal from Introducer Sheath, per artery (min) | 8 ± 5 (4; 22) |
| Distance from Ostium to First Bifurcation–Right (mm) | 47.7 ± 10.4 (32.0; 67.0) |
| Distance from Ostium to First Bifurcation–Left (mm) | 33.5 ± 12.0 (12.1; 51.0) |
| Distance to Ostium from Infusion Site–Right (mm) | 25.8 ± 7.3 (9.4; 35.0) |
| Distance to Ostium from Infusion Site–Left (mm) | 16.3 ± 6.8 (8.2; 29.9) |
| Volume of Alcohol Infused, per artery (mL) | 0.3 |
| Total Volume Contrast Used, per artery (mL) | 40 ± 18 (20; 100) |
| Total IV Fluid Used, per artery (mL) | 1003 ± 507 (0; 2058) |
| Fluoroscopic Time, per artery (min) | 6.0 ± 5.4 (2.0; 26.0) |
| Average Hospital Stay (Days)—Median (IQR) | 2 (2; 3) |
| Renal Lab Parameter | Baseline (Mean ± SD) n = 10 | 7-Day (Mean ± SD) n = 10 | 30-Day (Mean ± SD) n = 10 | 3-Month (Mean ± SD) n = 10 | 6-Month (Mean ± SD) n = 10 | 12-Month (Mean ± SD) n = 10 | 24-Month (Mean ± SD) n = 8 |
|---|---|---|---|---|---|---|---|
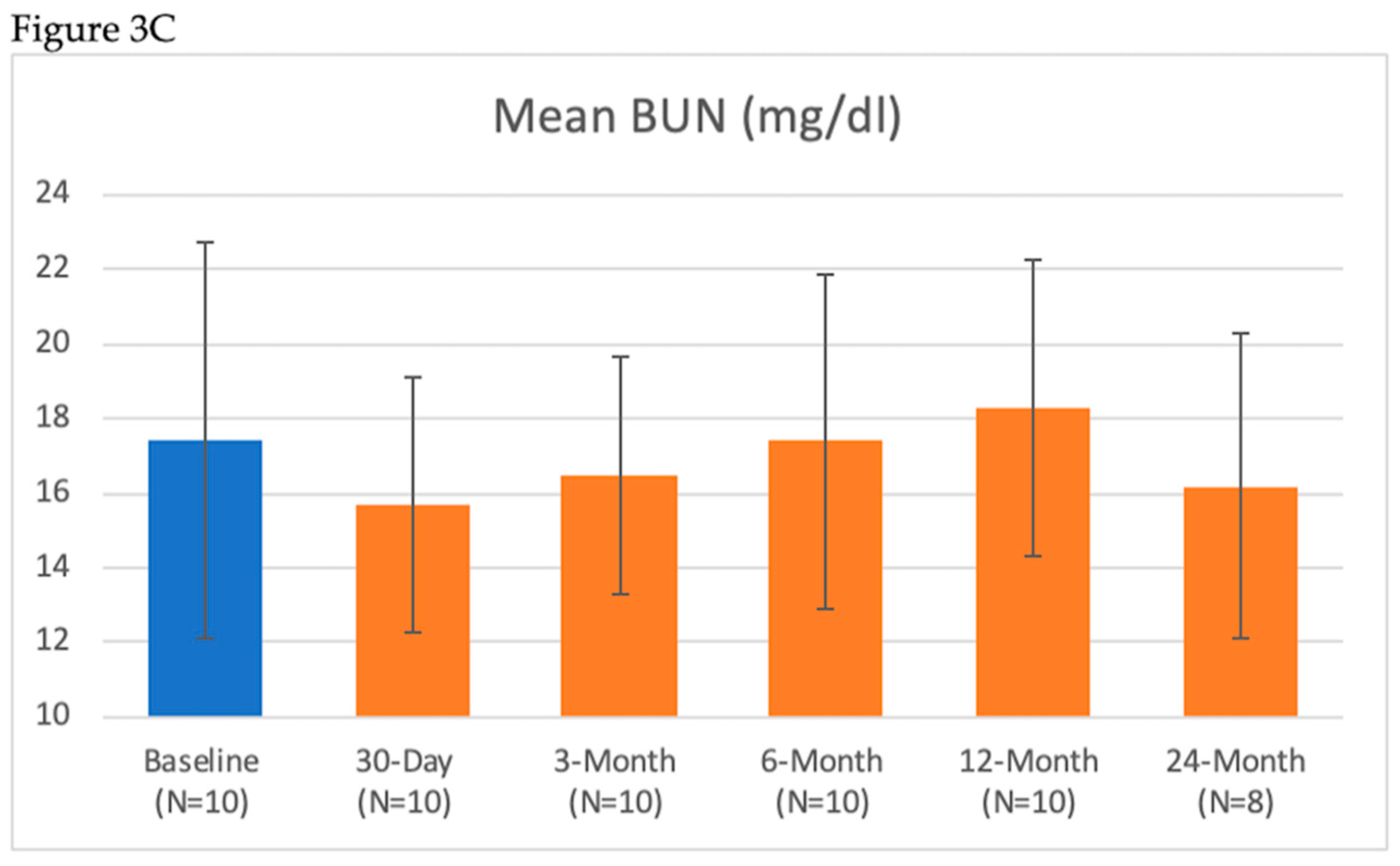
| BUN (mg/dL) | 17.4 ± 5.3 | 17.5 ± 3.8 | 15.7 ± 3.4 | 16.5 ± 3.2 | 17.4 ± 4.5 | 18.3 ± 4.0 | 16.2 ± 4.1 |
| Serum Creatinine (mg/dL) | 0.96 ± 0.37 | 0.96 ± 0.37 | 0.87 ± 0.22 | 0.90 ± 0.34 | 0.91 ± 0.30 | 0.94 ± 0.29 | 0.85 ± 0.22 |
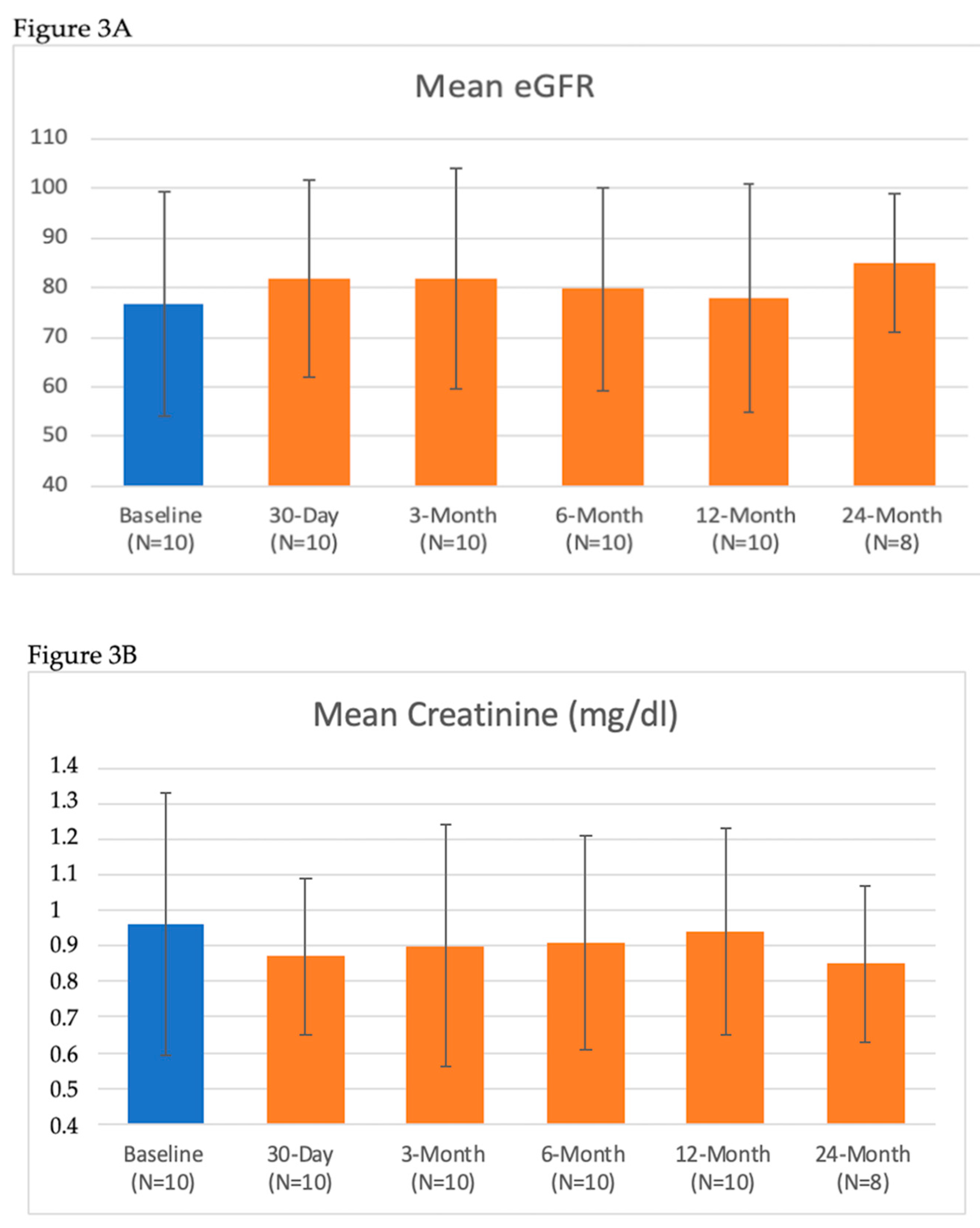
| eGFR (mL/min/1.73 m2) | 78 ± 21 | 76 ± 21 | 82 ± 20 | 82 ± 22 | 80 ± 20 | 77 ± 20 | 85 ± 14 |
| ≥25% Reduction in eGFR | 10% (1/10) | 0% (0/10) | 0% (0/10) | 0% (0/10) | 10% (1/10) | 0% (0/8) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janas, A.; Król, M.; Hochuł, M.; Jochymczyk, M.; Hayward-Costa, C.; Parise, H.; Haratani, N.; Fischell, T.; Wojakowski, W. Evaluation of Transcatheter Alcohol-Mediated Perivascular Renal Denervation to Treat Resistant Hypertension. J. Clin. Med. 2020, 9, 1881. https://doi.org/10.3390/jcm9061881
Janas A, Król M, Hochuł M, Jochymczyk M, Hayward-Costa C, Parise H, Haratani N, Fischell T, Wojakowski W. Evaluation of Transcatheter Alcohol-Mediated Perivascular Renal Denervation to Treat Resistant Hypertension. Journal of Clinical Medicine. 2020; 9(6):1881. https://doi.org/10.3390/jcm9061881
Chicago/Turabian StyleJanas, Adam, Marek Król, Mariusz Hochuł, Monika Jochymczyk, Claudia Hayward-Costa, Helen Parise, Nicole Haratani, Tim Fischell, and Wojciech Wojakowski. 2020. "Evaluation of Transcatheter Alcohol-Mediated Perivascular Renal Denervation to Treat Resistant Hypertension" Journal of Clinical Medicine 9, no. 6: 1881. https://doi.org/10.3390/jcm9061881
APA StyleJanas, A., Król, M., Hochuł, M., Jochymczyk, M., Hayward-Costa, C., Parise, H., Haratani, N., Fischell, T., & Wojakowski, W. (2020). Evaluation of Transcatheter Alcohol-Mediated Perivascular Renal Denervation to Treat Resistant Hypertension. Journal of Clinical Medicine, 9(6), 1881. https://doi.org/10.3390/jcm9061881





