High Preoperative Serum Syndecan-1, a Marker of Endothelial Glycocalyx Degradation, and Severe Acute Kidney Injury after Valvular Heart Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
2.3. Measurements
2.4. Statistical Analysis
3. Results
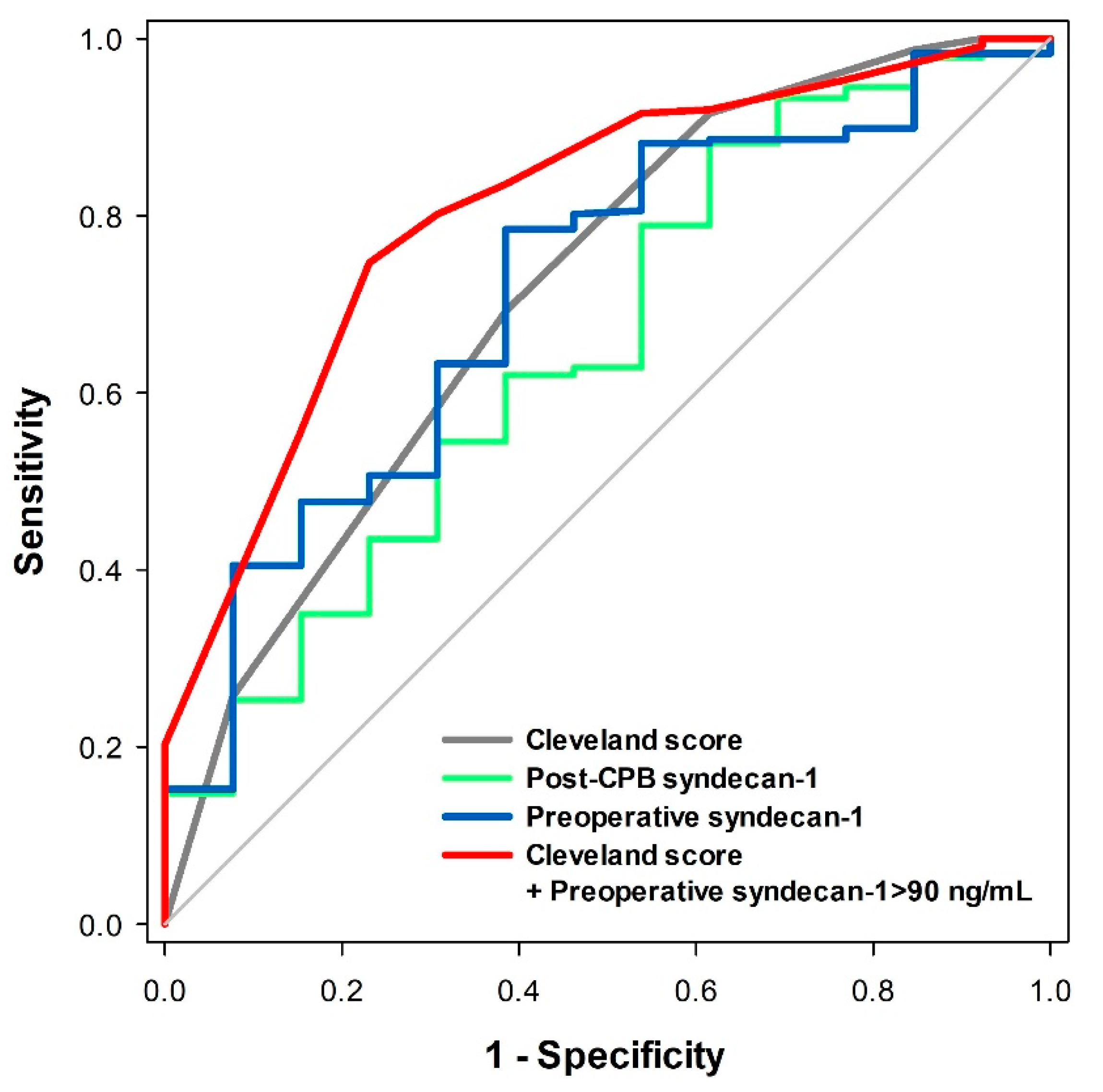
3.1. Prediction of Postoperative Severe AKI
3.2. Comparison Between Low and High Preoperative Syndecan-1 Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thiele, R.H.; Isbell, J.M.; Rosner, M.H. AKI associated with cardiac surgery. Clin. J. Am. Soc. Nephrol. 2015, 10, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Liotta, M.; Olsson, D.; Sartipy, U.; Holzmann, M.J. Minimal changes in postoperative creatinine values and early and late mortality and cardiovascular events after coronary artery bypass grafting. Am. J. Cardiol. 2014, 113, 70–75. [Google Scholar] [CrossRef]
- Robert, A.M.; Kramer, R.S.; Dacey, L.J.; Charlesworth, D.C.; Leavitt, B.J.; Helm, R.E.; Hernandez, F.; Sardella, G.L.; Frumiento, C.; Likosky, D.S.; et al. Cardiac surgery-associated acute kidney injury: A comparison of two consensus criteria. Ann. Thorac. Surg. 2010, 90, 1939–1943. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Shim, J.K.; Lee, S.; Song, J.W.; Choi, N.; Lee, S.; Kwak, Y.L. Chronic progression of cardiac surgery associated acute kidney injury: Intermediary role of acute kidney disease. J. Thorac. Cardiovasc. Surg. 2019. [Google Scholar] [CrossRef]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflügers Archiv. Eur. J. Physiol. 2007, 454, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Zullo, J.; Lipphardt, M.; Dragovich, M.; Zhang, F.X.; Fu, B.; Goligorsky, M.S. Endothelial glycocalyx-the battleground for complications of sepsis and kidney injury. Nephrol. Dial. Transplant. 2018, 33, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Kolarova, H.; Ambruzova, B.; Svihalkova Sindlerova, L.; Klinke, A.; Kubala, L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediat. Inflamm. 2014, 2014, 694312. [Google Scholar] [CrossRef] [PubMed]
- Alphonsus, C.S.; Rodseth, R.N. The endothelial glycocalyx: A review of the vascular barrier. Anaesthesia 2014, 69, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Molitoris, B.A. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin. Nephrol. 2015, 35, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Stensballe, J.; Rasmussen, L.S.; Ostrowski, S.R. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein c depletion, fibrinolysis, and increased mortality in trauma patients. Ann. Surg. 2011, 254, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Anand, D.; Ray, S.; Srivastava, L.M.; Bhargava, S. Evolution of serum hyaluronan and syndecan levels in prognosis of sepsis patients. Clin. Biochem. 2016, 49, 768–776. [Google Scholar] [CrossRef]
- Schmidt, E.P.; Overdier, K.H.; Sun, X.; Lin, L.; Liu, X.; Yang, Y.; Ammons, L.A.; Hiller, T.D.; Suflita, M.A.; Yu, Y.; et al. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2016, 194, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Berkestedt, I.; Schmidtchen, A.; Ljunggren, L.; Bodelsson, M. Increased levels of glycosaminoglycans during septic shock: Relation to mortality and the antibacterial actions of plasma. Shock 2008, 30, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Rehm, M.; Bruegger, D.; Christ, F.; Conzen, P.; Thiel, M.; Jacob, M.; Chappell, D.; Stoeckelhuber, M.; Welsch, U.; Reichart, B.; et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 2007, 116, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Svennevig, K.; Hoel, T.; Thiara, A.; Kolset, S.; Castelheim, A.; Mollnes, T.; Brosstad, F.; Fosse, E.; Svennevig, J. Syndecan-1 plasma levels during coronary artery bypass surgery with and without cardiopulmonary bypass. Perfusion 2008, 23, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Bruegger, D.; Rehm, M.; Abicht, J.; Paul, J.O.; Stoeckelhuber, M.; Pfirrmann, M.; Reichart, B.; Becker, B.F.; Christ, F. Shedding of the endothelial glycocalyx during cardiac surgery: On-pump versus off-pump coronary artery bypass graft surgery. J. Thorac. Cardiovasc. Surg. 2009, 138, 1445–1447. [Google Scholar] [CrossRef] [PubMed]
- Bruegger, D.; Brettner, F.; Rossberg, I.; Nussbaum, C.; Kowalski, C.; Januszewska, K.; Becker, B.F.; Chappell, D. Acute degradation of the endothelial glycocalyx in infants undergoing cardiac surgical procedures. Ann. Thorac. Surg. 2015, 99, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, C.; Haberer, A.; Tiefenthaller, A.; Januszewska, K.; Chappell, D.; Brettner, F.; Mayer, P.; Dalla Pozza, R.; Genzel-Boroviczeny, O. Perturbation of the microvascular glycocalyx and perfusion in infants after cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 2015, 150, 1474–1481.e1471. [Google Scholar] [CrossRef] [PubMed]
- Thakar, C.V.; Arrigain, S.; Worley, S.; Yared, J.P.; Paganini, E.P. A clinical score to predict acute renal failure after cardiac surgery. J. Am. Soc. Nephrol. 2005, 16, 162–168. [Google Scholar] [CrossRef]
- Qureshi, S.H.; Patel, N.N.; Murphy, G.J. Vascular endothelial cell changes in postcardiac surgery acute kidney injury. Am. J. Physiol. Ren. Physiol. 2018, 314, F726–F735. [Google Scholar] [CrossRef]
- de Melo Bezerra Cavalcante, C.T.; Castelo Branco, K.M.; Pinto Junior, V.C.; Meneses, G.C.; de Oliveira Neves, F.M.; de Souza, N.M.; Penaforte, K.L.; Martins, A.M.; Liborio, A.B. Syndecan-1 improves severe acute kidney injury prediction after pediatric cardiac surgery. J. Thorac. Cardiovasc. Surg. 2016, 152, 178–186.e172. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, E.; Passov, A.; Andersson, S.; Suojaranta, R.; Niemi, T.; Raivio, P.; Salmenpera, M.; Schramko, A. Glycocalyx degradation and inflammation in cardiac surgery. J. Cardiothorac. Vasc. Anesthesia 2019, 33, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Padberg, J.S.; Wiesinger, A.; di Marco, G.S.; Reuter, S.; Grabner, A.; Kentrup, D.; Lukasz, A.; Oberleithner, H.; Pavenstadt, H.; Brand, M.; et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis 2014, 234, 335–343. [Google Scholar] [CrossRef]
- Murphy, L.S.; Wickersham, N.; McNeil, J.B.; Shaver, C.M.; May, A.K.; Bastarache, J.A.; Ware, L.B. Endothelial glycocalyx degradation is more severe in patients with non-pulmonary sepsis compared to pulmonary sepsis and associates with risk of ards and other organ dysfunction. Ann. Intensive Care 2017, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Holst, D.P.; Kaye, D.M. Tricuspid regurgitation contributes to renal dysfunction in patients with heart failure. J. Card. Fail. 2008, 14, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Bruegger, D.; Jacob, M.; Rehm, M.; Loetsch, M.; Welsch, U.; Conzen, P.; Becker, B.F. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1993–H1999. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, A.R.; Jennings, C.A. Neutralisation of heparan sulphate and low molecular weight heparin by protamine. Thromb. Haemost. 1985, 53, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Passov, A.; Schramko, A.; Makisalo, H.; Nordin, A.; Andersson, S.; Pesonen, E.; Ilmakunnas, M. Graft glycocalyx degradation in human liver transplantation. PLoS ONE 2019, 14, e0221010. [Google Scholar] [CrossRef] [PubMed]
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L. World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, R.; Liu, S.; Yu, X.; Zou, J.; Ding, X. Global incidence and outcomes of adult patients with acute kidney injury after cardiac surgery: A systematic review and meta-analysis. J. Cardiothorac. Vasc. Anesthesia 2016, 30, 82–89. [Google Scholar] [CrossRef]
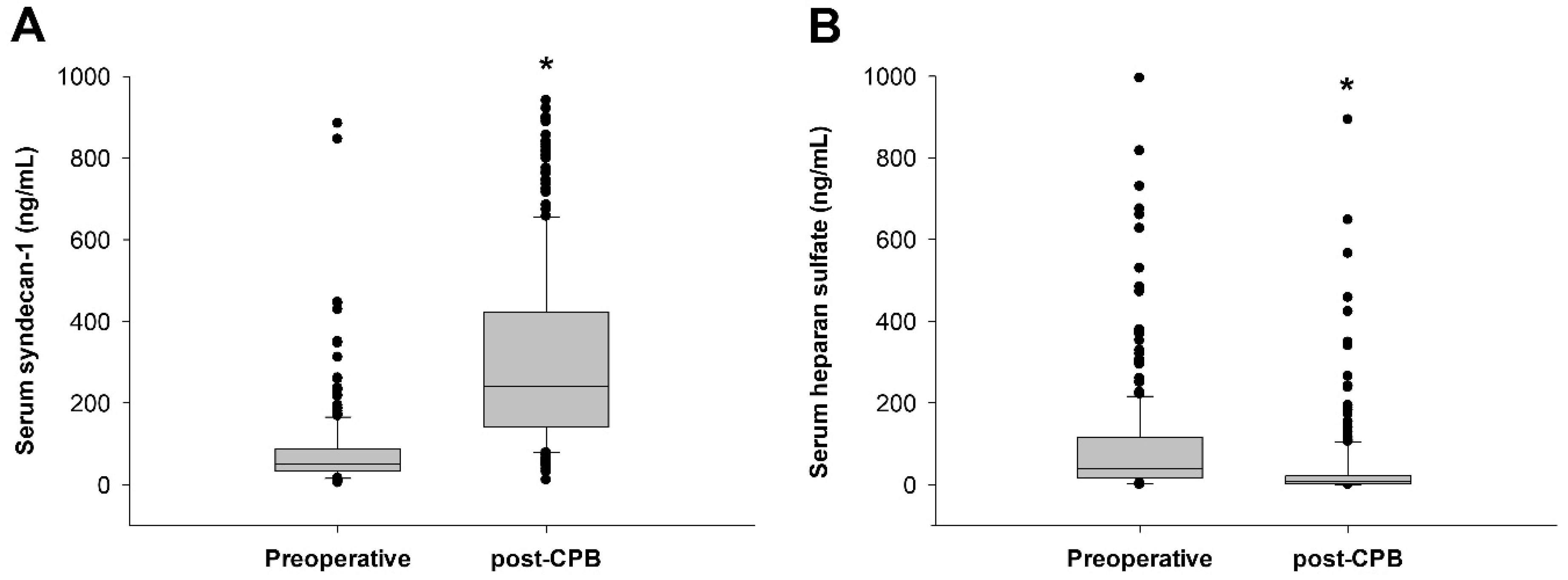
| Total (5–884 ng/mL) (N = 250) | Low SDC-1 Group (0–89 ng/mL) (N = 191) | High SDC-1 Group (90–884 ng/mL) (N = 59) | p Value | |
|---|---|---|---|---|
| Age (years) | 66 (57–73) | 66 (57–73) | 65 (56–74) | 0.648 |
| Male (n (%)) | 118 (47.2) | 87 (45.5) | 31 (52.5) | 0.347 |
| Body mass index (kg/m2) | 23.9 ± 3.8 | 24.0 ± 4.0 | 23.7 ± 2.9 | 0.567 |
| Hypertension | 133 (53.2) | 108 (56.5) | 25 (42.4) | 0.057 |
| Diabetes mellitus | 48 (19.2) | 36 (18.8) | 12 (20.3) | 0.799 |
| Chronic obstructive lung disease | 9 (3.6) | 6 (3.1) | 3 (5.1) | 0.690 |
| Preoperative serum Cr >1.2 mg/dL | 15 (6.0) | 7 (3.7) | 8 (13.6) | 0.010 |
| Prior myocardial infarction | 8 (3.2) | 6 (3.1) | 2 (3.4) | 0.924 |
| Congestive heart failure | 55 (22.0) | 38 (19.9) | 17 (28.8) | 0.148 |
| Coronary artery occlusive disease | 35 (14.0) | 28 (14.7) | 7 (11.9) | 0.589 |
| Peripheral artery occlusive disease | 3 (1.2) | 2 (1.0) | 1 (1.7) | >0.999 |
| Liver cirrhosis | 9 (3.6) | 4 (2.1) | 5 (8.5) | 0.036 |
| Preoperative steroid use | 5 (2.0) | 5 (2.6) | 0 (0.0) | 0.344 |
| Preoperative inotrope use | 9 (3.6) | 5 (2.6) | 4 (6.8) | 0.221 |
| Severe aortic stenosis | 62 (24.8) | 47 (24.6) | 15 (25.4) | 0.899 |
| Severe aortic regurgitation | 54 (21.6) | 44 (23.0) | 10 (16.9) | 0.321 |
| Severe mitral stenosis | 25 (10.0) | 15 (7.9) | 10 (16.9) | 0.042 |
| Severe mitral regurgitation | 75 (30) | 65 (34.0) | 10 (16.9) | 0.012 |
| Severe tricuspid regurgitation | 59 (23.6) | 34 (17.8) | 25 (42.4) | <0.001 |
| Type of surgery | 0.238 | |||
| Mitral valve repair/replacement | 116 (46.4) | 84 (44.0) | 32 (54.2) | |
| Aortic valve replacement | 72 (28.8) | 62 (32.5) | 10 (16.9) | |
| Double valve surgery | 23 (9.2) | 16 (8.4) | 7 (11.9) | |
| Valve + CABG | 22 (8.8) | 16 (8.4) | 6 (10.2) | |
| Valve + aorta | 17 (6.8) | 13 (6.8) | 4 (6.8) | |
| Left ventricular ejection fraction (%) | 62 ± 11 | 62 ± 12 | 60 ± 9 | 0.175 |
| LA volume index (mL/m2) | 64 (43–94) | 59 (39–88) | 67 (52–112) | 0.067 |
| RV systolic pressure (mmHg) | 36 (28–46) | 33 (27–43) | 41 (33–51) | 0.001 |
| Cleveland score | 2 (2–3) | 2 (1–3) | 2 (2–3) | 0.088 |
| EuroSCORE | 5 (3–7) | 5 (3–7) | 5 (3–8) | 0.371 |
| Pre-operative medication | ||||
| Beta-blockers | 95 (38.0) | 70 (36.6) | 25 (42.4) | 0.429 |
| RAS antagonists | 137 (54.8) | 109 (57.1) | 28 (47.5) | 0.195 |
| Calcium-channel blockers | 59 (23.6) | 51 (26.7) | 8 (13.6) | 0.053 |
| Antiplatelet agent | 57 (22.8) | 49 (25.7) | 8 (13.6) | 0.075 |
| Heparin | 77 (30.8) | 45 (23.6) | 32 (54.2) | <0.001 |
| Diuretics | 154 (61.6) | 110 (57.6) | 44 (74.6) | 0.019 |
| Statins | 94 (37.6) | 75 (39.3) | 19 (32.2) | 0.328 |
| Digoxin | 41 (16.4) | 28 (14.7) | 13 (22.0) | 0.181 |
| Total (N = 250) | Low SDC-1 (N = 191) | High SDC-1 (N = 59) | p Value | |
|---|---|---|---|---|
| Acute kidney injury | 47 (18.8) | 28 (14.7) | 19 (32.2) | 0.003 |
| Stage 1 | 34 (13.6) | 23 (12.0) | 11 (18.6) | |
| Stage 2 | 6 (2.4) | 2 (1.0) | 4 (6.8) | |
| Stage 3 | 7 (2.8) | 3 (1.6) | 4 (6.8) | |
| Oliguria | 7 (2.8) | 3 (1.5) | 4 (6.8) | 0.025 |
| Renal replacement therapy | 3 (1.2) | 0 (0.0) | 3 (5.1) | 0.013 |
| Serum creatinine (mg/dL) | ||||
| Baseline | 0.83 ± 0.23 | 0.81 ± 0.20 | 0.92 ± 0.27 | 0.024 |
| Postoperative 6 h | 0.74 ± 0.28 | 0.71 ± 0.26 | 0.84 ± 0.32 | 0.024 |
| Postoperative 24 h | 0.97 ± 0.71 | 0.91 ± 0.75 | 1.14 ± 0.52 | 0.048 |
| Postoperative 48 h | 0.93 ± 1.21 | 0.89 ± 1.36 | 1.05 ± 0.43 | 0.688 |
| Total (N = 250) | Low SDC-1 (N = 191) | High SDC-1 (N = 59) | p Value | |
|---|---|---|---|---|
| Duration of CPB (min) | 95 (70–120) | 90 (70–120) | 105 (75–136) | 0.131 |
| Crystalloid (mL) | 5859 (4784–6982) | 5791 (4703–5791) | 6406 (5248–7554) | 0.010 |
| Colloid (mL) | 600 (450–1000) | 600 (450–980) | 650 (450–1100) | 0.260 |
| Patients requiring pRBC transfusion (n (%)) | 148 (59.2) | 111 (58.1) | 37 (62.7) | 0.530 |
| Amount of pRBC transfusion (mL) | 280 (0–560) | 280 (0–280) | 280 (0–560) | 0.107 |
| Patients requiring FFP/cryoprecipitate/platelet transfusion (n (%)) | 91 (36.4) | 58 (30.3) | 33 (55.9) | <0.001 |
| Amount of FFP/cryoprecipitate/platelet transfusion (mL) | 0 (0–608) | 0 (0–260) | 260 (0–1280) | <0.001 |
| Urine output (mL) | 6358 (5430–7408) | 6175 (5340–7230) | 7000 (6165–7650) | 0.007 |
| Chest tube drainage (mL) | 470 (307–645) | 440 (290–617) | 532 (360–721) | 0.022 |
| Furosemide dose (mg) | 55 (25–80) | 50 (20–75) | 60 (40–100) | 0.019 |
| Norepinephrine dose (μg) | 972 (424–3329) | 882 (416–2875) | 1235 (469–3760) | 0.339 |
| Vasopressin dose (unit) | 1.0 (0–3.3) | 0.9 (0–3.2) | 1.0 (0–3.7) | 0.274 |
| Patients requiring inotrope (n (%)) | 112 (44.8) | 80 (41.8) | 32 (54.2) | 0.100 |
| Total (N = 250) | Low SDC-1 (N = 191) | High SDC-1 (N = 59) | p Value | |
|---|---|---|---|---|
| TNF-α (pg/mL) | ||||
| Post-induction | 1.58 (1.17–2.06) | 1.45 (1.14–1.92) | 1.85 (1.37–2.43) | <0.001 |
| Post-CPB | 6.00 (3.77–9.59) | 5.92 (3.39–9.61) | 6.20 (3.90–9.55) | 0.738 |
| IL-6 (pg/mL) | ||||
| Post-induction | 3.43 (2.18–6.72) | 3.10 (2.05–6.28) | 4.93 (2.71–9.68) | 0.018 |
| Post-CPB | 92.1 (26.6–283.7) | 84.9 (23.1–247.8) | 198.4 (53.1–308.7) | 0.062 |
| C-reactive protein (mg/L) | ||||
| Preoperative | 1.3 (0.6–2.9) | 1.2 (0.6–2.6) | 1.8 (0.9–5.4) | 0.060 |
| Postoperative 6 h | 10.3 (2.8–18.2) | 9.0 (3.2–19.9) | 10.4 (2.5–18.0) | >0.999 |
| Postoperative 24 h | 75.9 (52.7–105.5) | 75.2 (50.8–98.5) | 86.1 (65.5–147.1) | 0.040 |
| Postoperative 48 h | 164.7 (131.8–204.6) | 162.3 (131.0–206.3) | 175.6 (135.6–204.0) | >0.999 |
| Total (N = 250) | Low SDC-1 (N = 191) | High SDC-1 (N = 59) | p Value | |
|---|---|---|---|---|
| Stroke | 2 (0.8) | 1 (0.5) | 1 (1.7) | 0.417 |
| Sternal infection | 0 (0.0) | 0 (0.0) | 0 (0.0) | >0.999 |
| Hemostatic reoperation | 5 (2.0) | 4 (2.1) | 1 (1.7) | >0.999 |
| Mechanical ventilation >24 h | 15 (6.0) | 8 (4.2) | 7 (11.9) | 0.053 |
| Mortality | 2 (0.8) | 2 (1.0) | 0 (0.0) | >0.999 |
| Length of ICU stay (day) | 3 (2–3) | 3 (2–3) | 3 (2–4) | 0.019 |
| Length of hospital stay (day) | 14 (11–18) | 13 (11–17) | 16 (12–24) | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-B.; Soh, S.; Kwak, Y.-L.; Bae, J.C.; Kang, S.H.; Song, J.W. High Preoperative Serum Syndecan-1, a Marker of Endothelial Glycocalyx Degradation, and Severe Acute Kidney Injury after Valvular Heart Surgery. J. Clin. Med. 2020, 9, 1803. https://doi.org/10.3390/jcm9061803
Kim H-B, Soh S, Kwak Y-L, Bae JC, Kang SH, Song JW. High Preoperative Serum Syndecan-1, a Marker of Endothelial Glycocalyx Degradation, and Severe Acute Kidney Injury after Valvular Heart Surgery. Journal of Clinical Medicine. 2020; 9(6):1803. https://doi.org/10.3390/jcm9061803
Chicago/Turabian StyleKim, Hye-Bin, Sarah Soh, Young-Lan Kwak, Jae Chan Bae, Sang Hwa Kang, and Jong Wook Song. 2020. "High Preoperative Serum Syndecan-1, a Marker of Endothelial Glycocalyx Degradation, and Severe Acute Kidney Injury after Valvular Heart Surgery" Journal of Clinical Medicine 9, no. 6: 1803. https://doi.org/10.3390/jcm9061803
APA StyleKim, H.-B., Soh, S., Kwak, Y.-L., Bae, J. C., Kang, S. H., & Song, J. W. (2020). High Preoperative Serum Syndecan-1, a Marker of Endothelial Glycocalyx Degradation, and Severe Acute Kidney Injury after Valvular Heart Surgery. Journal of Clinical Medicine, 9(6), 1803. https://doi.org/10.3390/jcm9061803






