Electromyographic Analysis of Shoulder Neuromuscular Activity in Women Following Breast Cancer Treatment: A Cross-Sectional Descriptive Study
Abstract
1. Introduction
2. Experimental Section
2.1. Design
2.2. Participants
2.3. Sample Size Estimation
2.4. Data Collection
2.4.1. Shoulder Neuromuscular Activity
The Onset of Muscle Activity
- (1)
- Shoulder abduction movement: Elevation of the arm in the scapular plane from rest position, until 90 degrees was reached.
- (2)
- Shoulder forward flexion movement: Elevation of the arm in the sagittal plane of motion from rest position, until 90 degrees was reached.
- (3)
- Shoulder external rotation movement: External movement (outward) turning around the vertical axis in the transverse plane of motion with the arm at 90 degrees of elevation in the scapular plane and the elbow in the flexion position. Movement from 0 degrees until 30 degrees was reached.
The Amplitude of Muscle Activity
- (1)
- Shoulder abduction movement: A 5-s isometric contraction at 90 degrees of elevation of the arm in the scapular plane.
- (2)
- Shoulder forward flexion movement: A 5-s isometric contraction at 90 degrees of arm elevation in the sagittal plane of motion.
- (3)
- Shoulder external rotation movement: A 5-s isometric contraction at 0 degrees of external movement (outward) turning around the vertical axis in the transverse plane of motion, with the arm at 90 degrees of elevation in the scapular plane and the elbow in the flexion position. Prior to isometric contraction, women maintained that arm elevation and flexion elbow position during 5-s to re-establish a baseline signal.
2.4.2. Other Outcomes
2.5. Data Analysis
3. Results
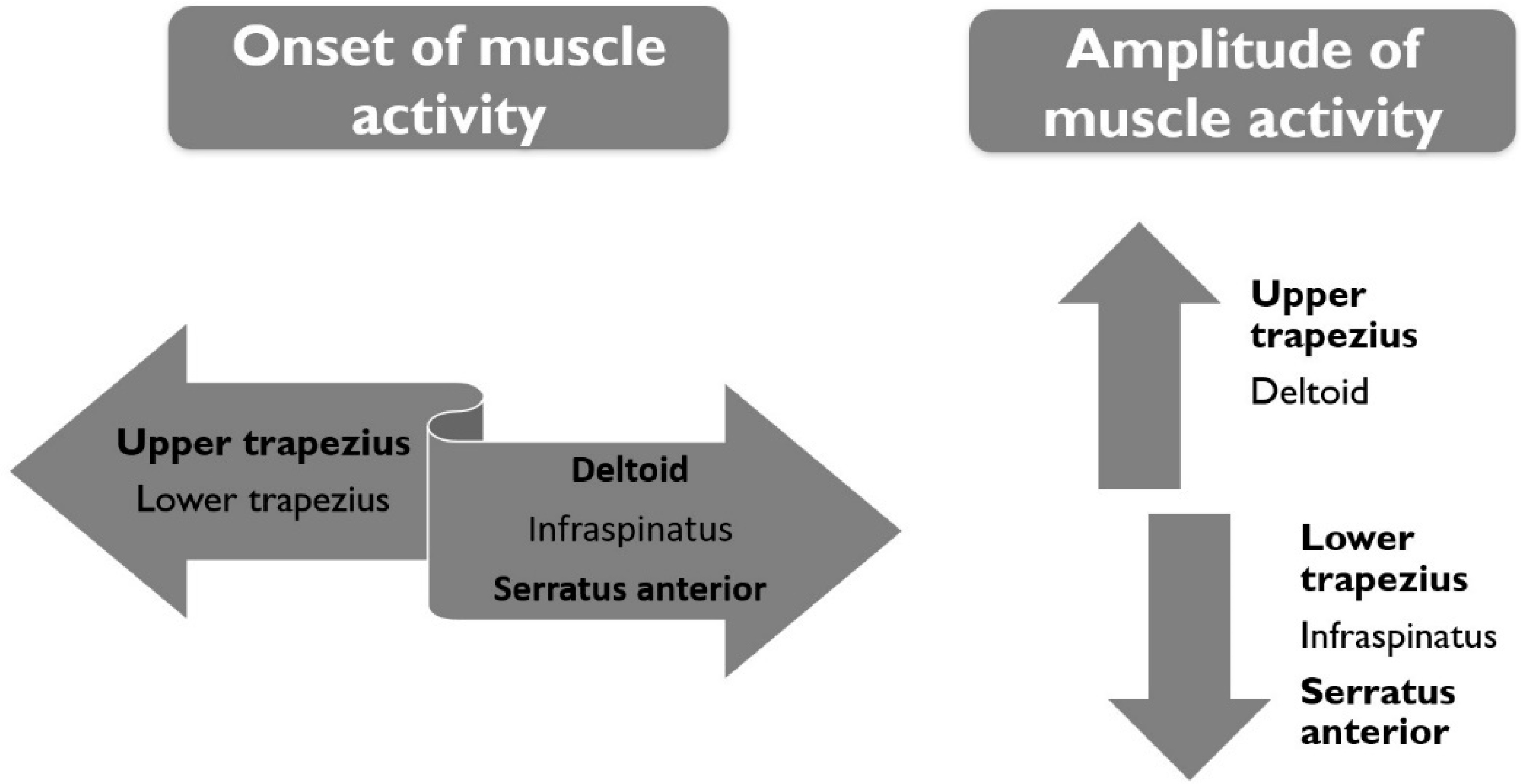
3.1. The Onset of Muscle Activity
3.1.1. Shoulder Abduction Movement
3.1.2. Shoulder Forward Flexion Movement
3.1.3. Shoulder External Rotation Movement
3.2. The Amplitude of Muscle Activity (RMS%)
3.2.1. Shoulder Abduction Movement
3.2.2. Shoulder Forward Flexion Movement
3.2.3. Shoulder External Rotation Movement
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuste, M.J.; Torres, M.; Sánchez, B.; Prieto, D.; Pacheco, S.; Cerezo, E.; Zapico, A. Health-related quality of life improvement in breast cancer patients: Secondary outcome from a simple blinded, randomised clinical trial. Breast J. 2014, 24, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, J.; Kilbreath, S.; Dylke, E.; Refshauge, M.; Nicholson, L.; Beith, J.; Spillane, A.J.; White, K. Effects of Mastectomy on Shoulder and Spinal Kinematics During Bilateral Upper-Limb Movement. Phys. Ther. 2010, 90, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, S.; Haddad, C.; Giron, P.; Pinheiro, T.; Nazário, A.; Facina, G. Winged scapula incidence and upper limb morbidity after surgery for breast cancer with axillary dissection. Support Care Cancer 2016, 24, 2707–2715. [Google Scholar] [CrossRef] [PubMed]
- Fong, S.S.M.; Ng, S.S.M.; Luk, W.S.; Chung, J.W.Y.; Chung, L.M.Y.; Tsang, W.W.N.; Chow, L.P.Y. Shoulder Mobility, Muscular Strength, and Quality of Life in Breast Cancer Survivors with and without Tai Chi Qigong Training. Evid. Based Complement. Alternat. Med. 2013, 2013, 787169. [Google Scholar] [CrossRef] [PubMed]
- Shamley, D.; Lascurain-Aguirrebeña, I.; Oskrochi, R.; Srinaganathan, R. Shoulder morbidity after treatment for breast cancer is bilateral and greater after mastectomy. Acta Oncol. 2012, 51, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Harrington, S.; Padua, D.; Battaglini, C.; Michener, L.; Giuliani, C.; Myers, J.; Groff, D. Comparison of shoulder flexibility, strength, and function between breast cancer survivors and healthy participants. J. Cancer Surviv. 2011, 5, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, N.; De Ridder, M.; Lievens, P.; Van Parijs, H.; Vanhoeij, M.; Miedema, G.; Voordeckers, M.; Versmessen, H.; Storme, G.; Lamote, J.; et al. Scapula alata in early breast cancer patients enrolled in a randomized clinical trial of post-surgery short-course image-guided radiotherapy. World J. Surg. Oncol. 2012, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Shamley, D.; Srinanaganathan, R.; Weatherall, R.; Oskrochi, R.; Watson, M.; Ostlere, S.; Sugden, E. Changes in shoulder muscle size and activity following treatment for breast cancer. Breast Cancer Res. Treat. 2007, 106, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Torres Lacomba, M.; Mayoral del Moral, O.; Coperias Zazo, J.L.; Gerwin, R.D.; Goni, A.Z. Incidence of myofascial pain syndrome in breast cancer surgery: A prospective study. Clin. J. Pain 2010, 26, 320–325. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, I.; Torres, M.; Acosta, P.; Orive, I.G.; Nee, R.J.; de la Villa, P.; Andrés-Esteban, E.M.; Sánchez-Sánchez, B. Protective myoelectric activity at performing upper limb neurodynamic test 1 in breast cancer survivors. A cross-sectional observational study. Musculoskelet. Sci. Prac. 2018, 36, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Brookham, R.L.; Cudlip, A.C.; Dickerson, C.R. Quantification of upper limb electromyographic measures and dysfunction of breast cancer survivors during performance of functional dynamic tasks. Clin. Biomech. 2018, 52, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ebaugh, D.; Spinelli, B.; Schmitz, K. Shoulder impairments and their association with symptomatic rotator cuff disease in breast cancer survivors. Med. Hypotheses 2011, 77, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Borstad, J.D.; Szucs, K.A. Three-dimensional scapula kinematics and shoulder function examined before and after surgical treatment for breast cancer. Hum. Mov. Sci. 2012, 31, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Huang, C.; Ou, H.L.; Lin, J. Scapular Dyskinesis: Patterns, Functional Disability and Associated Factors in People with Shoulder Disorders. Man. Ther. 2016, 26, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Castelein, B.; Cagnie, B.; Cools, A. Scapular muscle dysfunction associated with subacromial pain syndrome. J. Hand Ther. 2017, 30, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.T.; Yin-fat, G.; Shing Chung, C.; Ngor, S. Rotator cuff tendinopathy alters the muscle activity onset and kinematics of scapula. J. Electromyogr. Kinesiol. 2017, 35, 40–46. [Google Scholar] [CrossRef]
- Ludewig, P.; Braman, P. Shoulder impingement: Biomechanical considerations in rehabilitation. Man. Ther. 2010, 16, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Naef, F.; Grace, S.; Crowley-McHattan, Z.; Hardy, D.; McLeod, A. The effect of chronic shoulder pain on maximal force of shoulder abduction. J. Bodyw. Mov. Ther. 2014, 19, 410–416. [Google Scholar] [CrossRef]
- Shamley, D.; Srinaganathan, R.; Oskrochi, R.; Lascurain-Aguirrebeña, I.; Sugden, E. Three-dimensional scapulothoracic motion following treatment for breast cancer. Breast Cancer Res. Treat. 2009, 118, 315–322. [Google Scholar] [CrossRef]
- Shamley, D.; Lascurain-Aguirrebeña, I.; Oskrochi, R. Clinical anatomy of the shoulder after treatment for breast cancer. Clin. Anat. 2014, 27, 467–477. [Google Scholar] [CrossRef]
- Heuberer, P.; Kranzl, A.; Laky, B.; Anderl, W.; Wurnig, C. Electromyographic analysis: Shoulder muscle activity revisited. Arch. Orthop. Trauma Surg. 2015, 135, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Wickham, J.; Stansfeld, T.; Burnside, K.; Watson, A. Quantifying ‘normal’ shoulder muscle activity during abduction. J. Electromyogr. Kinesiol. 2009, 20, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, R.F.; Hooks, T.R.; Wilk, K.E. Optimal management of shoulder impingement syndrome. Open Access J. Sports Med. 2014, 5, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Struyf, F.; Lluch, E.; Falla, D.; Meeus, M.; Noten, S.; Nijs, J. Influence of shoulder pain on muscle function: Implications for the assessment and therapy of shoulder disorders. Eur. J. Appl. Physiol. 2015, 115, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Borstad, J.; Woeste, C. The role of sensitization in musculoskeletal shoulder pain. Braz. J. Phys. Ther. 2015, 19, 251–257. [Google Scholar] [CrossRef]
- Dean, B.J.F.; Gwilym, S.E.; Carr, A.J. Why does my shoulder hurt? A review of the neuroanatomical and biochemical basis of shoulder pain. Br. J. Sports Med. 2013, 47, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, R.; Higgins, J.; Bourbonnais, D. Is neuroplasticity in the central nervous system the missing link to our understanding of chronic musculoskeletal disorders? BMC Musculoskelet. Disord. 2015, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Vargas, A.; Roldan-Jimenez, C.; Neblett, R.; Gatchel, R. Cross-cultural adaptation and validity of the Spanish Central Sensitization Inventory. Springerplus 2016, 5, 1–8. [Google Scholar] [CrossRef]
- Torres-Lacomba, M.; Sánchez-Sánchez, B.; Prieto-Gómez, V.; Pacheco-da-Costa, S.; Yuste-Sánchez, M.J.; Navarro-Brazález, B.; Gutiérrez-Ortega, C. Spanish cultural adaptation and validation of the shoulder pain and disability index, and the oxford shoulder score after breast cancer surgery. Health Qual. Life Out. 2015, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Karen, L.; Peter, R.; Polus, B. Muscle activation patterns in the scapular positioning muscles during loaded scapular plane elevation: The effects of Latent Myofascial Trigger Points. Clin. Biomech. 2010, 25, 765–770. [Google Scholar] [CrossRef]
- Larsson, B.; Karlberg, C.; Elert, J.; Gerdle, B. Reproducibility of surface EMG during dynamic shoulder forward flexions: A study of clinically healthy subjects. Clin. Physiol. 1999, 19, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Clisby, E.; Bitter, N.; Sandow, M.; Jones, M.; Magarey, M.; Jaberzadeh, S. Relative contributions of the infraspinatus and deltoid during external rotation in patients with symptomatic subacromial impingement. J. Shoulder Elbow Surg. 2008, 17, S92. [Google Scholar] [CrossRef] [PubMed]
- Cram, J.R.; Kasman, G.S.; Holtz, J. Introduction to Surface Electronyography; Aspen Publishers: Gaitersburg, MD, USA, 1998. [Google Scholar]
- Villarroya-Aparicio, M. Electromiografía cinesiológica. Rehabilitación 2005, 39, 9. [Google Scholar] [CrossRef]
- Mousa, H.; Khademi, M. A Review on Technical and Clinical Impact of Microsoft Kinect on Physical Therapy and Rehabilitation. J. Med. Eng. Technol. 2014, 16. [Google Scholar] [CrossRef]
- García, J.R.; Barea, R.; López, E.; Prieto, V.; Yuste, M.; De La Villa, P.; Bergasa, L.M.; Ocaña, M. 12º Workshop. Robótica Cognitiva. Robocity 2030. Capítulo 11: Sistema de Captura de Movimientos con Sensor Kinect y Acelerómetros para Rehabilitación Funcional y Cognitiva de Cáncer de Mama; Universidad Nacional de Educación a Distancia—UNED: Madrid, Spain, 2013; pp. 155–169. ISBN 978-84-695-8175-9. [Google Scholar]
- Nijs, J.; Leysen, L.; Adriaenssens, N.; Aguilar Ferrándiz, M.E.; Devoogdt, N.; Tassenoy, A.; Ickmans, K.; Goubert, D.; van Wilgen, C.P.; Wijma, A.J.; et al. Pain following cancer treatment: Guidelines for the clinical classification of predominant neuropathic, nociceptive and central sensitization pain. Acta Oncol. 2016, 55, 659–663. [Google Scholar] [CrossRef] [PubMed]
- De Mey, K.; Danneels, L.A.; Cagnie, B.; Huyghe, L.; Seyns, E.; Cools, A.M. Conscious correction of scapular orientation in overhead athletes performing selected shoulder rehabilitation exercises: The effect on trapezius muscle activation measured by surface electromyography. J. Orthop. Sports Phys. Ther. 2013, 43, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Struyf, F.; Cagnie, B.; Cool, A.; Baert, I.; Brempt, J.V.; Struyf, P.; Meeus, M. Scapulothoracic muscle activity and recruitment timing in patients with shoulder impingement symptoms and glenohumeral instability. J. Electromyogr. Kinesiol. 2013, 24, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, P.M.; Heneghan, N.R. Motor control and the management of musculoskeletal dysfunction. Man. Ther. 2006, 11, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Tintignac, L.A.; Brenner, H.; Rüegg, M.A. Mechanisms Regulating Neuromuscular Junction Development and Function and Causes of Muscle Wasting. Physiol. Rev. 2015, 95, 809–852. [Google Scholar] [CrossRef] [PubMed]
- Page, P.; Frank, C.C.; Lardner, R. (Eds.) Structural and functional approaches to muscle imbalance. In Assessment and Treatment of Muscle Imbalance. The Janda Approach; Human Kinetics: Windsor, ON, Canada, 2009; pp. 3–11. [Google Scholar]
- Morais, N. The pectoralis minor muscle and shoulder movement-related impairments and pain: Rationale, assessment and management. Phys. Ther. Sport 2015, 17, 1–13. [Google Scholar] [CrossRef]
- Struyf, F.; Nijs, J.; De Graeve, J.; Mottram, S.; Meeusen, R. Scapular positioning in overhead athletes with and without shoulder pain: A case–control study. Scand. J. Med. Sci. Sports 2011, 21, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Timmons, M.K.; Thigpen, C.A.; Seitz, A.L.; Karduna, A.R.; Arnold, B.L.; Michener, L.A. Scapular Kinematics and Subacromial-Impingement Syndrome: A Meta-Analysis. J. Sport Rehabil. 2012, 21, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Matias, R.; Pascoal, A.G. The unstable shoulder in arm elevation: A three-dimensional and electromyographic study in subjects with glenohumeral instability. Clin. Biomech. 2006, 21, S58. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Gómez, V.; Torres-Lacomba, M.; Navarro-Brazález, B.; Yuste-Sánchez Carazo-Díaz, C.; Falla, D. Is multimodal physiotherapy more effective than exercise alone in women with persistent pain following breast cancer treatment? A randomized clinical trial. J. Physiother. under review.
- Galiano-Castillo, N.; Fernández-Lao, C.; Cantarero-Villanueva, I.; Fernández-De-Las-Peñas, C.; Menjón-Beltrán, S.; Arroyo-Morales, M. Altered pattern of cervical muscle activation during performance of a functional upper limb task in breast cancer survivors. Am. J. Phys. Med. Rehabil. 2011, 90, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.; Szeto, G.; Lee, R. Altered spinal kinematics and muscle recruitment pattern of the cervical and thoracic spine in people with chronic neck pain during functional task. J. Electromyogr. Kinesiol. 2013, 24, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.V.; Henry, S.M.; Jones, S.L.; Hitt, J.R.; Bunn, J.Y. A history of low back pain associates with altered electromyographic activation patterns in response to perturbations of standing balance. J. Neurophysiol. 2011, 106, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Torres-Lacomba, M.; Mayoral-del-Moral, O.; Coperías-Zazo, J.L.; Yuste-Sanchez, M.J.; Ferrandez, J.C.; Zapico-Goni, A. Axillary web syndrome after axillary dissection in breast cancer: A prospective study. Breast Cancer Res. Treat. 2009, 117, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Torres-Lacomba, M.; Yuste-Sanchez, M.J.; Zapico-Goni, A.; Prieto-Merino, D.; Mayoral-del-Moral, O.; Cerezo-Tellez, E.; Mogollón, E.M. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: Randomised, single blinded, clinical trial. BMJ 2010, 340, b5396. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Lluch Girbés, E.; Lundberg, M.; Malfliet, A.; Sterling, M. Exercise therapy for chronic musculoskeletal pain: Innovation by altering pain memories. Man. Ther. 2015, 20, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Camargo, P.R.; Neumann, D.A. Kinesiologic considerations for targeting activation of scapulothoracic muscles—Part 2: Trapezius. Braz. J. Phys. Ther. 2019, 23, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, R.A.; Donatelli, R.A.; Soderberg, G.L. Surface electromyographic analysis of exercises for the trapezius and serratus anterior muscles. J. Orthop. Sports Phys. Ther. 2003, 33, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, P.M.; Hoff, M.S.; Osowski, E.E.; Meschke, S.A.; Rundquist, P.J. Relative Balance of Serratus Anterior and Upper Trapezius Muscle Activity during Push-Up Exercises. Am. J. Sports Med. 2004, 32, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.A.; Camargo, P.R. Kinesiologic considerations for targeting activation of scapulothoracic muscles: Part 1: Serratus anterior. Braz. J. Phys. Ther. 2019, 23, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Castelein, B.; Cagnie, B.; Parlevliet, T.; Cools, A. Serratus anterior or pectoralis minor: Which muscle has the upper hand during protraction exercises? Man. Ther. 2016, 22, 158–164. [Google Scholar] [CrossRef] [PubMed]
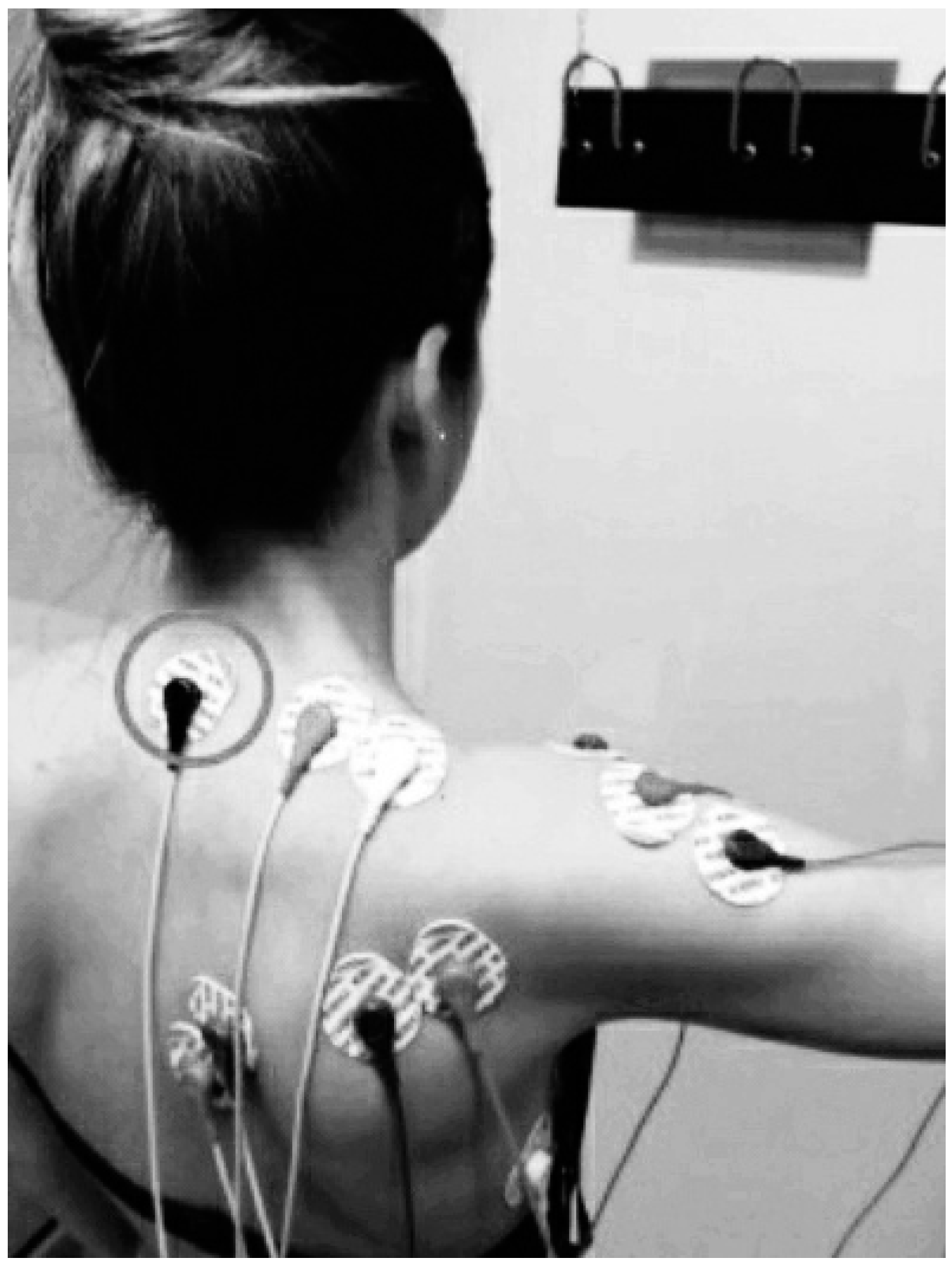
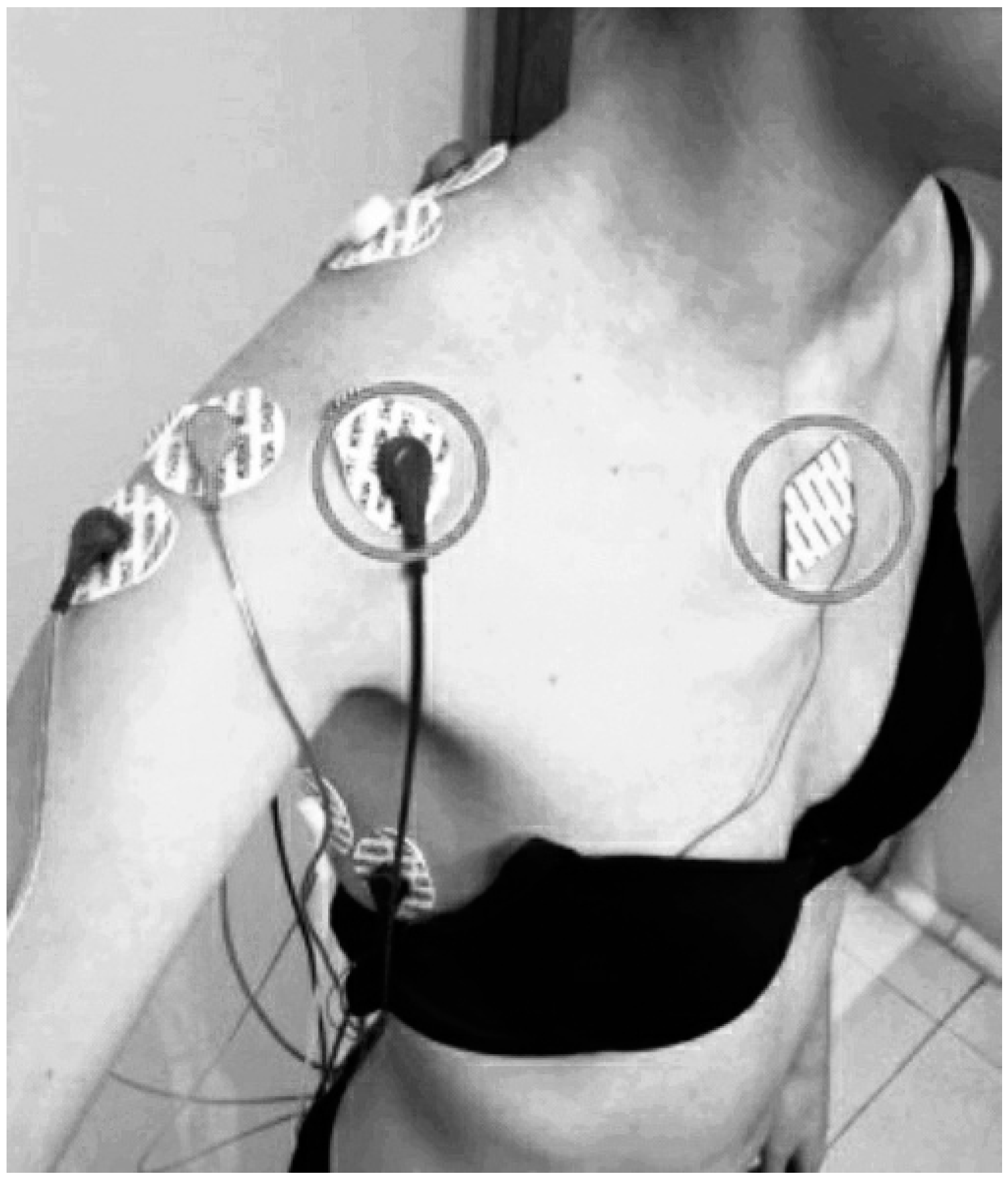
| Outcomes | Group 1 (N = 30) | Group 2 (N = 30) | Group 3 (N = 30) | p |
|---|---|---|---|---|
| Age (years, X (SD)) | 56 (8) | 54 (8) | 55 (8) | 0.788 * |
| BMI (Kg/m2, X (SD)) | 25.4 (5.1) | 24.4 (5) | 23.5 (4.2) | 0.283 * |
| Occupation | ||||
| Remunerated job (n (%)) | 12 (40) | 9 (30) | 10 (33.3) | 0.484 ** |
| Retired (n (%)) | 9 (30) | 15 (50) | 10 (33.3) | |
| Disable (n (%)) | 3 (10) | 0 | 2 (6.7) | |
| Jobless (n (%)) | 6 (20) | 6 (20) | 8 (26.7) | |
| Education level | ||||
| No studies (n (%)) | 3 (10) | 7 (23) | 1 (3.3) | 0.156 ** |
| Primary (n (%)) | 9 (30) | 8 (26.7) | 10 (33.3) | |
| Secondary (n (%)) | 6 (20) | 10 (33.3) | 9 (30) | |
| Bachelor (n (%)) | 8 (26.7) | 4 (13.3) | 10 (33.3) | |
| Superior grade (n (%)) | 3 (10) | 1 (3.3) | 0 | |
| Master/doctorate (n (%)) | 1 (3.3) | 0 | 0 | |
| Surgical procedure | ||||
| Lumpectomy (n (%)) | 26 (86.7) | 24 (80) | - | 0.488 ** |
| Quadrantectomy (n (%)) | 4 (13.3) | 6 (20) | ||
| Axillary surgical | ||||
| Lymphadenectomy (n (%)) | 29 (96.7) | 29 (96.7) | - | - |
| SNLB (n (%)) | 1 (3.3) | 1 (3.3) | ||
| Chemotherapy (n (%)) | 30 (100) | 30 (100) | - | - |
| Radiotherapy (n (%)) | 30 (100) | 30 (100) | - | - |
| Hormonal Therapy (n (%)) | 25 (83.3) | 23 (76.7) | - | 0.784 ** |
| Axillary web syndrome after surgery (n (%)) | 28 (93.3) | 29 (96.7) | - | NS ** |
| Lymphocele (n (%)) | 1 (3.3) | 2 (6.7) | - | NS ** |
| MPS (n (%)) | 30 (100) | - | ||
| VAS (cm, X (SD)) | 7.20 (1.12) | |||
| Characteristics of pain | ||||
| Predominant neuropathic pain (n (%)) | 25 (83.3) | - | ||
| Mix (neuropathic & nociceptive pain) (n (%)) | 30 (100) | |||
| Mm | Group 1 X (SD) | Group 2 X (SD) | Group 3 X (SD) | p * | Group 1 vs. Group 2 | Group 1 vs. Group 3 | Group 2 vs. Group 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dif X | 95% CI | p ** | Dif X | 95% CI | p ** | Dif X | 95% CI | p ** | ||||||||
| LL | UL | LL | UL | LL | UL | |||||||||||
| UT | −1.51 (2.24) | −0.36 (0.33) | −0.41 (0.37) | 0.001 | 1.15 | 0.31 | 1.98 | 0.004 | 1.10 | 0.26 | 1.94 | 0.005 | 0.04 | −0.79 | 0.88 | NS *** |
| LT | −0.23 (0.2) | 0.16 (0.69) | 1.12 (0.09) | <0.001 | 0.40 | 0.13 | 0.66 | 0.001 | 1.35 | 1.09 | 1.62 | <0.001 | 0.95 | 0.69 | 1.22 | <0.001 |
| MD | 1.85 (0.86) | 0.76 (0.65) | 0.22 (0.15) | <0.001 | 1.09 | 0.69 | 1.49 | <0.001 | 1.62 | 1.23 | 2.02 | <0.001 | 0.53 | 0.13 | 0.93 | 0.004 |
| I | 1.01 (1.81) | 0.90 (0.27) | −0.10 (1.13) | 0.001 | 0.10 | −0.67 | 0.89 | NS *** | 1.12 | 0.33 | 1.90 | 0.002 | 1.01 | 0.23 | 1.80 | 0.006 |
| SA | 2.08 (1.08) | 1.15 (0.54) | 1.09 (0.21) | <0.001 | 0.93 | 0.48 | 1.38 | <0.001 | 0.99 | 0.54 | 1.44 | <0.001 | 0.06 | −0.38 | 0.50 | NS *** |
| Mm | Group 1 X (SD) | Group 2 X (SD) | Group 3 X (SD) | p * | Group 1 vs. Group 2 | Group 1 vs. Group 3 | Group 2 vs. Group 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dif X | 95% CI | p ** | Dif X | 95% CI | p ** | Dif X | 95% CI | p ** | ||||||||
| LL | UL | LL | UL | LL | UL | |||||||||||
| UT | −0.33 (0.16) | −0.14 (0.13) | −0.1 (0.14) | <0.001 | 0.18 | 0.09 | 0.28 | <0.001 | 0.22 | 0.13 | 0.32 | <0.001 | 0.03 | −0.13 | 0.05 | 0.924 |
| LT | 0.59 (0.29) | 0.86 (0.3) | 0.98 (0.21) | <0.001 | 0.26 | 0.09 | 0.43 | 0.001 | 0.39 | 0.21 | 0.56 | <0.001 | 0.12 | 0.04 | 0.30 | 0.228 |
| AD | 0.99 (0.61) | 0.24 (0.37) | 0.04 (0.14) | <0.001 | 0.74 | 0.48 | 1.01 | <0.001 | 0.95 | 0.68 | 1.22 | <0.001 | 0.20 | −0.05 | 0.47 | 0.178 |
| I | 0.03 (0.18) | 0.19 (0.23) | 0.3 (0.26) | <0.001 | 0.15 | 0.01 | 0.3 | 0.028 | 0.26 | 0.12 | 0.41 | <0.001 | 0.11 | 0.03 | 0.25 | 0.204 |
| SA | 1.25 (0.38) | 0.75 (0.36) | 0.51 (0.3) | <0.001 | 0.50 | 0.27 | 0.72 | <0.001 | 0.74 | 0.52 | 0.96 | <0.001 | 0.24 | 0.02 | 0.46 | 0.026 |
| Mm | Group 1 X (SD) | Group 2 X (SD) | Group 3 X (SD) | p * | Group 1 vs. Group 2 | Group 1 vs. Group 3 | Group 2 vs. Group 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dif X | 95% CI | p ** | Dif X | 95% CI | p ** | Dif X | 95% CI | p ** | ||||||||
| LL | UL | LL | UL | LL | UL | |||||||||||
| UT | −0.09 (0.18) | 0.009 (0.29) | 0.09 (0.14) | 0.005 | 0.10 | 0.02 | 0.24 | 0.178 | 0.18 | 0.05 | 0.32 | 0.003 | 0.08 | 0.05 | 0.31 | 0.453 |
| LT | 2.01 (0.74) | 1.5 (0.46) | 1.49 (0.36) | 0.001 | 0.45 | 0.10 | 0.79 | 0.006 | 0.52 | 0.17 | 0.86 | 0.001 | 0.06 | −0.27 | 0.41 | NS *** |
| PD | 0.82 (0.47) | 0.67 (0.38) | 0.46 (0.29) | 0.002 | 0.15 | −0.09 | 0.40 | 0.378 | 0.36 | 0.11 | 0.61 | 0.002 | 0.20 | −0.03 | 0.45 | 0.129 |
| I | 0.29 (0.52) | 0.11 (0.36) | −0.03 (0.08) | 0.003 | 0.18 | −0.05 | 0.41 | 0.177 | 0.33 | 0.10 | 0.56 | 0.002 | 0.15 | −0.08 | 0.38 | 0.340 |
| SA | 1.68 (0.54) | 1.29 (0.54) | 1.04 (0.33) | <0.001 | 0.39 | 0.08 | 0.69 | 0.006 | 0.64 | 0.33 | 0.94 | <0.001 | 0.24 | −0.05 | 0.55 | 0.154 |
| Mm | Group 1 X (SD) | Group 2 X (SD) | Group 3 X (SD) | p * | Group 1 vs. Group 2 | Group 1 vs. Group 3 | Group 2 vs. Group 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dif X | 95% CI | p ** | Dif X | 95% CI | p ** | Dif X | 95% CI | p ** | ||||||||
| LL | UL | LL | UL | LL | UL | |||||||||||
| UP | 58.24 (10.31) | 38.50 (9.18) | 34.11(12.99) | <0.001 | 19.74 | 12.82 | 26.65 | <0.001 | 24.13 | 17.21 | 31.04 | <0.001 | 4.39 | −2.52 | 11.30 | 0.374 |
| LT | 14.72 (5.37) | 15.94 (3.85) | 19.64 (4.30) | <0.001 | 1.21 | 1.65 | 4.09 | 0.909 | 4.92 | 2.04 | 7.79 | <0.001 | 3.70 | 0.83 | 6.57 | 0.007 |
| MD | 75.41 (10.19) | 65.97 (9.89) | 59.74 (8.86) | <0.001 | 9.44 | 3.35 | 15.54 | 0.001 | 15.67 | 9.58 | 21.77 | <0.001 | 6.23 | 0.13 | 12.32 | 0.043 |
| I | 6.52 (2.63) | 10.07 (3.01) | 10.99 (3.27) | <0.001 | 3.55 | 1.67 | 5.43 | <0.001 | 4.47 | 2.59 | 6.35 | <0.001 | 0.92 | −2.8 | 0.96 | 0.709 |
| SA | 4.75 (2.25) | 13.77 (5.9) | 22.63 (5.31) | <0.001 | 9.02 | 6.01 | 12.02 | <0.001 | 17.87 | 14.87 | 20.87 | <0.001 | 8.85 | 5.85 | 11.85 | <0.001 |
| Mm | Group 1 X (SD) | Group 2 X (SD) | Group 3 X (SD) | p * | Group 1 vs. Group 2 | Group 1 vs. Group 3 | Group 2 vs. Group 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dif X | 95% CI | p ** | Dif X | 95% CI | p ** | Dif X | 95% CI | p ** | ||||||||
| LL | UL | LL | UL | LL | UL | |||||||||||
| UT | 37.09 (10.25) | 20.95 (6.75) | 17.38 (5.11) | <0.001 | 16.14 | 11.30 | 20.98 | <0.001 | 19.71 | 14.87 | 24.55 | <0.001 | 3.57 | −1.27 | 8.41 | 0.226 |
| LT | 11.21 (3.99) | 15.24 (4.36) | 22.89 (7.68) | <0.001 | 4.03 | 0.50 | 7.56 | 0.020 | 11.68 | 8.14 | 15.21 | <0.001 | 7.64 | 4.11 | 11.17 | <0.001 |
| AD | 80.79 (9.68) | 65.43 (13.70) | 61.90 (12.17) | <0.001 | 15.35 | 7.8 | 22.89 | <0.001 | 18.89 | 11.34 | 26.43 | <0.001 | 3.53 | −4.008 | 11.08 | 0.767 |
| I | 11.17 (5.67) | 22.6 (12.41) | 46.88 (11.49) | <0.001 | 11.42 | 4.93 | 12.92 | <0.001 | 35.70 | 29.21 | 42.2 | <0.001 | 24.28 | 17.78 | 30.77 | <0.001 |
| SA | 6.8 (3.54) | 17.32 (7.7) | 38.23 (11.04) | <0.001 | 10.45 | 5.39 | 15.52 | <0.001 | 31.36 | 26.30 | 36.43 | <0.001 | 20.91 | 15.84 | 25.97 | <0.001 |
| Mm | Group 1 X (SD) | Group 2 X (SD) | Group 3 X (SD) | p * | Group 1 vs. Group 2 | Group 1 vs. Group 3 | Group 2 vs. Group 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dif X | 95% CI | p ** | Dif X | 95 % CI | p ** | Dif X | 95% CI | p ** | ||||||||
| LL | UL | LL | UL | LL | UL | |||||||||||
| UT | 33.15 (7.89) | 22.01 (8.6) | 18.45 (7.89) | <0.001 | 11.14 | 6.01 | 16.26 | <0.001 | 14.7 | 9.57 | 19.82 | <0.001 | 3.56 | −1.56 | 8.68 | 0.281 |
| LT | 17.14 (3.71) | 28.84 (12.39) | 32.57 (12.1) | <0.001 | 11.7 | 5.25 | 18.15 | <0.001 | 15.43 | 8.98 | 21.88 | <0.001 | 3.73 | −10.17 | 2.71 | 0.484 |
| PD | 22.39 (7.48) | 42.81 (16.95) | 48.5 (12.02) | <0.001 | 20.42 | 12.38 | 28.46 | <0.001 | 26.11 | 18.07 | 34.14 | <0.001 | 5.68 | −13.72 | 2.35 | 0.263 |
| I | 20.91 (7.34) | 50.20 (20.73) | 72.55 (8.62) | <0.001 | 29.29 | 20.69 | 37.89 | <0.001 | 51.64 | 43.02 | 60.24 | <0.001 | 22.35 | 13.75 | 30.94 | <0.001 |
| SA | 17.09 (6.60) | 28.67 (10.75) | 59.86 (10.92) | <0.001 | 11.58 | 5.5 | 17.65 | <0.001 | 42.77 | 36.69 | 48.84 | <0.001 | 31.19 | 25.11 | 37.26 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto-Gómez, V.; Navarro-Brazález, B.; Sánchez-Méndez, Ó.; de-la-Villa, P.; Sánchez-Sánchez, B.; Torres-Lacomba, M. Electromyographic Analysis of Shoulder Neuromuscular Activity in Women Following Breast Cancer Treatment: A Cross-Sectional Descriptive Study. J. Clin. Med. 2020, 9, 1804. https://doi.org/10.3390/jcm9061804
Prieto-Gómez V, Navarro-Brazález B, Sánchez-Méndez Ó, de-la-Villa P, Sánchez-Sánchez B, Torres-Lacomba M. Electromyographic Analysis of Shoulder Neuromuscular Activity in Women Following Breast Cancer Treatment: A Cross-Sectional Descriptive Study. Journal of Clinical Medicine. 2020; 9(6):1804. https://doi.org/10.3390/jcm9061804
Chicago/Turabian StylePrieto-Gómez, Virginia, Beatriz Navarro-Brazález, Óscar Sánchez-Méndez, Pedro de-la-Villa, Beatriz Sánchez-Sánchez, and María Torres-Lacomba. 2020. "Electromyographic Analysis of Shoulder Neuromuscular Activity in Women Following Breast Cancer Treatment: A Cross-Sectional Descriptive Study" Journal of Clinical Medicine 9, no. 6: 1804. https://doi.org/10.3390/jcm9061804
APA StylePrieto-Gómez, V., Navarro-Brazález, B., Sánchez-Méndez, Ó., de-la-Villa, P., Sánchez-Sánchez, B., & Torres-Lacomba, M. (2020). Electromyographic Analysis of Shoulder Neuromuscular Activity in Women Following Breast Cancer Treatment: A Cross-Sectional Descriptive Study. Journal of Clinical Medicine, 9(6), 1804. https://doi.org/10.3390/jcm9061804








