Sinus Rhythm Conduction Properties across Bachmann’s Bundle: Impact of Underlying Heart Disease and Atrial Fibrillation
Abstract
1. Introduction
2. Methods
2.1. Study Population
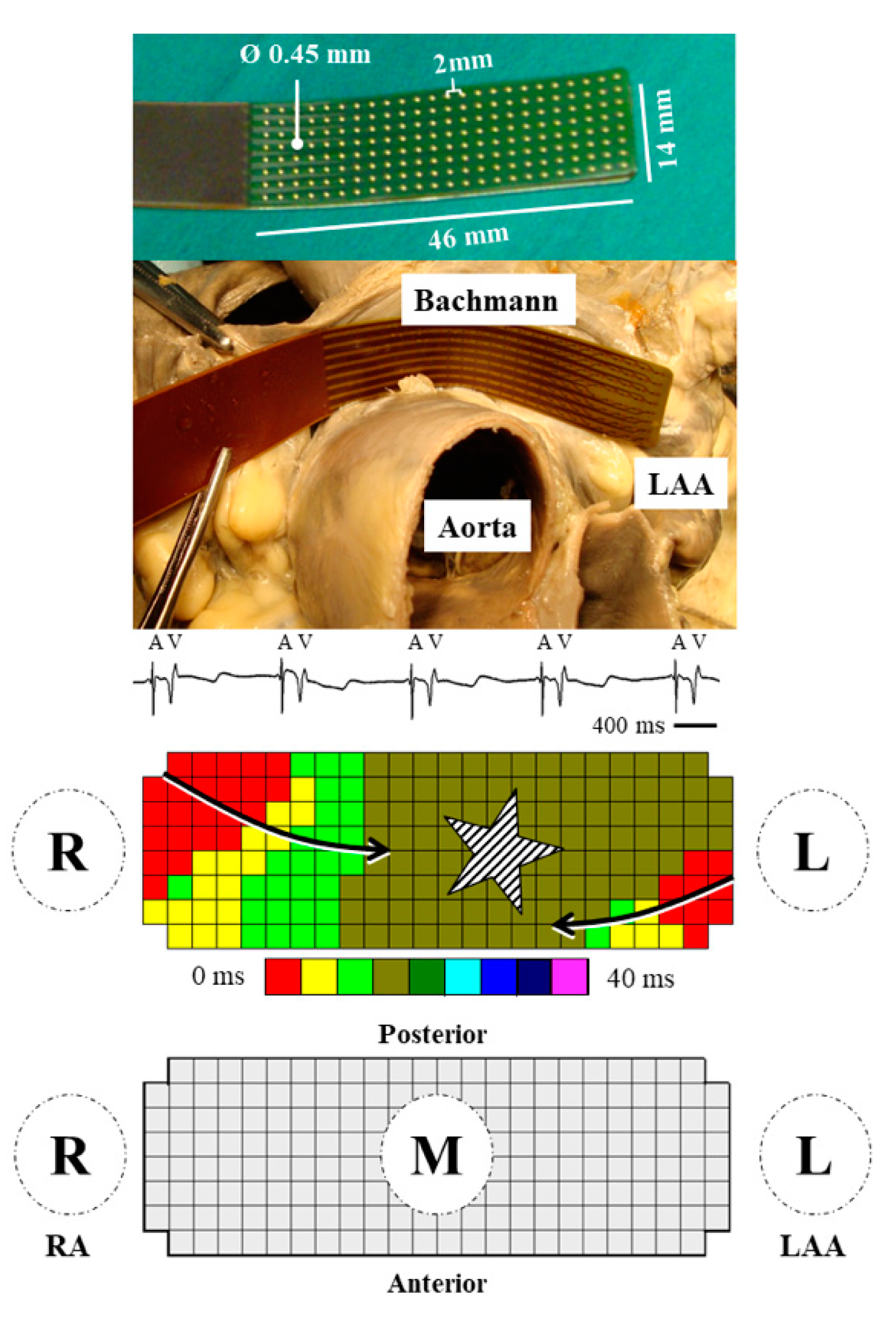
2.2. Mapping Procedure
2.3. Mapping Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Study Population
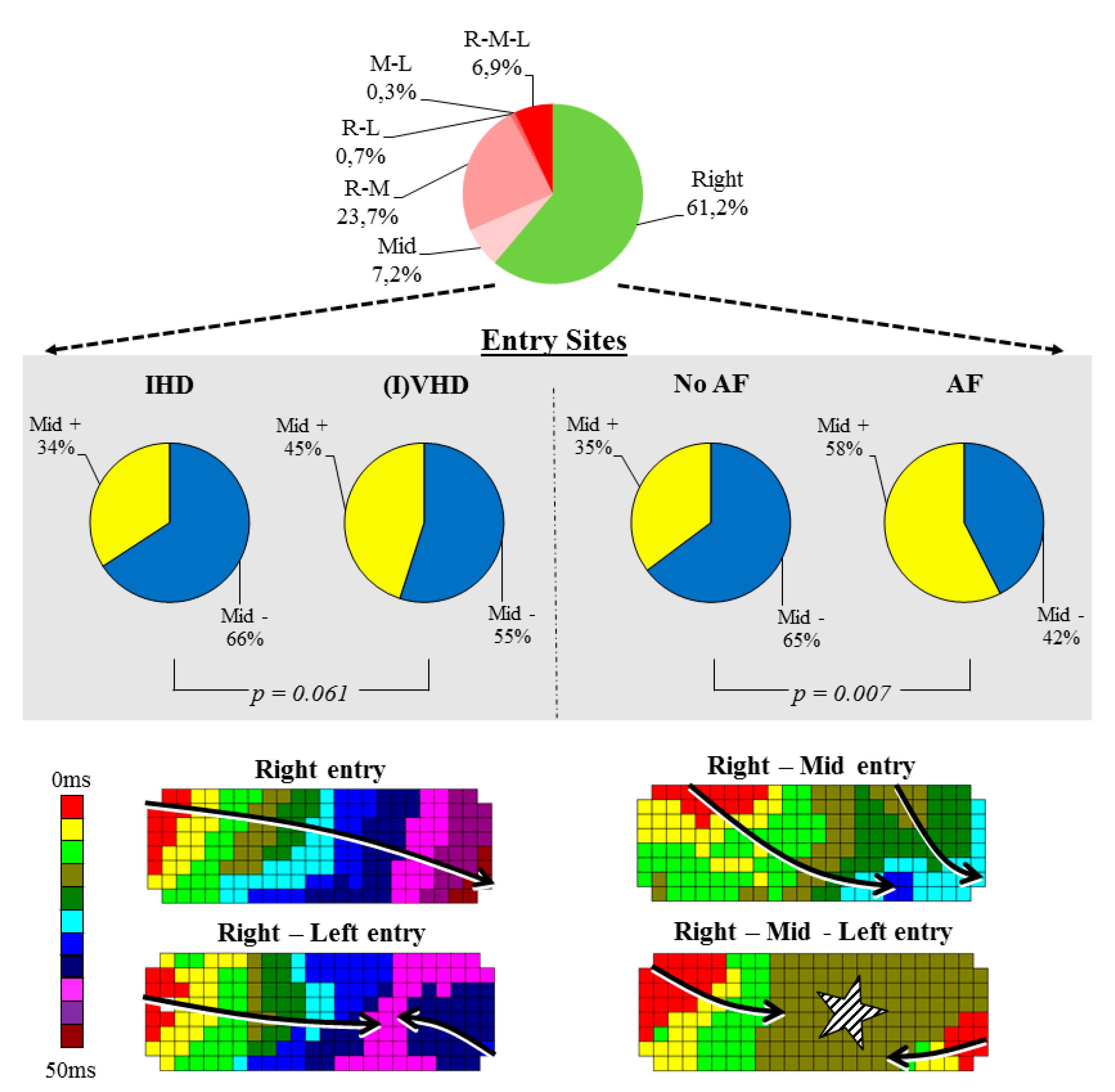
3.2. Impact of Heart Disease and Atrial Fibrillation on Entry Sites
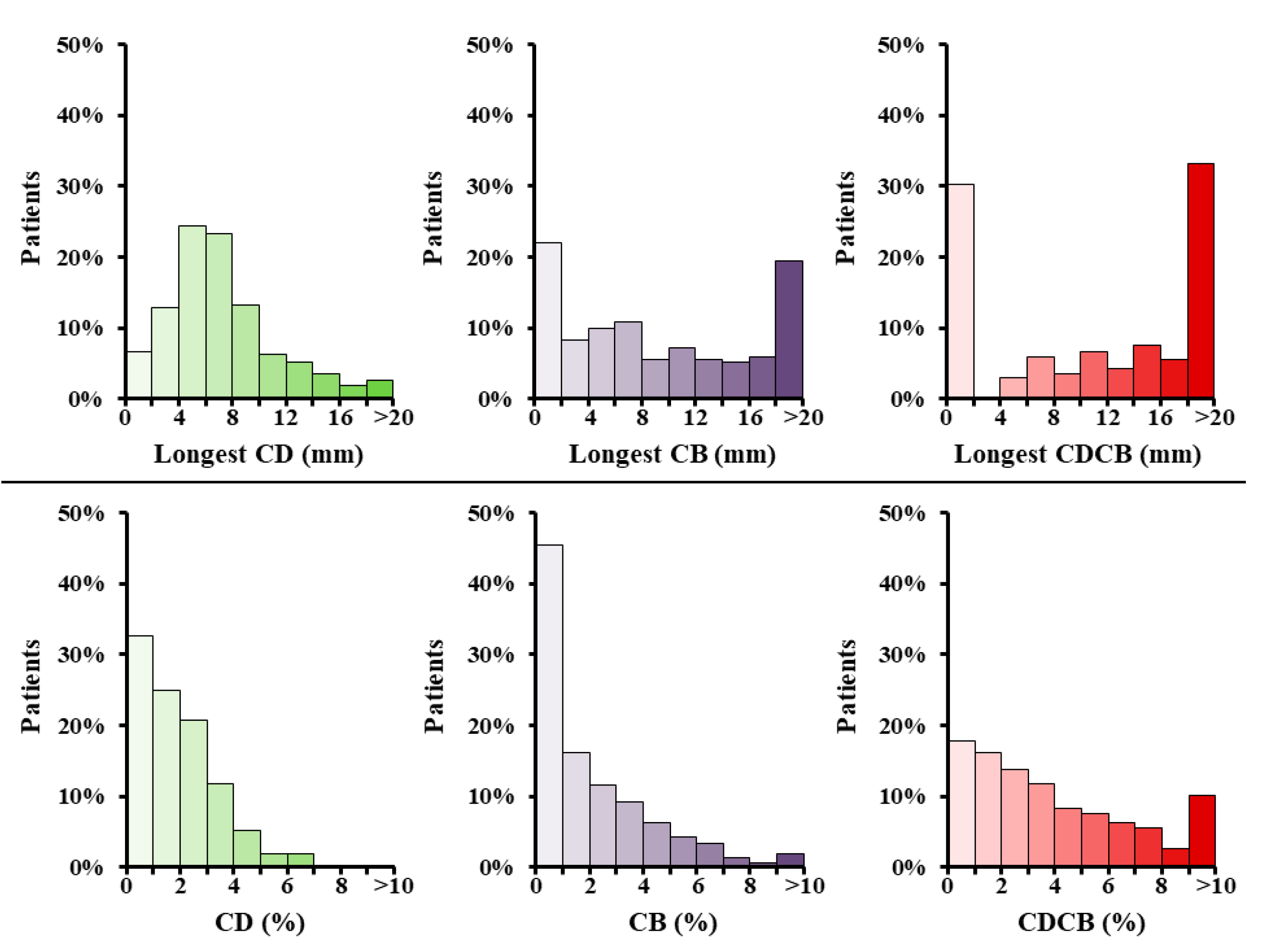
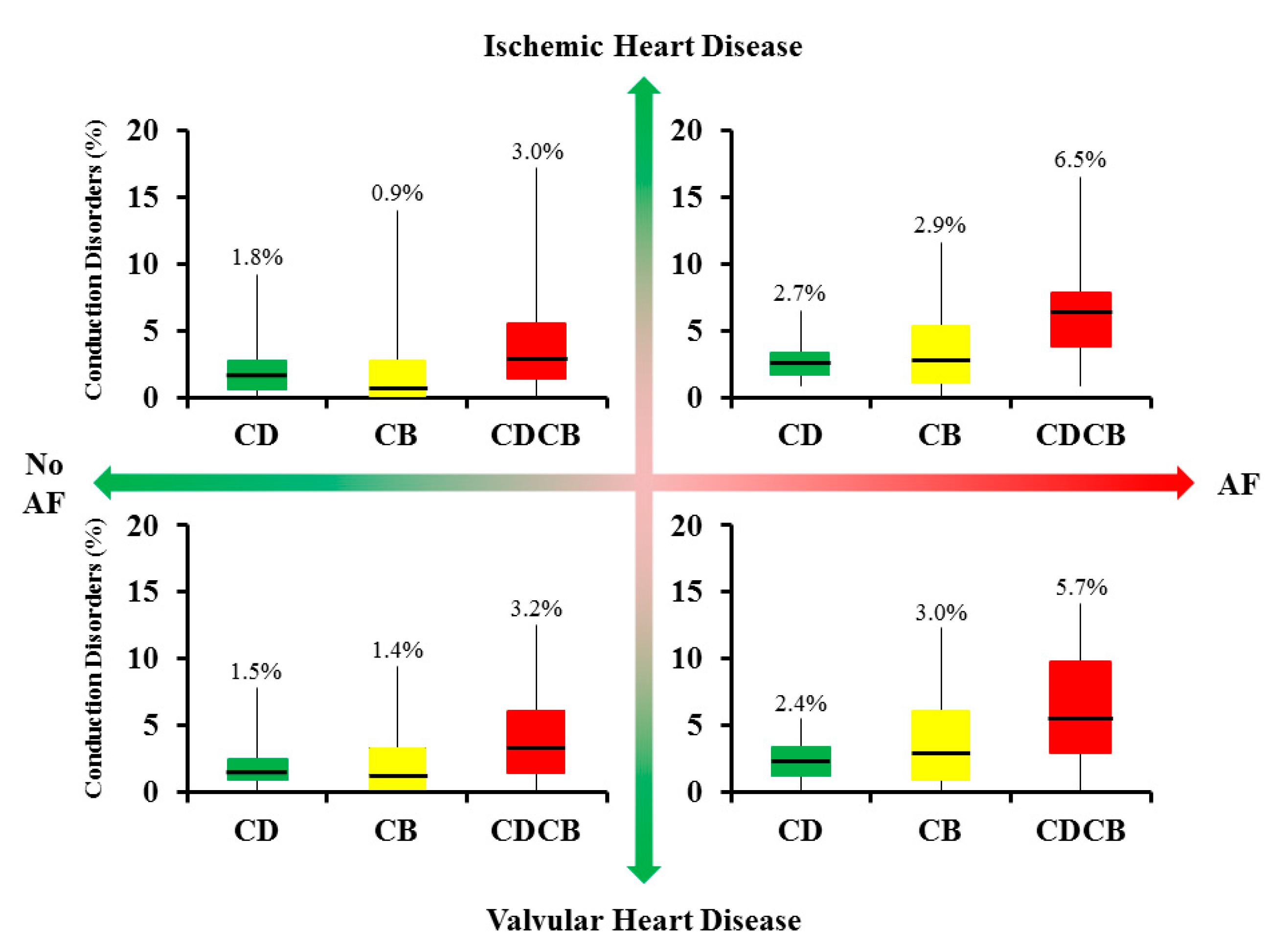
3.3. Correlation between Heart Disease or Atrial Fibrillation with Conduction Disorders
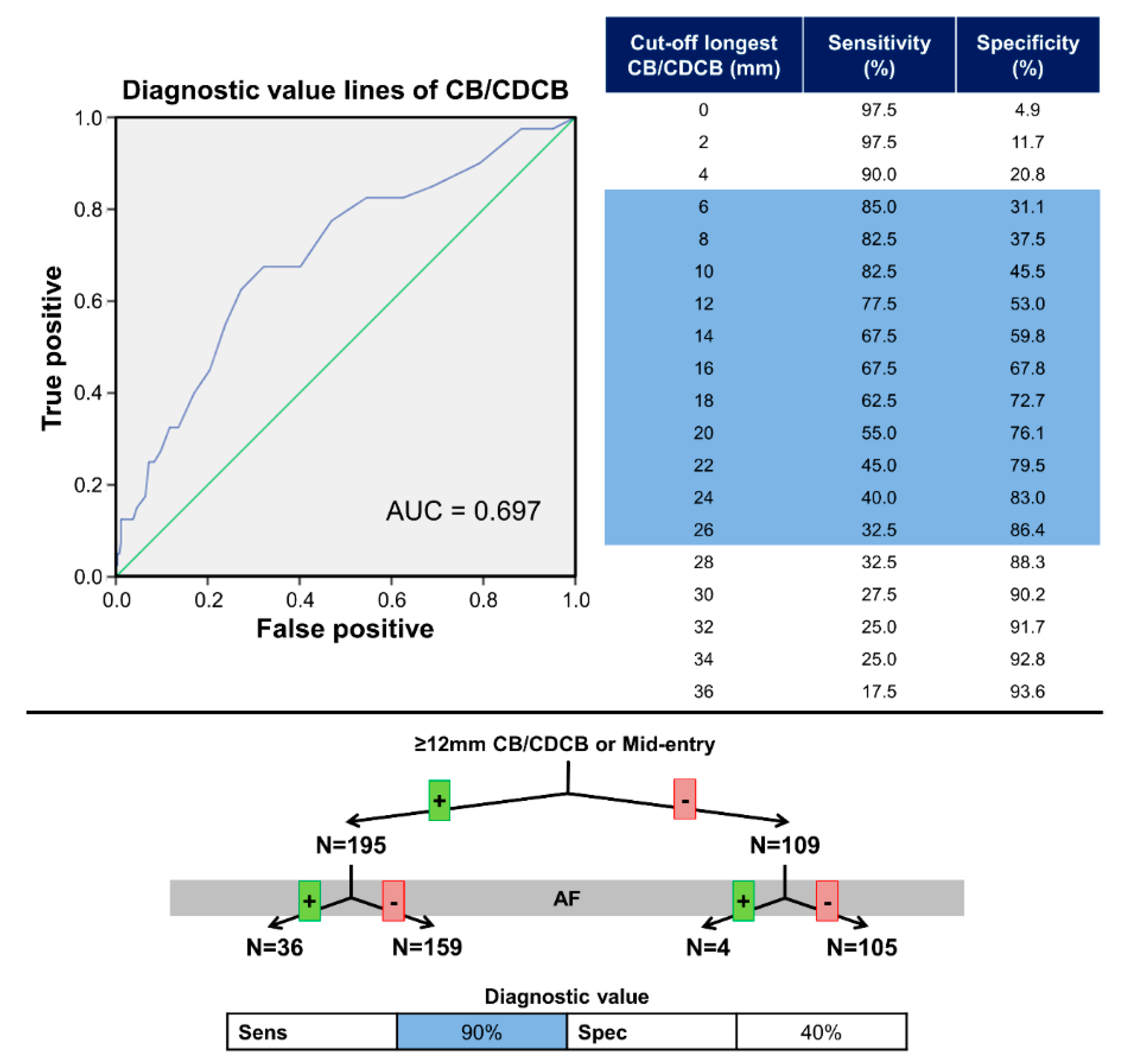
3.4. Diagnostic Value for Atrial Fibrillation
4. Discussion
4.1. Atrial Remodeling in Atrial Fibrillation
4.2. Relation between Mid-Entry and Patients with Atrial Fibrillation
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Markides, V.; Schilling, R.J.; Ho, S.Y.; Chow, A.W.; Davies, D.W.; Peters, N.S. Characterization of left atrial activation in the intact human heart. Circulation 2003, 107, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Teuwen, C.P.; Yaksh, A.; Lanters, E.A.; Kik, C.; Van Der Does, L.J.; Knops, P.; Taverne, Y.J.; Van De Woestijne, P.C.; Oei, F.B.; Bekkers, J.A.; et al. Relevance of Conduction Disorders in Bachmann’s Bundle During Sinus Rhythm in Humans. Circ. Arrhythmia Electrophysiol. 2016, 9, e003972. [Google Scholar] [CrossRef] [PubMed]
- Khaja, A.; Flaker, G. Bachmann’s Bundle: Does It Play a Role in Atrial Fibrillation? Pacing Clin. Electrophysiol. 2005, 28, 855–863. [Google Scholar] [CrossRef] [PubMed]
- van Campenhout, M.J.; Yaksh, A.; Kik, C.; de Jaegere, P.P.; Ho, S.Y.; Allessie, M.A.; de Groot, N.M. Bachmann’s bundle: A key player in the development of atrial fibrillation? Circ. Arrhythm. Electrophysiol. 2013, 6, 1041–1046. [Google Scholar] [CrossRef]
- Baranchuk, A. Interatrial Block and Supraventricular Arrhythmias: Clinical Implications of Bayés’ Syndrome; Cardiotext Publishing: Minneapolis, MN, USA, 2017. [Google Scholar]
- Bailin, S.; Adler, S.; Giudici, M. Prevention of chronic atrial fibrillation by pacing in the region of Bachmann’s bundle: Results of a multicenter randomized trial. J. Cardiovasc. Electrophysiol. 2001, 12, 912–917. [Google Scholar] [CrossRef]
- Nigro, G.; Russo, V.; Politano, L.; Della Cioppa, N.; Rago, A.; Arena, G.; Papa, A.A.; Paoli, L.D.; De Chiara, A.; Russo, V.; et al. Does Bachmann’s bundle pacing prevent atrial fibrillation in myotonic dystrophy type 1 patients? A 12 months follow-up study. Europace 2010, 12, 1219–1223. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Levy, D.; Vaziri, S.M.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A. Independent Risk Factors for Atrial Fibrillation in a Population-Based Cohort. JAMA 1994, 271, 840–844. [Google Scholar] [CrossRef]
- Lanters, E.; Yaksh, A.; Teuwen, C.P.; Van Der Does, L.J.; Kik, C.; Knops, P.; Van Marion, D.M.S.; Brundel, B.J.J.M.; Bogers, A.J.J.C.; A Allessie, M.; et al. Spatial distribution of conduction disorders during sinus rhythm. Int. J. Cardiol. 2017, 249, 220–225. [Google Scholar] [CrossRef]
- Knol, W.G.; Teuwen, C.P.; Kleinrensink, G.-J.; Bogers, A.J.J.C.; De Groot, N.M.; Taverne, Y.J. The Bachmann bundle and interatrial conduction: Comparing atrial morphology to electrical activity. Heart Rhythm 2019, 16, 606–614. [Google Scholar] [CrossRef]
- Moe, G.; Abildskov, J. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am. Heart J. 1959, 58, 59–70. [Google Scholar] [CrossRef]
- Groot, N.M.; Van Der Does, L.; Yaksh, A.; Lanters, E.; Teuwen, C.; Knops, P.; Van De Woestijne, P.; Bekkers, J.; Kik, C.; Bogers, A.; et al. Direct Proof of Endo-Epicardial Asynchrony of the Atrial Wall During Atrial Fibrillation in Humans. Circ. Arrhythmia Electrophysiol. 2016, 9, e003648. [Google Scholar] [CrossRef]
- Müller-Edenborn, B.; Chen, J.; Allgeier, J.; Didenko, M.V.; Moreno-Weidmann, Z.; Neumann, F.-J.; Lehrmann, H.; Weber, R.; Arentz, T.; Jadidi, A. Amplified sinus-P-wave reveals localization and extent of left atrial low-voltage substrate: Implications for arrhythmia freedom following pulmonary vein isolation. Europace 2019, 22, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Legato, M.J.; Bull, M.B.; Ferrer, M.I. Atrial Ultrastructure in Patients with Fixed Intra-atrial Block. Chest 1974, 65, 252–261. [Google Scholar] [CrossRef] [PubMed]
- van der Does, L.J.; Lanters, E.A.; Teuwen, C.P.; Mouws, E.M.; Yaksh, A.; Knops, P.; de Groot, N.M.; Kik, C.; Bogers, A.J.J.C. The Effects of Valvular Heart Disease on Atrial Conduction during Sinus Rhythm. Prelim. Results 2019. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, M.C.; Kirchhof, C.J.; Dorland, R.; Allessie, M.A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995, 92, 1954–1968. [Google Scholar] [CrossRef]
- Ausma, J.; Wijffels, M.; Wouters, L.; Allessie, M.; Borgers, M.; Thoné, F. Structural Changes of Atrial Myocardium due to Sustained Atrial Fibrillation in the Goat. Circulation 1997, 96, 3157–3163. [Google Scholar] [CrossRef]
- Dispersyn, G.D.; Ausma, J.; Thoné, F.; Flameng, W.; Vanoverschelde, J.-L.; Allessie, M.A.; Ramaekers, F.C.; Borgers, M. Cardiomyocyte remodelling during myocardial hibernation and atrial fibrillation: Prelude to apoptosis. Cardiovasc. Res. 1999, 43, 947–957. [Google Scholar] [CrossRef]
- Lin, C.-S.; Pan, C.-H. Regulatory mechanisms of atrial fibrotic remodeling in atrial fibrillation. Cell. Mol. Life Sci. 2008, 65, 1489–1508. [Google Scholar] [CrossRef]
- Frustaci, A.; Chimenti, C.; Bellocci, F.; Morgante, E.; Russo, M.A.; Maseri, A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997, 96, 1180–1184. [Google Scholar] [CrossRef]
- Aguero, J.; Galán-Arriola, C.; Fernández-Jiménez, R.; Sanchez-Gonzalez, J.; Ajmone, N.; Delgado, V.; Solis, J.; Lopez, G.J.; De Molina-Iracheta, A.; Hajjar, R.J.; et al. Atrial Infarction and Ischemic Mitral Regurgitation Contribute to Post-MI Remodeling of the Left Atrium. J. Am. Coll. Cardiol. 2017, 70, 2878–2889. [Google Scholar] [CrossRef]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef] [PubMed]
- Platonov, P.G.; Mitrofanova, L.; Ivanov, V.; Ho, S.Y. Substrates for intra-atrial and interatrial conduction in the atrial septum: Anatomical study on 84 human hearts. Heart Rhythm 2008, 5, 1189–1195. [Google Scholar] [CrossRef]
- Dobrzynski, H.; Li, J.; Tellez, J.; Greener, I.D.; Nikolski, V.P.; Wright, S.E.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. Computer three-dimensional reconstruction of the sinoatrial node. Circulation 2005, 111, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Sánchez-Quintana, D. Anatomy and pathology of the sinus node. J. Interv. Card. Electrophysiol. 2015, 46, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hansen, B.J.; Csepe, T.A.; Zhao, J.; Ignozzi, A.J.; Sul, L.V.; Zakharkin, S.O.; Kalyanasundaram, A.; Davis, J.P.; Biesiadecki, B.J.; et al. Redundant and diverse intranodal pacemakers and conduction pathways protect the human sinoatrial node from failure. Sci. Transl. Med. 2017, 9, eaam5607. [Google Scholar] [CrossRef]
| Total | IHD | (I)VHD | p-Value | |
|---|---|---|---|---|
| Patients, n | 304 | 193 | 111 | |
| Age, years (mean ± SD) | 66.0 ± 10.1 | 65.5 ± 9.2 | 66.8 ± 11.4 | 0.415 |
| Male gender, n (%) | 237 (78.0) | 163 (84.5) | 74 (66.7) | <0.001 |
| BSA, m2 (mean ± SD) | 2.02 ± 0.21 | 2.05 ± 0.20 | 1.96 ± 0.21 | 0.564 |
| Hypertension, n (%) | 170 (55.9) | 120 (62.2) | 50 (45.0) | 0.004 |
| Hypercholesterolemia, n (%) | 111 (36.5) | 84 (43.5) | 27 (24.3) | 0.001 |
| Diabetes mellitus, n (%) | 85 (28.0) | 68 (35.2) | 17 (15.3) | <0.001 |
| AAD, n (%) | 230 (75.7) | 166 (86.0) | 64 (57.7) | <0.001 |
| PCI, n (%) | 70 (23.0) | 58 (30.1) | 12 (10.8) | <0.001 |
| Myocardial infarction, n (%) | 94 (30.9) | 85 (44.0) | 9 (8.1) | <0.001 |
| Indication VHD, n (%) | ||||
| VHD | 62 (20.4) | 62 (55.9) | ||
| IVHD | 49 (16.1) | 49 (44.1) | ||
| Aortic valve stenosis | 70 (23.0) | 70 (63.1) | ||
| Aortic valve insufficiency | 20 (6.6) | 20 (18.0) | ||
| Mitral valve disease | 3 (1.0) | 3 (2.7) | ||
| Mitral valve insufficiency | 41 (13.5) | 41 (36.9) | ||
| Left ventricular function | 0.618 | |||
| Normal | 234 (77.0) | 146 (75.6) | 88 (79.3) | |
| Mild dysfunction | 60 (19.7) | 39 (20.2) | 21 (18.9) | |
| Moderate dysfunction | 8 (2.6) | 6 (3.1) | 2 (1.8) | |
| Severe dysfunction | 2 (0.7) | 2 (1.0) | 0 | |
| Left atrial dilatation >45 mm, n (%) | 54 (17.8) | 27 (14.0) | 27 (24.3) | 0.001 |
| History of AF, n (%) | 40 (13.2) | 14 (7.3) | 26 (23.4) | <0.001 |
| Paroxysmal | 32 (10.5) | 14 (7.3) | 18 (16.2) | |
| Persistent | 7 (2.3) | 0 | 7 (6.3) | |
| Longstanding persistent | 1 (0.3) | 0 | 1 (0.9) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teuwen, C.P.; Does, L.J.M.E.v.d.; Kik, C.; Mouws, E.M.J.P.; Lanters, E.A.H.; Knops, P.; Taverne, Y.J.H.J.; Bogers, A.J.J.C.; de Groot, N.M.S. Sinus Rhythm Conduction Properties across Bachmann’s Bundle: Impact of Underlying Heart Disease and Atrial Fibrillation. J. Clin. Med. 2020, 9, 1875. https://doi.org/10.3390/jcm9061875
Teuwen CP, Does LJMEvd, Kik C, Mouws EMJP, Lanters EAH, Knops P, Taverne YJHJ, Bogers AJJC, de Groot NMS. Sinus Rhythm Conduction Properties across Bachmann’s Bundle: Impact of Underlying Heart Disease and Atrial Fibrillation. Journal of Clinical Medicine. 2020; 9(6):1875. https://doi.org/10.3390/jcm9061875
Chicago/Turabian StyleTeuwen, Christophe P., Lisette J.M.E. van der Does, Charles Kik, Elisabeth M.J.P. Mouws, Eva A.H. Lanters, Paul Knops, Yannick J.H.J. Taverne, Ad J.J.C. Bogers, and Natasja M.S. de Groot. 2020. "Sinus Rhythm Conduction Properties across Bachmann’s Bundle: Impact of Underlying Heart Disease and Atrial Fibrillation" Journal of Clinical Medicine 9, no. 6: 1875. https://doi.org/10.3390/jcm9061875
APA StyleTeuwen, C. P., Does, L. J. M. E. v. d., Kik, C., Mouws, E. M. J. P., Lanters, E. A. H., Knops, P., Taverne, Y. J. H. J., Bogers, A. J. J. C., & de Groot, N. M. S. (2020). Sinus Rhythm Conduction Properties across Bachmann’s Bundle: Impact of Underlying Heart Disease and Atrial Fibrillation. Journal of Clinical Medicine, 9(6), 1875. https://doi.org/10.3390/jcm9061875






