The Effect of Probiotics and Synbiotics on Risk Factors Associated with Cardiometabolic Diseases in Healthy People—A Systematic Review and Meta-Analysis with Meta-Regression of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Inclusion Criteria
2.2. Data Abstraction
2.3. Outcomes
2.4. Data Synthesis and Statistical Analysis
3. Results
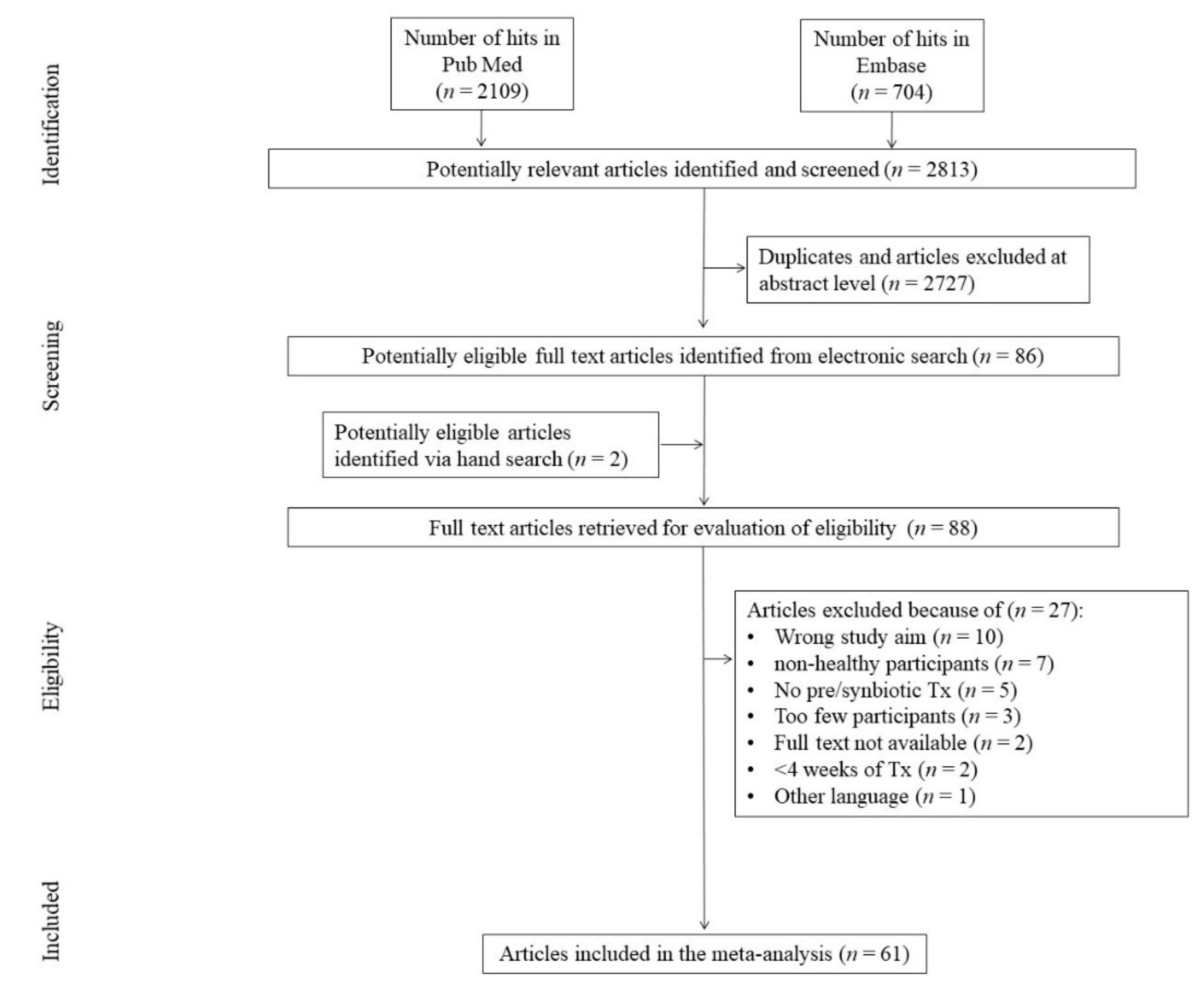
3.1. Search Results
3.2. Study, Treatment, and Patient Characteristics
3.3. Risk of Bias Assessment
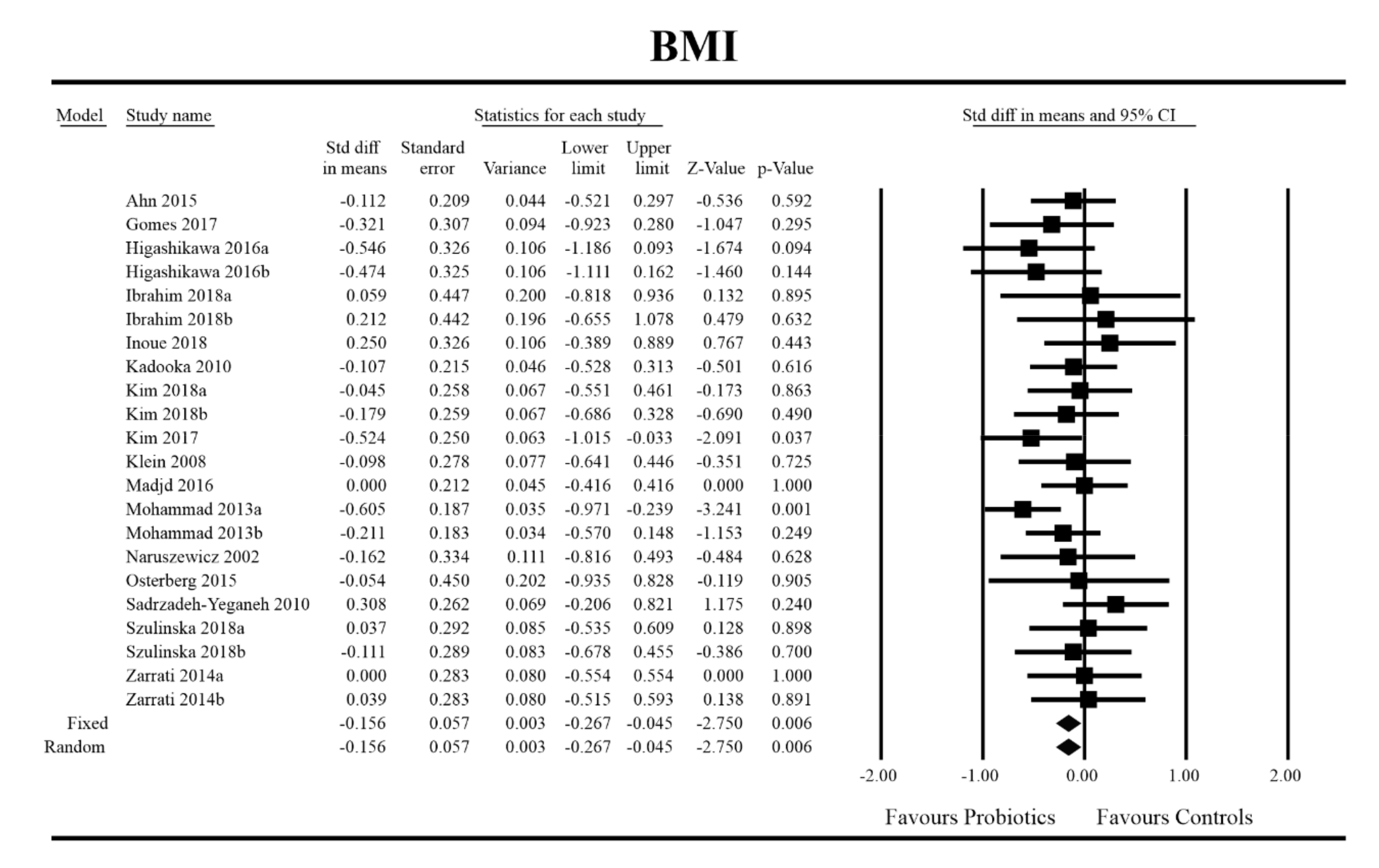
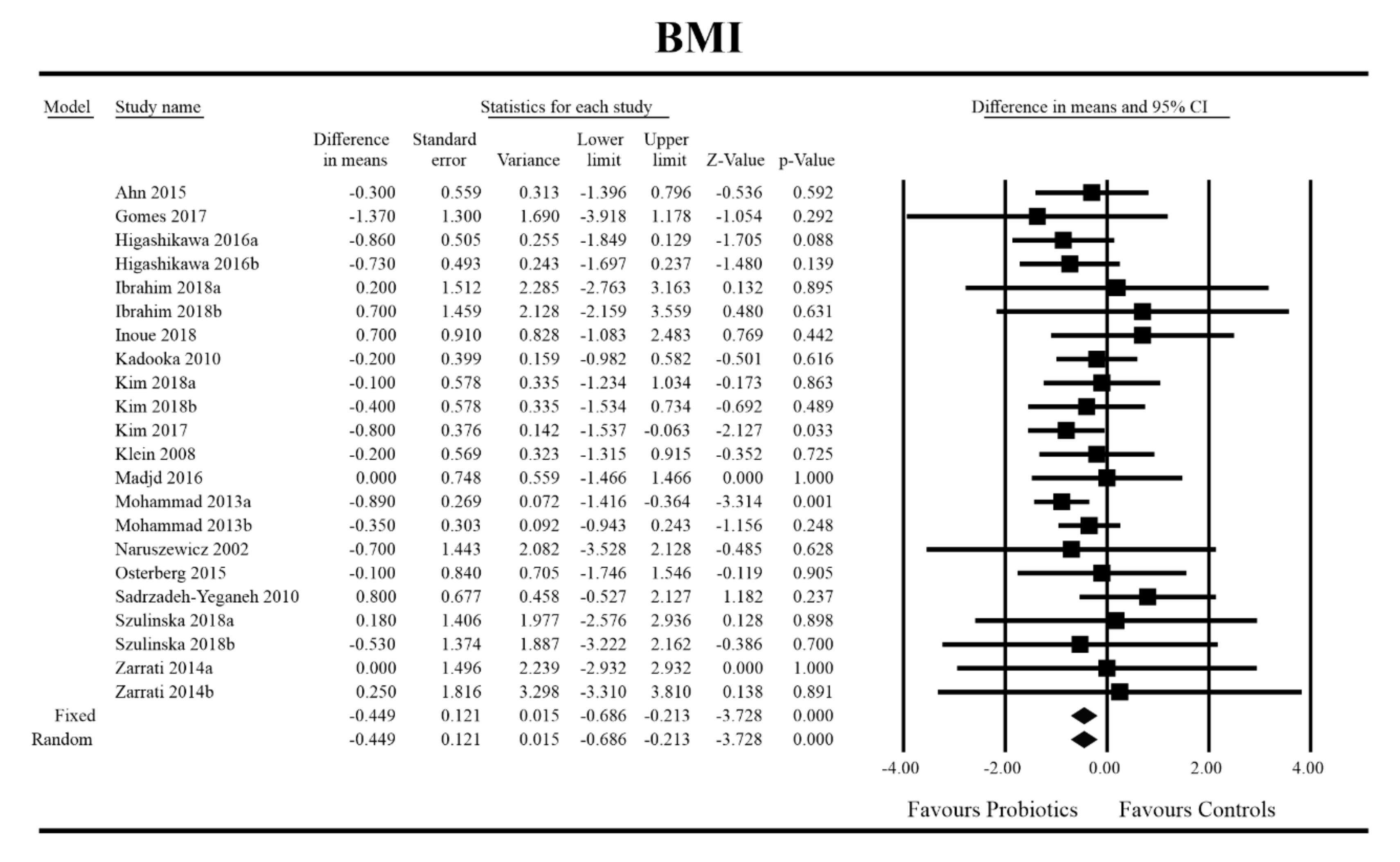
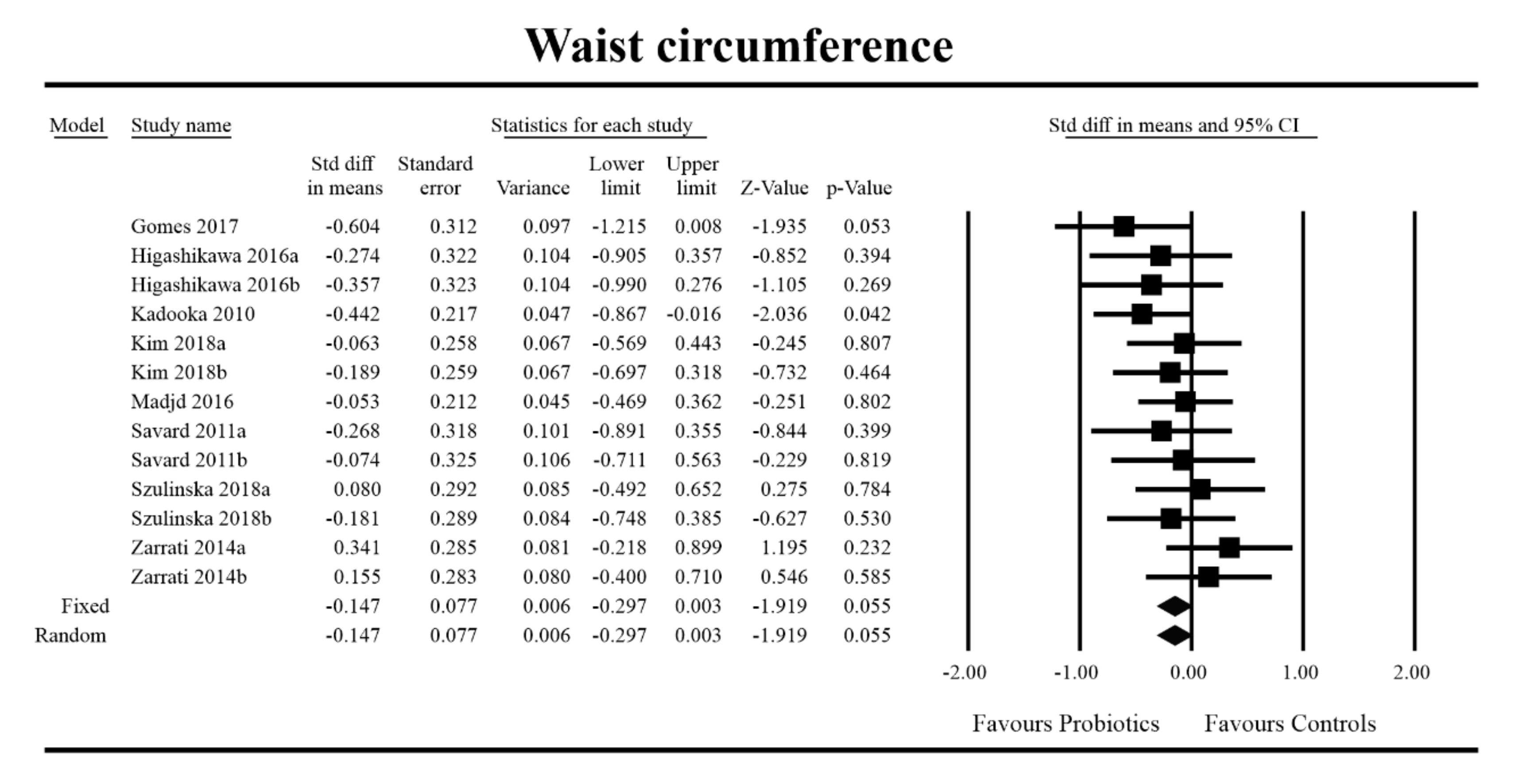
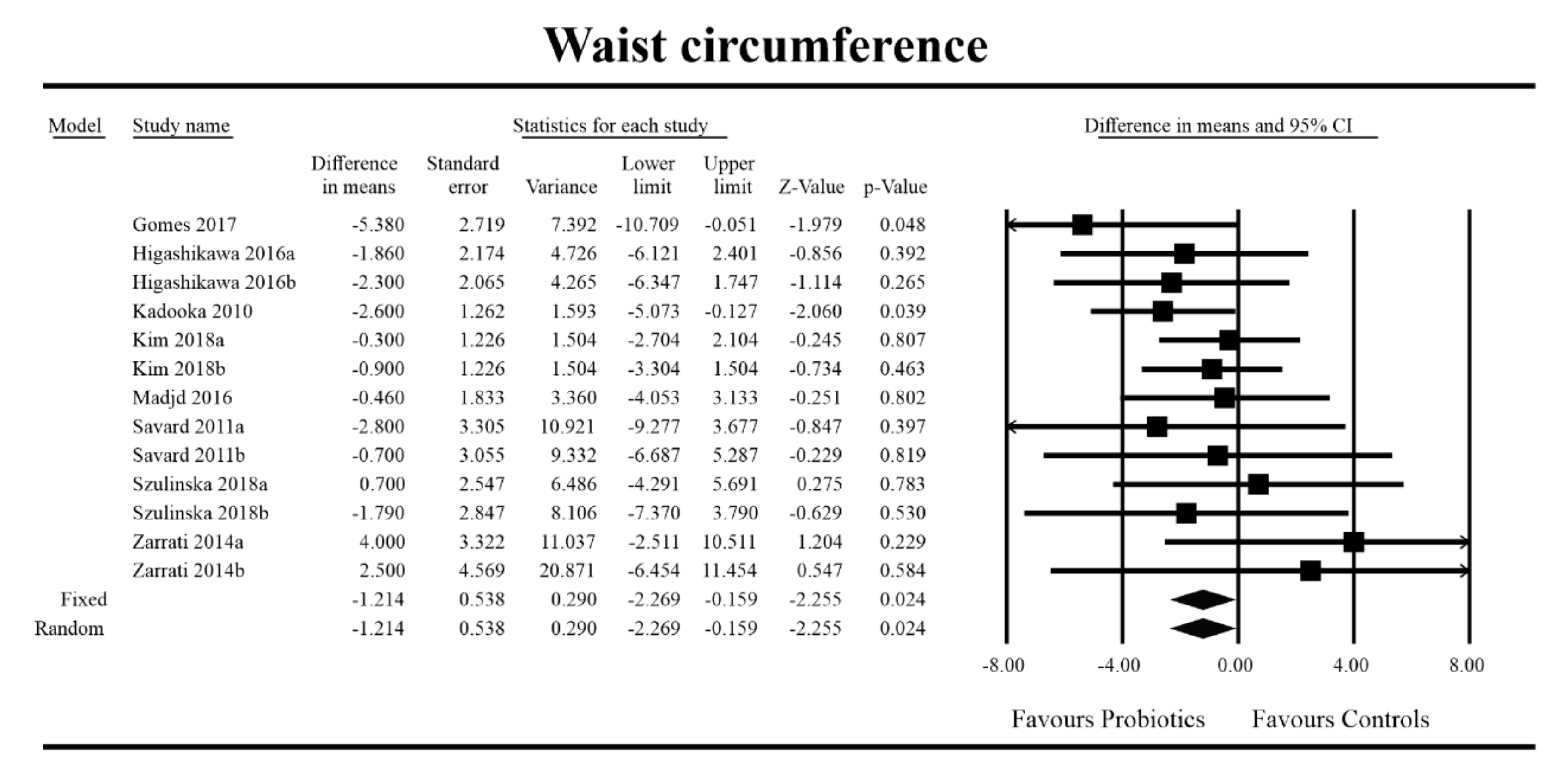
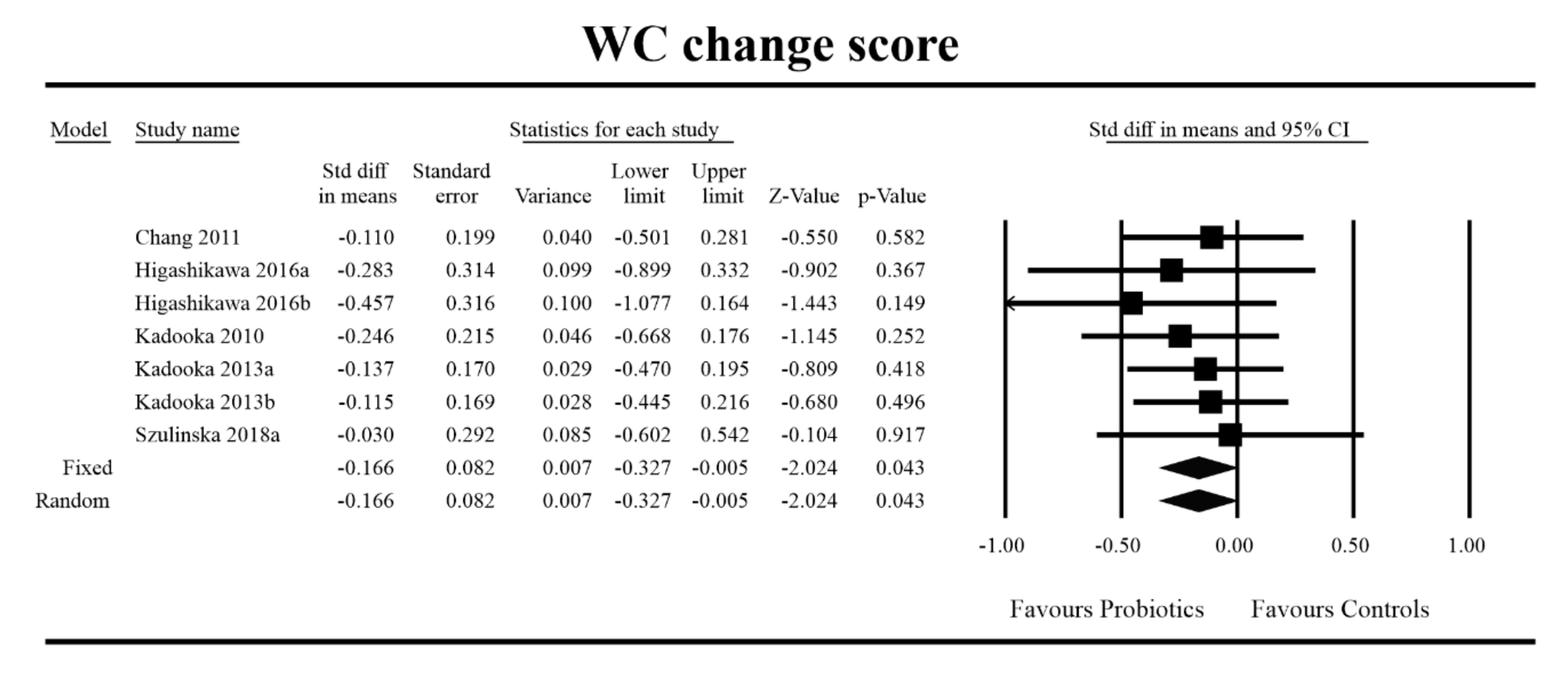
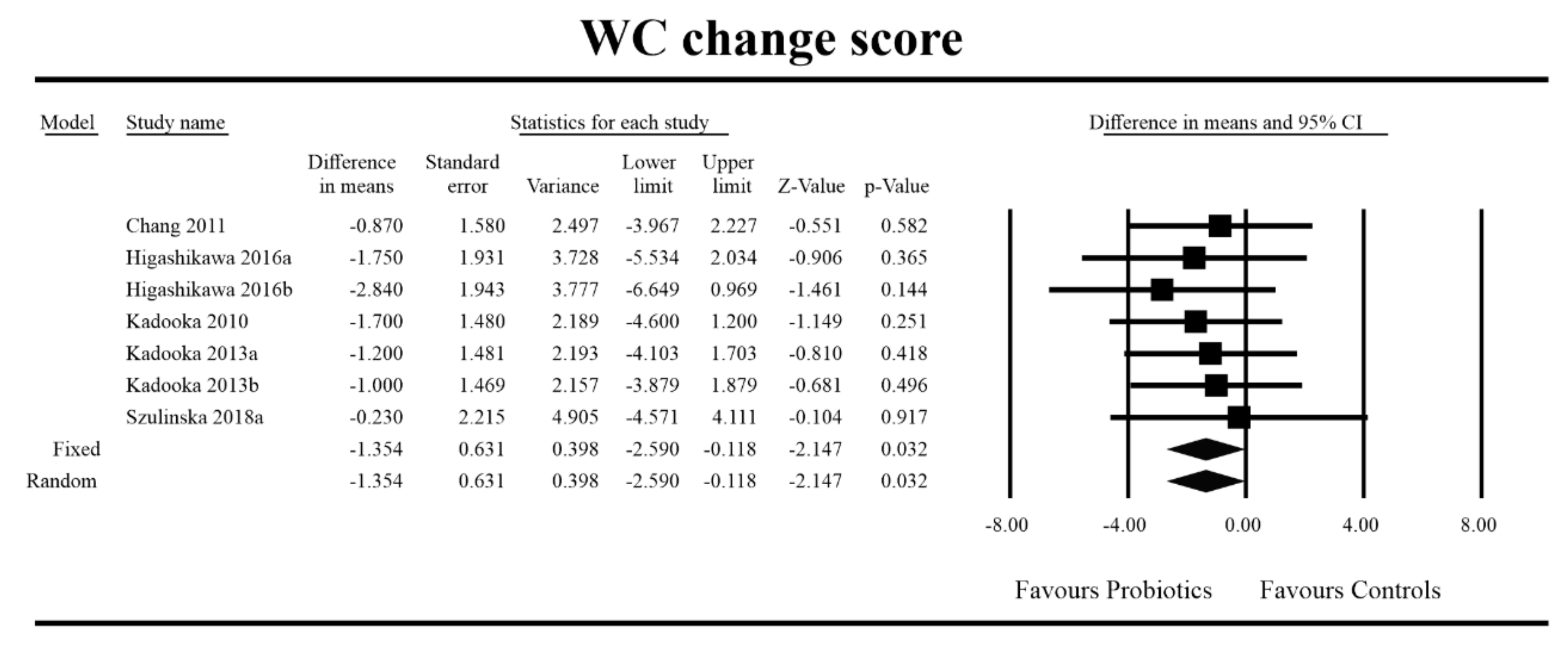
3.4. Effects on Metabolic Indices
3.5. Effects on Metabolic Indices Regarding Obesity Status
3.6. Metaregression Analyses
3.7. Microbiota Parameters
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Cercato, C.; Fonseca, F.A. Cardiovascular risk and obesity. Diabetol. Metab. Syndr. 2019, 11, 74. [Google Scholar] [CrossRef]
- Zhang, Y.; Vittinghoff, E.; Pletcher, M.J.; Allen, N.B.; Hazzouri, A.Z.A.; Yaffe, K.; Balte, P.P.; Alonso, A.; Newman, A.B.; Ives, D.G.; et al. Associations of Blood Pressure and Cholesterol Levels During Young Adulthood With Later Cardiovascular Events. J. Am. Coll. Cardiol. 2019, 74, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Costa Pereira, L.M.; Aidar, F.J.; de Matos, D.G.; de Farias Neto, J.P.; de Souza, R.F.; Sobral Sousa, A.C.; de Almeida, R.R.; Prado Nunes, M.A.; Nunes-Silva, A.; da Silva Júnior, W.M. Assessment of Cardiometabolic Risk Factors, Physical Activity Levels, and Quality of Life in Stratified Groups up to 10 Years after Bariatric Surgery. Int. J. Environ. Res. Public Health 2019, 16, 1975. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Aguilar-Salinas, C.A.; Viveros-Ruiz, T. Recent advances in managing/understanding the metabolic syndrome. F1000Res. 2019, 8. [Google Scholar] [CrossRef]
- Tzika, E.; Dreker, T.; Imhof, A. Epigenetics and Metabolism in Health and Disease. Front. Genet. 2018, 9, 361. [Google Scholar] [CrossRef]
- Dhurandhar, E.J.; Keith, S.W. The aetiology of obesity beyond eating more and exercising less. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 533–544. [Google Scholar] [CrossRef]
- Parekh, P.J.; Balart, L.A.; Johnson, D.A. The Influence of the Gut Microbiome on Obesity, Metabolic Syndrome and Gastrointestinal Disease. Clin. Transl. Gastroenterol. 2015, 6, e91. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A.; Education Research and Assessment (OPERA) Group on behalf of the Obesity Programs of Nutrition. Gut microbiota: A new path to treat obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Shi, B. Gut microbiota as a potential target of metabolic syndrome: The role of probiotics and prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef]
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araújo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J. Nutr. Biochem. 2017, 50, 16–25. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, M.; Chen, L.; Bhochhibhoya, A. Probiotic Foods and Supplements Interventions for Metabolic Syndromes: A Systematic Review and Meta-Analysis of Recent Clinical Trials. ANM 2019, 74, 224–241. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Suganthy, N.; Chaiyasut, C. A Review on Role of Microbiome in Obesity and Antiobesity Properties of Probiotic Supplements. Available online: https://www.hindawi.com/journals/bmri/2019/3291367/ (accessed on 3 December 2019).
- Koutnikova, H.; Genser, B.; Monteiro-Sepulveda, M.; Faurie, J.-M.; Rizkalla, S.; Schrezenmeir, J.; Clément, K. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: A systematic review and meta-analysis of randomised controlled trials. BMJ. Open 2019, 9, e017995. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Kaczmarczyk, M.; Łoniewski, I.; Lara, L.F.; Koulaouzidis, A.; Misera, A.; Maciejewska, D.; Marlicz, W. A Systematic Review, Meta-Analysis, and Meta-Regression Evaluating the Efficacy and Mechanisms of Action of Probiotics and Synbiotics in the Prevention of Surgical Site Infections and Surgery-Related Complications. J. Clin. Med. 2018, 7, 556. [Google Scholar] [CrossRef]
- Marlicz, W.; Skonieczna-Zydecka, K.; Yung, D.E.; Loniewski, I.; Koulaouzidis, A. Endoscopic findings and colonic perforation in microscopic colitis: A systematic review. Dig. Liver Dis. 2017, 49, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Łoniewski, I.; Misera, A.; Stachowska, E.; Maciejewska, D.; Marlicz, W.; Galling, B. Second-generation antipsychotics and metabolism alterations: A systematic review of the role of the gut microbiome. Psychopharmacology 2018, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011, 343, d5928. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Agerbaek, M.; Gerdes, L.U.; Richelsen, B. Hypocholesterolaemic effect of a new fermented milk product in healthy middle-aged men. Eur. J. Clin. Nutr. 1995, 49, 346–352. [Google Scholar]
- Agerholm-Larsen, L.; Raben, A.; Haulrik, N.; Hansen, A.S.; Manders, M.; Astrup, A. Effect of 8 week intake of probiotic milk products on risk factors for cardiovascular diseases. Eur. J. Clin. Nutr. 2000, 54, 288–297. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Kim, M.; Ahn, Y.-T.; Sim, J.-H.; Choi, I.-D.; Lee, S.-H.; Lee, J.H. The triglyceride-lowering effect of supplementation with dual probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032: Reduction of fasting plasma lysophosphatidylcholines in nondiabetic and hypertriglyceridemic subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 724–733. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Kim, M.; Chae, J.S.; Ahn, Y.-T.; Sim, J.-H.; Choi, I.-D.; Lee, S.-H.; Lee, J.H. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduces fasting triglycerides and enhances apolipoprotein A-V levels in non-diabetic subjects with hypertriglyceridemia. Atherosclerosis 2015, 241, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Borges, N. Effect of fermented milk containing Lactobacillus acidophilus and Bifidobacterium longum on plasma lipids of women with normal or moderately elevated cholesterol. J. Dairy Res. 2009, 76, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Bjerg, A.T.; Kristensen, M.; Ritz, C.; Stark, K.D.; Holst, J.J.; Leser, T.D.; Wellejus, A.; Astrup, A. Four weeks supplementation with Lactobacillus paracasei subsp. paracasei L. casei W8® shows modest effect on triacylglycerol in young healthy adults. Benef. Microbes 2015, 6, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Boesmans, L.; Valles-Colomer, M.; Wang, J.; Eeckhaut, V.; Falony, G.; Ducatelle, R.; Van Immerseel, F.; Raes, J.; Verbeke, K. Butyrate Producers as Potential Next-Generation Probiotics: Safety Assessment of the Administration of Butyricicoccus pullicaecorum to Healthy Volunteers. mSystems 2018, 3, e00094-18. [Google Scholar] [CrossRef]
- Brahe, L.K.; Le Chatelier, E.; Prifti, E.; Pons, N.; Kennedy, S.; Blædel, T.; Håkansson, J.; Dalsgaard, T.K.; Hansen, T.; Pedersen, O.; et al. Dietary modulation of the gut microbiota--a randomised controlled trial in obese postmenopausal women. Br. J. Nutr. 2015, 114, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, H.; Pieczul-Mróz, J.; Jastrzebska, M.; Chełstowski, K.; Naruszewicz, M. Decrease in fibrinogen and LDL-cholesterol levels upon supplementation of diet with Lactobacillus plantarum in subjects with moderately elevated cholesterol. Atherosclerosis 1998, 137, 437–438. [Google Scholar]
- Chang, B.J.; Park, S.U.; Jang, Y.S.; Ko, S.H.; Joo, N.M.; Kim, S.I.; Kim, C.-H.; Chang, D.K. Effect of functional yogurt NY-YP901 in improving the trait of metabolic syndrome. Eur. J. Clin. Nutr. 2011, 65, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; West, N.P.; Horn, P.L.; Lehtinen, M.J.; Koerbin, G.; Pyne, D.B.; Lahtinen, S.J.; Fricker, P.A.; Cripps, A.W. Effects of probiotic supplementation over 5 months on routine haematology and clinical chemistry measures in healthy active adults. Eur. J. Clin. Nutr. 2014, 68, 1255–1257. [Google Scholar] [CrossRef]
- de Roos, N.M.; van Hemert, S.; Rovers, J.M.P.; Smits, M.G.; Witteman, B.J.M. The effects of a multispecies probiotic on migraine and markers of intestinal permeability-results of a randomized placebo-controlled study. Eur. J. Clin. Nutr. 2017, 71, 1455–1462. [Google Scholar] [CrossRef]
- Fabian, E.; Elmadfa, I. Influence of daily consumption of probiotic and conventional yoghurt on the plasma lipid profile in young healthy women. Ann. Nutr. Metab. 2006, 50, 387–393. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Oliveira, M.; McCauley, T.; Tauler, P.; Lawrence, C. Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Gohel, M.K.; Prajapati, J.B.; Mudgal, S.V.; Pandya, H.V.; Singh, U.S.; Trivedi, S.S.; Phatak, A.G.; Patel, R.M. Effect of Probiotic Dietary Intervention on Calcium and Haematological Parameters in Geriatrics. J. Clin. Diagn. Res. 2016, 10, LC05–LC09. [Google Scholar] [CrossRef]
- Gomes, A.C.; de Sousa, R.G.M.; Botelho, P.B.; Gomes, T.L.N.; Prada, P.O.; Mota, J.F. The additional effects of a probiotic mix on abdominal adiposity and antioxidant Status: A double-blind, randomized trial. Obesity (Silver Spring) 2017, 25, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Greany, K.A.; Bonorden, M.J.L.; Hamilton-Reeves, J.M.; McMullen, M.H.; Wangen, K.E.; Phipps, W.R.; Feirtag, J.; Thomas, W.; Kurzer, M.S. Probiotic capsules do not lower plasma lipids in young women and men. Eur. J. Clin. Nutr. 2008, 62, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Guillemard, E.; Tanguy, J.; Flavigny, A.; de la Motte, S.; Schrezenmeir, J. Effects of consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114 001 on common respiratory and gastrointestinal infections in shift workers in a randomized controlled trial. J. Am. Coll. Nutr. 2010, 29, 455–468. [Google Scholar] [CrossRef]
- Hatakka, K.; Mutanen, M.; Holma, R.; Saxelin, M.; Korpela, R. Lactobacillus rhamnosus LC705 together with Propionibacterium freudenreichii ssp shermanii JS administered in capsules is ineffective in lowering serum lipids. J. Am. Coll. Nutr. 2008, 27, 441–447. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Yde, C.C.; Ziegler, M.L.; Honoré, A.H.; Saarinen, M.T.; Lahtinen, S.; Stahl, B.; Jensen, H.M.; Stenman, L.K. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef]
- Higashikawa, F.; Noda, M.; Awaya, T.; Danshiitsoodol, N.; Matoba, Y.; Kumagai, T.; Sugiyama, M. Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2016, 70, 582–587. [Google Scholar] [CrossRef]
- Ibrahim, N.S.; Muhamad, A.S.; Ooi, F.K.; Meor-Osman, J.; Chen, C.K. The effects of combined probiotic ingestion and circuit training on muscular strength and power and cytokine responses in young males. Appl. Physiol. Nutr. Metab. 2018, 43, 180–186. [Google Scholar] [CrossRef]
- Inoue, T.; Kobayashi, Y.; Mori, N.; Sakagawa, M.; Xiao, J.-Z.; Moritani, T.; Sakane, N.; Nagai, N. Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef. Microbes 2018, 9, 843–853. [Google Scholar] [CrossRef]
- Ito, M.; Kusuhara, S.; Yokoi, W.; Sato, T.; Ishiki, H.; Miida, S.; Matsui, A.; Nakamori, K.; Nonaka, C.; Miyazaki, K. Streptococcus thermophilus fermented milk reduces serum MDA-LDL and blood pressure in healthy and mildly hypercholesterolaemic adults. Benef. Microbes 2017, 8, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Lewis, J.R.; Thompson, P.L.; Prince, R.L. The effects of probiotic bacteria on glycaemic control in overweight men and women: A randomised controlled trial. Eur. J. Clin. Nutr. 2014, 68, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Thompson, P.L.; Stojceski, B.; Prince, R.L. The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 46–51. [Google Scholar] [CrossRef]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: A randomized controlled trial. Eur. J. Clin. Nutr. 2012, 66, 1234–1241. [Google Scholar] [CrossRef]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef]
- Kadooka, Y.; Sato, M.; Ogawa, A.; Miyoshi, M.; Uenishi, H.; Ogawa, H.; Ikuyama, K.; Kagoshima, M.; Tsuchida, T. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br. J. Nutr. 2013, 110, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; Hashimoto, H.; Hosoda, M.; Morita, H.; Hosono, A. Effect of administration of fermented milk containing whey protein concentrate to rats and healthy men on serum lipids and blood pressure. J. Dairy Sci. 2000, 83, 255–263. [Google Scholar] [CrossRef]
- Kim, J.; Yun, J.M.; Kim, M.K.; Kwon, O.; Cho, B. Lactobacillus gasseri BNR17 Supplementation Reduces the Visceral Fat Accumulation and Waist Circumference in Obese Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Med. Food 2018, 21, 454–461. [Google Scholar] [CrossRef]
- Kim, M.; Kim, M.; Kang, M.; Yoo, H.J.; Kim, M.S.; Ahn, Y.-T.; Sim, J.-H.; Jee, S.H.; Lee, J.H. Effects of weight loss using supplementation with Lactobacillus strains on body fat and medium-chain acylcarnitines in overweight individuals. Food Funct. 2017, 8, 250–261. [Google Scholar] [CrossRef]
- Klein, A.; Friedrich, U.; Vogelsang, H.; Jahreis, G. Lactobacillus acidophilus 74-2 and Bifidobacterium animalis subsp lactis DGCC 420 modulate unspecific cellular immune response in healthy adults. Eur. J. Clin. Nutr. 2008, 62, 584–593. [Google Scholar] [CrossRef]
- Lambert, M.N.T.; Thorup, A.C.; Hansen, E.S.S.; Jeppesen, P.B. Combined Red Clover isoflavones and probiotics potently reduce menopausal vasomotor symptoms. PLoS ONE 2017, 12, e0176590. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ba, Z.; Roberts, R.F.; Rogers, C.J.; Fleming, J.A.; Meng, H.; Furumoto, E.J.; Kris-Etherton, P.M. Effects of Bifidobacterium animalis subsp. lactis BB-12® on the lipid/lipoprotein profile and short chain fatty acids in healthy young adults: A randomized controlled trial. Nutr. J. 2017, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Ayres, J.W.; Winkler, W.; Sandine, W.E. Lactobacillus effects on cholesterol: In vitro and in vivo results. J. Dairy Sci. 1989, 72, 2885–2899. [Google Scholar] [CrossRef]
- Macfarlane, S.; Cleary, S.; Bahrami, B.; Reynolds, N.; Macfarlane, G.T. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: A randomised, double-blind, placebo-controlled crossover study. Aliment. Pharmacol. Ther. 2013, 38, 804–816. [Google Scholar] [CrossRef]
- Madjd, A.; Taylor, M.A.; Mousavi, N.; Delavari, A.; Malekzadeh, R.; Macdonald, I.A.; Farshchi, H.R. Comparison of the effect of daily consumption of probiotic compared with low-fat conventional yogurt on weight loss in healthy obese women following an energy-restricted diet: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 323–329. [Google Scholar] [CrossRef]
- Mohammad Moradi, S.; Javidan, A.; Naji Isfahani, H. Effects of probiotic ultra-filtered feta cheese and raw chicory root extract on lipid profile in healthy adult volunteers: A triple-blinded randomized controlled trial. Mediterr. J. Nutr. Metab. 2013, 6, 199–206. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Johansson, M.-L.; Zapolska-Downar, D.; Bukowska, H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am. J. Clin. Nutr. 2002, 76, 1249–1255. [Google Scholar] [CrossRef]
- Nishiyama, K.; Kobayashi, T.; Sato, Y.; Watanabe, Y.; Kikuchi, R.; Kanno, R.; Koshizuka, T.; Miyazaki, N.; Ishioka, K.; Suzutani, T. A Double-Blind Controlled Study to Evaluate the Effects of Yogurt Enriched with Lactococcus lactis 11/19-B1 and Bifidobacterium lactis on Serum Low-Density Lipoprotein Level and Antigen-Specific Interferon-γ Releasing Ability. Nutrients 2018, 10, 1778. [Google Scholar] [CrossRef]
- Nova, E.; Viadel, B.; Wärnberg, J.; Carreres, J.E.; Marcos, A. Beneficial effects of a synbiotic supplement on self-perceived gastrointestinal well-being and immunoinflammatory status of healthy adults. J. Med. Food 2011, 14, 79–85. [Google Scholar] [CrossRef]
- Ostan, R.; Béné, M.C.; Spazzafumo, L.; Pinto, A.; Donini, L.M.; Pryen, F.; Charrouf, Z.; Valentini, L.; Lochs, H.; Bourdel-Marchasson, I.; et al. Impact of diet and nutraceutical supplementation on inflammation in elderly people. Results from the RISTOMED. study, an open-label randomized control trial. Clin. Nutr. 2016, 35, 812–818. [Google Scholar] [CrossRef]
- Osterberg, K.L.; Boutagy, N.E.; McMillan, R.P.; Stevens, J.R.; Frisard, M.I.; Kavanaugh, J.W.; Davy, B.M.; Davy, K.P.; Hulver, M.W. Probiotic supplementation attenuates increases in body mass and fat mass during high-fat diet in healthy young adults. Obesity (Silver Spring) 2015, 23, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, H.; Mahmood, N.; Kumar, M.; Varikuti, S.R.; Challa, H.R.; Myakala, S.P. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: A randomized, controlled trial. Mediators Inflamm. 2014, 2014, 348959. [Google Scholar] [CrossRef]
- Sadrzadeh-Yeganeh, H.; Elmadfa, I.; Djazayery, A.; Jalali, M.; Heshmat, R.; Chamary, M. The effects of probiotic and conventional yoghurt on lipid profile in women. Br. J. Nutr. 2010, 103, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Lepage, M.; Rezzonico, E.; Ngom-Bru, C.; Berger, B.; Philippe, L.; Ammon-Zuffrey, C.; et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 2014, 111, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Savard, P.; Lamarche, B.; Paradis, M.-E.; Thiboutot, H.; Laurin, É.; Roy, D. Impact of Bifidobacterium animalis subsp. lactis BB-12 and, Lactobacillus acidophilus LA-5-containing yoghurt, on fecal bacterial counts of healthy adults. Int. J. Food Microbiol. 2011, 149, 50–57. [Google Scholar] [CrossRef]
- Simon, M.-C.; Strassburger, K.; Nowotny, B.; Kolb, H.; Nowotny, P.; Burkart, V.; Zivehe, F.; Hwang, J.-H.; Stehle, P.; Pacini, G.; et al. Intake of Lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: A proof of concept. Diabetes Care 2015, 38, 1827–1834. [Google Scholar] [CrossRef]
- Simons, L.A.; Amansec, S.G.; Conway, P. Effect of Lactobacillus fermentum on serum lipids in subjects with elevated serum cholesterol. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 531–535. [Google Scholar] [CrossRef]
- Stenman, L.K.; Lehtinen, M.J.; Meland, N.; Christensen, J.E.; Yeung, N.; Saarinen, M.T.; Courtney, M.; Burcelin, R.; Lähdeaho, M.-L.; Linros, J.; et al. Probiotic With or Without Fiber Controls Body Fat Mass, Associated With Serum Zonulin, in Overweight and Obese Adults-Randomized Controlled Trial. EBioMedicine 2016, 13, 190–200. [Google Scholar] [CrossRef]
- Szulińska, M.; Łoniewski, I.; Skrypnik, K.; Sobieska, M.; Korybalska, K.; Suliburska, J.; Bogdański, P. Multispecies Probiotic Supplementation Favorably Affects Vascular Function and Reduces Arterial Stiffness in Obese Postmenopausal Women-A 12-Week Placebo-Controlled and Randomized Clinical Study. Nutrients 2018, 10, 1672. [Google Scholar] [CrossRef]
- Szulińska, M.; Łoniewski, I.; van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; D’Avino, M.; Ciampaglia, R.; Maisto, M.; Schisano, C.; Bocchino, B.; Novellino, E. Lactofermented Annurca Apple Puree as a Functional Food Indicated for the Control of Plasma Lipid and Oxidative Amine Levels: Results from a Randomised Clinical Trial. Nutrients 2019, 11, 122. [Google Scholar] [CrossRef]
- Trautvetter, U.; Ditscheid, B.; Kiehntopf, M.; Jahreis, G. A combination of calcium phosphate and probiotics beneficially influences intestinal lactobacilli and cholesterol metabolism in humans. Clin. Nutr. 2012, 31, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Usinger, L.; Jensen, L.T.; Flambard, B.; Linneberg, A.; Ibsen, H. The antihypertensive effect of fermented milk in individuals with prehypertension or borderline hypertension. J. Hum. Hypertens 2010, 24, 678–683. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valentini, L.; Pinto, A.; Bourdel-Marchasson, I.; Ostan, R.; Brigidi, P.; Turroni, S.; Hrelia, S.; Hrelia, P.; Bereswill, S.; Fischer, A.; et al. Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota - The “RISTOMED. project”: Randomized controlled trial in healthy older people. Clin. Nutr. 2015, 34, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Välimäki, I.A.; Vuorimaa, T.; Ahotupa, M.; Kekkonen, R.; Korpela, R.; Vasankari, T. Decreased training volume and increased carbohydrate intake increases oxidized LDL levels. Int. J. Sports Med. 2012, 33, 291–296. [Google Scholar] [CrossRef]
- Venkataraman, R.; Juwal, J.; Princy, J. The effect of probiotics on glycemic index. Panminerva Med. 2018, 60, 234–235. [Google Scholar] [CrossRef]
- Xiao, J.Z.; Kondo, S.; Takahashi, N.; Miyaji, K.; Oshida, K.; Hiramatsu, A.; Iwatsuki, K.; Kokubo, S.; Hosono, A. Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers. J. Dairy Sci. 2003, 86, 2452–2461. [Google Scholar] [CrossRef]
- Zarrati, M.; Salehi, E.; Nourijelyani, K.; Mofid, V.; Zadeh, M.J.H.; Najafi, F.; Ghaflati, Z.; Bidad, K.; Chamari, M.; Karimi, M.; et al. Effects of probiotic yogurt on fat distribution and gene expression of proinflammatory factors in peripheral blood mononuclear cells in overweight and obese people with or without weight-loss diet. J. Am. Coll. Nutr. 2014, 33, 417–425. [Google Scholar] [CrossRef]
- Rajkumar, H.; Kumar, M.; Das, N.; Kumar, S.N.; Challa, H.R.; Nagpal, R. Effect of Probiotic Lactobacillus salivarius UBL S22 and Prebiotic Fructo-oligosaccharide on Serum Lipids, Inflammatory Markers, Insulin Sensitivity, and Gut Bacteria in Healthy Young Volunteers: A Randomized Controlled Single-Blind Pilot Study. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 289–298. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Piera-Mardemootoo, C.; Lambert, P.; Faillie, J.-L. Efficacy of metformin on glycemic control and weight in drug-naive type 2 diabetes mellitus patients: A systematic review and meta-analysis of placebo-controlled randomized trials. Therapie 2018. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Fei, X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina 2016, 52, 28–34. [Google Scholar] [CrossRef]
- Pan, J.; Pan, Q.; Chen, Y.; Zhang, H.; Zheng, X. Efficacy of probiotic supplement for gestational diabetes mellitus: A systematic review and meta-analysis. J. Matern. Fetal. Neonatal. Med. 2019, 32, 317–323. [Google Scholar] [CrossRef]
- Ardeshirlarijani, E.; Tabatabaei-Malazy, O.; Mohseni, S.; Qorbani, M.; Larijani, B.; Baradar Jalili, R. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: A meta-analysis of randomized trials. Daru 2019, 27, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Ostrowski, M.; Ferreira, D.M.T.P.; Rosado, E.L.; Soares-Mota, M. Effect of Lactobacillus on body weight and body fat in overweight subjects: A systematic review of randomized controlled clinical trials. Int. J. Obes. (Lond) 2017, 41, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- John, G.K.; Wang, L.; Nanavati, J.; Twose, C.; Singh, R.; Mullin, G. Dietary Alteration of the Gut Microbiome and Its Impact on Weight and Fat Mass: A Systematic Review and Meta-Analysis. Genes (Basel) 2018, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- (12) (PDF) Lactobacillus rhamnosus CNCMI-4317 Modulates Fiaf/Angpt14 in Intestinal Epithelial Cells and Circulating Level in Mice. Available online: https://www.researchgate.net/publication/282646122_Lactobacillus_rhamnosus_CNCMI-4317_Modulates_FiafAngpt14_in_Intestinal_Epithelial_Cells_and_Circulating_Level_in_Mice (accessed on 16 February 2020).
- Borgeraas, H.; Johnson, L.K.; Skattebu, J.; Hertel, J.K.; Hjelmesaeth, J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2018, 19, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Omar, J.M.; Chan, Y.-M.; Jones, M.L.; Prakash, S.; Jones, P.J.H. Lactobacillus fermentum and Lactobacillus amylovorus as probiotics alter body adiposity and gut microflora in healthy persons. J. Funct. Foods 2013, 5, 116–123. [Google Scholar] [CrossRef]
- Raygan, F.; Rezavandi, Z.; Bahmani, F.; Ostadmohammadi, V.; Mansournia, M.A.; Tajabadi-Ebrahimi, M.; Borzabadi, S.; Asemi, Z. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol. Metab. Syndr. 2018, 10, 51. [Google Scholar] [CrossRef]
- Samah, S.; Ramasamy, K.; Lim, S.M.; Neoh, C.F. Probiotics for the management of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2016, 118, 172–182. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef] [PubMed]
- Akram Kooshki, A.; Tofighiyan, T.; Rakhshani, M.H. Effects of Synbiotics on Inflammatory Markers in Patients With Type 2 Diabetes Mellitus. Glob. J. Health Sci. 2015, 7, 1–5. [Google Scholar] [CrossRef][Green Version]
- Zheng, H.J.; Guo, J.; Jia, Q.; Huang, Y.S.; Huang, W.-J.; Zhang, W.; Zhang, F.; Liu, W.J.; Wang, Y. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 142, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Ostadmohammadi, V.; Lankarani, K.B.; Akbari, M.; Akbari, H.; Vakili, S.; Shokrpour, M.; Kolahdooz, F.; Rouhi, V.; Asemi, Z. The effects of probiotic and synbiotic supplementation on inflammatory markers among patients with diabetes: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2019, 852, 254–264. [Google Scholar] [CrossRef]
- Wang, L.; Guo, M.-J.; Gao, Q.; Yang, J.-F.; Yang, L.; Pang, X.-L.; Jiang, X.-J. The effects of probiotics on total cholesterol. Medicine (Baltimore) 2018, 97, e9679. [Google Scholar] [CrossRef]
- Cho, Y.A.; Kim, J. Effect of Probiotics on Blood Lipid Concentrations: A Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore) 2015, 94, e1714. [Google Scholar] [CrossRef]
- Shimizu, M.; Hashiguchi, M.; Shiga, T.; Tamura, H.; Mochizuki, M. Meta-Analysis: Effects of Probiotic Supplementation on Lipid Profiles in Normal to Mildly Hypercholesterolemic Individuals. PLoS ONE 2015, 10, e0139795. [Google Scholar] [CrossRef]
- Ishimwe, N.; Daliri, E.B.; Lee, B.H.; Fang, F.; Du, G. The perspective on cholesterol-lowering mechanisms of probiotics. Mol. Nutr. Food Res. 2015, 59, 94–105. [Google Scholar] [CrossRef]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension 2014, 64, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Newson, L. Menopause and cardiovascular disease. Post. Reprod. Health 2018, 24, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
| No. | Study Description | Intervention | Study Characteristisc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference/Year/Country/Sponsorship | Blinding/Crossover (Y/N)/Multiarm > 2 | Focus on | ROB | Form/Probiotic Strain/Prebiotic | Probiotic Dose CFU | Duration of Probiotic Administration (Days)/Comparator | N Total Randomized and Allocated to Intervention/Analyzed | Age Years (Mean ± SD) | Males (n/%) | BMI Baseline (kg/m2): Probiotic Group (Mean ± SD) | BMI Baseline (kg/m2): Control group (Mean ± SD) | |
| 1 | Agerbaek et al./1995/Denmark/Industry [29] | DB/N/N | Lipoprotein levels | 2 | Naturally fermented milk/Enterococcus faecium (1 strain), Streptococcus termophilus (2 strains) | Daily: Enterococcus faecium 4 × 1010 ; Streptococcus termophilus 1.4 × 1011 | 42/chemically fermented milk | 58/57 | 44 (±ND) | 57/100 | 24.3 (±2) | 24.1 (±1.7) |
| 2a | Agerholm-Larsen et al./2000/Denmark/Industry [30] | DB/N/Y: 5 arms: 3 probiotic groups and 2 placebo groups (3 probiotic and PBO tablet arms were analyzed) | Risk factors for cardiovascular disease | 2 | Yoghurt/2 strains of Streptococcus thermophilus and 2 strains of Lactobacillus acidophilus | 3 days/week: Streptococcus thermophilus 4.5 × 1010, Lactobacillus acidophilus 9 × 109 | 56/placebo tablets | 2626 | 38.49 (±2.58) | 7/26.92 | 30 (±2.8) | 29.9 (±3.48) |
| 2b | Yoghurt/2 strains of Streptococcus thermophilus and 1 strain of Lactobacillus rhamnosus | 3 days/week: Streptococcus thermophiles 3.6 × 1011, Lactobacillus rhamnosus 9 × 1010 | 56/placebo tablets | 2424 | 38.07 (±2.77) | 7/29.17 | 30.2 (±2.62) | 29.9 (±3.48) | ||||
| 2c | Yoghurt/1 strain of Enterococcus faecium and 2 strains of Streptococcus termophilus | 3 days/week: Enterococcus faecium 2.7 × 1010, Streptococcus thermophilus 4.5 × 1011 | 56/placebo tablets | 26/26 | 37.99 (±2.54) | 7/26.92 | 30.1 (±2.4) | 29.9 (±3.48) | ||||
| 3 | Ahn et al./2015/South Korea/Non-industry [31] | DB/N/N | Triglyceride level and fasting plasma metabolome | 2 | Powder/Lactobacillus curvatus HY7601, Lactobacillus plantarum KY1032 | Daily: Lactobacillus curvatus HY7601 5 × 109 and Lactobacillus plantarum KY1032 5 × 109 | 84/placebo powder | 92/92 | 53.4 (±8.38) | 30/32.61 | 24.7 (±2.91) | 24.9 (±2.26) |
| 4 | Ahn et al./2015a/South Korea/Non-industry [32] | DB/N/N | Triglyceride and apolipoprotein A-V levels | 2 | Powder/Lactobacillus curvatus HY7601, L. Plantarum KY1032 | Daily: Lactobacillus curvatus HY7601 0.5 × 1010 and Lactobacillus plantarum KY1032 0.5 × 1010 | 84/placebo powder | 128/121 | 52.87 (±9.02) | 33/27.27 | 24.9 (±3.2) | 24.8 (±2.62) |
| 5a | Andrade and Borges/2009/Portugal/industry [33] | DB/Y/N | Plasma lipids concentration | 0 | Fermented milk/L. Acidophilus 145 and Bifidobacterium longum BB536 | Daily: Lactobacillus acidophilus 145 5.25–7.88 × 1010 and Bifidobacterium longum BB536 1.01–3.75 × 1010 | 28-7 washout-28/regular yoghurt) | 41/34 | 35.44 (±11.17) | 0/0 | Baseline: Group probiotic-placebo 24.6 (±3.5) Group placebo-probiotic 24.9 (±3.40) | |
| 5b | ||||||||||||
| 6 | Bjerg et al./2015/Denamark/industry [34] | DB/N/N | Blood lipids, fatty acids levels and stearoyl-coa desaturase−1 (SCD1) activity | 3 | Capsules/L. Casei W8 | Daily: 1 × 1010 | 28/placebo capsules contained rice flour | 70/64 | Range: 20–45 | 34/48.57 | 23.7 (±) | 23.7 (±) |
| 7 | Boesmans et al./2018/Belgium/Non-industry [35] | DB/Y/N | Blood parameters, fecal microbiota composition and metabolites | 7 | Capsules/Butyricicoccus pullicaecorum 25-3T | Daily: 1 × 108 | 28-21 washout-28-21 washout/placebo capsules | 30/28 | Group probiotic-placebo 32 (26–45) Group placebo- probiotic 28 (25–33) 30(±ND) Albo Range: 25–45 | 14/46.67 | Baseline: Group probiotic-placebo 23.6 (±2.1) Group placebo-probiotic 22.1 (±1.9) | |
| 8 | Brahe et al./2015/Multicenter/Academic/industry [36] | SB/N/Y 3 arms: 1 probiotic 1 prebiotic and 1 placebo group (Probiotic and placebo groups were analyzed) | The gut microbiota composition, fecal SCFA concentration and metabolic risk markers in obesity | 3 | Powder/Lactobacillus paracasei F19 | Daily: 9.4 × 1010 | 42/placebo powder | 39/35 | 59.92 (±6.09) | 0/0 | 34.2 (±3.1) | 34.3 (±3.8) |
| 9 | Bukowska et al./1998/poland/Non-industry [37] | DB/N/N | Metabolic parmeteres | 2 | Drink/Lactobacillus plantarum 299 v/oat fibers | Daily: Lactobacillus plantarum 299v 1 × 1010 and 160 mg oat fibers | 42/control drink (rose hip drink) | 30/30 | 42.65 (±2.57) | 30/100 | 26.6 (±3.7) | 25.9 (±2.6) |
| 10 | Chang et al./2011/South Korea/Non-industry/industry [38] | DB/N/N | Metabolic parameters | 2 | The functional yogurt: starters: S. thermophilus, L. acidophilus, B. infantis; probiotics: Enterococcus faecalis FK-23, Bifidobacterium breve; fibersol-2 (resistant maltodextrin); pine needle extract; whey protein hydroxylate; Rice germ extract powder; Yucca schidigera and Quillaja saponaria extract | ND | 87/(control yoghurt with starters) | 103/101 | 36.78 (±9.45) | 31/30.69 | 22.63 (±3.26) | 22.13 (±2.8) |
| 11a | Cox et al./2014/Multicenter/industry [39] | DB/N/Y: 3 arms: 2 probiotic groups and 1 placebo group | Routine haematology and clinical chemistry measures | 2 | Powder/Bifidobacterium animalis subsp. Lactis Bl-04, | Daily: 2 × 109 | 150/placebo powder | 87/84 | 40.43 (±13.72) | 44/52.38 | 24.6 (±3.2) | 24.1 (±3.1) |
| 11b | Powder/Lactobacillus acidophilus NCFM, Bifidobacterium animalis subsp. Lactis Bi-07 | Daily: total dose1 × 1010 (equal amount of each strain) | 91/90 | 38.14 (±11.17) | 42/50 | 24.4 (±3.8) | 24.1 (±3.1) | |||||
| 12 | De Roos et al./2017/netherlands/Non-industry [40] | DB/N/N | Migraine symptom reduction, an effect on intestinal permeability and inflammation markers | 4 | Powder/Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcuslactis W19, Lactococcus lactis W58 | Daily: 5 × 109 | 84/placebo powder | 63/60 | 40.07, Range: 18–70 | 4/6.67 | 24.2 (±NA) | 25.6 (±NA) |
| 13 | Fabian et al./2006/Austria/industry [41] | ND/N/N | Plasma lipid profile | 0 | Yoghurt/starter cultures: Streptococcus thermophilus and Lactobacillus bulgaricus; probiotic: L. casei DN-114 001 | Daily: Days 1–14: 3.6 × 1010/Days 15–28: 7.2 × 1010 | 28/placebo regular yoghurt | 34/33 | 24 ± 2.56 | 0/0 | 20.7 (±3) | 21 (±2.7) |
| 14 | Gleeson et al./2012/United Kingdom/Industry [42] | DB/N/N | Incidence of upper respiratory tract infections (URTI) and mucosal immune markers | 1 | Powder/Lactobacillus salivarius | Daily: 2 × 1010 | 112/placebo powder | 66/54 | 23.9 (±4.7) On PRO age 25 ± 5 years PBO age 24 ± 4 years | 28/42.42 (during randomization) | 24.2 (±3.4) | 23.2 (±2.7) |
| 15 | Gohel et al./2016/india/Non-industry [43] | DB/Y/N | Calcium level and haematological parameters | 3 | Fermented milk/Lactobacillus helveticus MTCC 5463 | Daily: min. 2 × 1010 | 28-28 washout-28-28 washout/placebo milk | 76/59 | 68.93 (±4.1) | 38/50 | ND | ND |
| 16 | Gomes et al./2017/Brazil/Non-industry [44] | DB/N/N | Body composition, lipid profile, endotoxemia, inflammation, and antioxidant profile | 5 | Powder/Lactobacillusacidophilus LA-14, Lactobacillus casei LC-11, Lactococcus lactis LL-23, Bifidobacterium bifidum BB-06, and Bifidobacteriumlactis BL-4 | Daily: 2 × 1010 (equal amount of each strain) | 56/(placebo powder) | 60/43 | Range: 20–59 | 0/0 | 31.7 (±3.9) | 33.34 (±4.69) |
| 17 | Greany et al./2008/USA/Non-industry/industry [45] | SB/N/N | Plasma lipids | 2 | Capsules/Lactobacillus acidophilus DDS-1, Bifidobacterium longum UABL-14/FOS | Daily: L. acidophilus DDS-1: 3.75 × 109; B. longum UABL-14: 3.75 109 plus 30–45 mg FOS | 52/placebo capsules | 64/55 | 26.77 (±5.07) | 22/40 | 24.1 (±3.1) | 22.8 (±3.5) |
| 18 | Guillemard et al.2010/Multicenter/Industry [46] | DB/N/N | Incidence of respiratory and gastrointestinal common infectious diseases (cids) and immune functions | 3 | Fermented dairy drink/Lactobacillus casei DN-114 001 | Daily: 2 × 1010 | 84/placebo diary drink) | 1000/962 | 32.15 (±8.91) | 435/43.5 (during randomization) | 24 (±2.8) | 24.2 (±2.9) |
| 19 | Hatakka et al./2008/Finland/Industry [47] | DB/Y/N | Serum cholesterol and triglyceride levels | 2 | Capsules/L. rhamnosus LC705 and P. freudenreichii JS | Daily: L. rhamnosus LC70 5 2 × 1010; P. freudenreichii JS 2 × 1010 | 28-28/(placebo capsules) | 38/38 | 42 (±7.28) | 38/100 | Baseline: Group probiotic-placebo 25.2 (±3.4) Group placebo-probiotic 24.5 (±2.6) | |
| 20a | Hibberd et al./2019/Multicenter/industry [48] | DB/N/Y: 4 arms: 1 probiotic group, 1 synbiotic group and 2 control groups (Prebiotic and synbiotic and placebo arms were analyzed) | Body fat mass and obesity-related markers | 1 | Powder/Bifidobacterium animalis subsp. Lactis 420 | Daily: Bifidobacterium animalis subsp. Lactis 420, 1 × 1010 | 168/placebo powder | 61/61 | 48.63 (±10.09) | 17/27.87 | 30.9 (±1.9) | 31 (±2.2) |
| 20b | Powder/Bifidobacterium animalis subsp. Lactis 420 and Litesse Ultra (refined polydextrose) | Daily: Bifidobacterium animalis subsp. Lactis 420, 1 × 1010 + 12 g Litesse Ultra (refined polydextrose) | 73/73 | 47.69 (±9.85) | 16/21.92 | 31.2 (±2) | 31 (±2.2) | |||||
| 21a | Higashikawa et al./2016/Japan/Non-industry [49] | DB/N/Y: 3 arms: probiotic group, killed bacteria group and control group | Body fat and body weight | 6 | Powder/Pediococcus pentosaceus LP28 (live) | Daily: LP28 1 × 1011 | 84/placebo powder | 41/41 | 52.65 (±11.7) | 15/36.58 | 26.84 (±1.15) | 27.37 (±1.43) |
| 21b | Powder/Pediococus pentosaceus LP28 (heat-killed) | 41/41 | 54.18 (±10.89) | 27.1 (±1.24) | ||||||||
| 22a | Ibrahim et al./2018/Malaysia/Non-industry/industry [50] | ND/N/Y: 4 arms: 2 sedentary groups: probiotic group, placebo group; 2 circuit training groups: probiotic, placebo | Muscular strength and power and cytokine responses | 1 | Powder/Lacidophilusacidophilus BCMC 12130, L. casei BCMC 12313, L. lactis BCMC 12451, Bifidobacterium bifidum BCMC 02290, B. infantis BCMC 02129, and B. longum BCMC 02120) | Sedentary groups Daily: 6 × 1010 | 84/placebo powder | 24/20 | 22.5 (±1.66) | 24/100 | 21.8 (±3.4) | 21.1 (±2.8) |
| 22b | Circuit training groups Daily: 6 × 1010 | 84/placebo powder plus circuit training | 24/21 | 21.43 (±2.53) | 22.1 ±3.4 | 21.1 ±2.7 | ||||||
| 23 | Inoue et al./2018/Japan/Non-industry [51] | DB/N/N | Cognitive function, mental state, body composition, and bowel movement were measured | 4 | Powder/B. longum BB536, B. infantis M-63, B. breve M-16V and B. breve B-3 | Daily: 1.25 × 1010 of each strain | 84/placebo powder+ | 39/38 | 70.3 (±3.1) | 14/36.82 | 24 (±2.8) | 23 (±2.7) |
| 24 | Ito et al./2017/Japan/Non-industry [52] | DB/N/N | Serum lipids level | 5 | Fermented milk/Streptococcus thermophilus YIT 2001 | Daily: ≥1 × 1011 | 84/placebo non-fermented milk | 60/59 | 47.35 (±8.25) | 30/50.84 | 22.4 (±2.8) | 23.3 (±2.8) |
| 25 | Ivey et al./2014/Australia/Non-industry/Industry [53] | DB/N/Y: 4 arms: 2 probiotic yoghurt groups: probiotic capsules group, placebo group; 2 control milk: probiotic capsules, placebo (probiotic yoghurt, control milk | Biomarkers of glycaemic control | 4 | Yoghurt and capsules/Lactobacillus acidophilus La5, Bifidobacterium animalis subsp lactis Bb12 | Daily: 3 × 109 (both yoghurt and capsules) | 42/probiotic yoghurt placebo capsules, | 77/77 | 68.4 (±8.25) | 50/64.93 | 30.6 (±3.8) | 30.2 (±4.3) |
| Capsules/Lactobacillus acidophilus La5, Bifidobacterium animalis subsp lactis Bb12 | 42/control milk, placebo capsules | 79/79 | 65.05 (±7.79) | 46/58.23 | 30.8 (±3.5) | 30.8 (±3.5) | ||||||
| 26 | Ivey et al./2015/Australia/Non-industry/industry [54] | DB/N/Y: 4 arms: 2 probiotic yoghurt groups: probiotic capsules group, placebo group; 2 control milk groups: probiotic capsules, placebo | Blood pressure and serum lipid profile | 4 | Yoghurt and capsules/Lactobacillus acidophilus La5, Bifidobacterium animalis subsp lactis Bb12 | Daily: 3 × 109 (both yoghurt and capsules) | 42/placebo capsules, control milk | Probiotic yoghurt 77/77 | 68 (±8.34) | 50/64.93 | 31 (±4) | 30 (±4) |
| Control milk 79/79 | 65 (±7.52) | 46/58.23 | 31 (±4) | 31 (±4) | ||||||||
| 27 | Jones et al./2016/Canada/industry [55] | DB/N/N | Blood cholesterol concentration | 5 | Capsules/Lactobacillus. reuteri NCIMB 30242 | Daily: min. 4.0 × 109 | 63/(placebo capsules) | 131/127 | 49.09 (±13.57) | 55/43.31 | 26.83 (±3.05) | 27.62 (±2.81) |
| 28 | Kadooka et al./2010/Japan/ND [56] | DB/N | Abdominal adiposity, body weight and other body measures in adults with obese tendencies | 2 | Fermented milk/Lactobacillus gasseri SBT2055 (LG2055) | Daily: 1 × 1011 | 84/placebo fermented milk | 87/87 | 48.76 (±9.21) | 59/67.82 | 27.5 (±1.67) | 27.2 (±1.69) |
| 29a | Kadooka et al./2013/Japan/Non-industry [57] | DB/N/Y: 3 arms: lower dose probiotic group, higher dose probiotic group, control group | Abdominal adiposity, anthropometric measures and body composition | 2 | Fermented milk/Lactobacillus gasseri SBT2055 | Daily: 2 × 109 | 84/fermented placebo milk | 139/139 | 47.15 (±7.21) | 69/49.64 | 27.5 (±1.9) | 27.2 (±1.9) |
| 29b | Daily: 2 × 108 | 141/141 | 47.29 (±7.21) | 72/51.06 | 27.2 (±1.8) | 27.2 (±1.9) | ||||||
| 30 | Kawase et al./2000/Japan/Non-industry [58] | SB/N/N | Serum lipid level | 2 | Fermented milk/Lactobacillus casei subsp. casei TMC0409, Streptococcus thermophilus TMC1543 | Daily: L. casei TMC0409 2.44 × 1011, S. thermophilus TMC1543 1.04 × 1010 | 59/fermented placebo milk | 20/20 | 40.1 (±ND) | 20/100 | ND | ND |
| 31a | Kim et al./2018/South Korea/Non-industry/Non-industry [59] | DB/N/Y: 3 arms: lower dose probiotic group, higher dose probiotic group, control group | Adiposity | 5 | Capsules/Lactobacillus gasseri BNR17 | Daily: 1 × 109 | 84/placebo capsules | 60/60 | 38.7 (±11.76) | 20/33.3 | 27.9 (±1.07) | 28.6 (±1.96) |
| 31b | Daily: 1 × 1010 | 60/60 | 38 (±10.4) | 21/35 | 28.8 (±2.24) | 28.6 (±1.96) | ||||||
| 32 | Kim et al./2017/South Korea/Non-industry [60] | DB/N/N | Adiposity parameters and metabolomic profile | 5 | Powder/L. curvatus HY7601 and L. Plantarum KY1032 | Daily: Lactobacillus curvatus HY7601 2.5 × 109, Lactobacillus plantarum KY1032 2.5 × 109 | 84/placebo powder | 120/66 | 38.99 (±1.93) | ND | 26.6 (±1.3) | 27.1 (±1.57) |
| 33 | Klein et al./2008/Germany/Non-industry [61] | DB/Y/N | Blood lipids, faecal microbiota, and immunological parameters | 3 | Yoghurt/B. lactis DGCC420, L. acidophilus 74-2 | Daily:B. lactis 9 × 108; L. acidophilus 74-2 2.79 × 1011 | 35-35/placebo yoghurt | 26/26 | 25 (±3) | 13/50 | Baseline: Group probiotic-placebo 21.3(±2.1) Group placebo-probiotic21.5(±2.0) | |
| 34 | Lambert et al./2017/Denmark/industry/Non-industry [62] | DB/N/N | Anthropometric data, lipids level, and menopausal symptoms | 4 | Drink/Heterogeneous culture of probiotic lactic acid bacteria in red clover drink | ND | 84/placebo water based drink | 62/59 | 52.34 (±3.66) | 0/0 | 26.02 (±5.38) | 25.45 (±3.34) |
| 35a | Lee et al./2017/USA/Non-industry [63] | Partially SB/Y/Y: 4 arms: (1) placebo yogurt, (2) yogurt with probiotic added pre-fermentation, (3) yogurt with probiotic added post-fermentation, and (4) probiotic capsules | Blood lipids level and fecal excretion of scfas | 3 | Yoghurt, capsules/Bifidobacterium animalis subsp. Lactis BB-12® | Daily: 3.16 × 109 | Each treatment period 28-washout 14/placebo capsules | 36/30 | 28.2 (±6.4) | 11/36.67 | All crossover groups 24.2 (±2.6) | |
| 35b | ||||||||||||
| 35c | ||||||||||||
| 36 | Lin et al./1989/USA/industry [64] | DB/Y/N | Serum lipids level | 4 | Tablets/L. acidophilus (ATCC 4962) and L. Bulgaricus (ATCC 33409) | Daily: 8 × 106 | 42-21 washout-42/placebo tablets | 334/334 | ND | ND | ND | |
| 37 | Macfarlane et al./2013/United Kingdom/Non-industry [65] | DB/Y/N | Colonic microbiota composition, immune function and health status | 5 | Capsules/Bifidobacterium longum/Powder/Synergy I mixture of inulin and oligofructose (DP2-60) | Daily: 4 × 1011 B. longum + 12 gof prebiotic) | 28-28 washout-28/placebo capsules and powder | 47/43 | 71.9 (±5.4) | 21/48.83 | All crossover groups 26.9 (±4.2) | |
| 38 | Madjd et al./2016/Multicenter/Non-industry [66] | SB/N/N | Body weight and cardiometabolic risk factors | 3 | Yoghurt/Lactobacillus acidophilus LA5, Bifidobacterium lactis BB12 | ND | 84/low fat yoghurt | 89/89 | 31.98 (±6.88) | 0/0 | 32.14 (±3.2) | 32.05 (±3.94) |
| 39a | Mohammad Moradi et al./2015.Iran/non-industry [67] | TB/N/Y: 3 arms: (1) probiotic cheese and extract of chicory root, (2) probiotic cheese, and (3) control | Lipid profile | 5 | Cheese/Lactobacillus acidophilus LA5, Bifidobacterium lactis BB12and raw chicory root | ND | 49/no intervention | 120/120 | 37.55 (±15.97) | 60/50 | 22.38 (±2.01) | 22.14 (±0.97) |
| 39b | Cheese/Lactobacillus acidophilus LA5, Bifidobacterium lactis BB12 | 120/120 | 39.4 (±15.92) | 60/50 | 21.94 (±2.19) | 22.14 (±0.97) | ||||||
| 40 | Naruszewicz et al./2002/poland/Non-industry [68] | DB/N/N | Lipid profiles, inflammatory markers, and monocyte function | 3 | Drink/Lactobacillus plantarum 299v | Daily: 2 × 1010 | 42/placebo drink | 36/36 | 42.3 (±3.9) | 18/50 | 24.8 (±4.8) | 25.8 (±3.7) |
| 41 | Nishiyama et al./2018/Japan/industry [69] | DB/N/N | Immunity and metabolic syndrome parameters | 2 | Yoghurt/L. lactis 11/19-B1, B. Lactis BB-12 | No data available | 56/placebo yoghurt | 79/76 | 42.35 (±11.15) | 29/38.15 | ND | ND |
| 42 | Nova et al./2011/Spain/Non-industry [70] | DB/N/N | Self-perceived gastrointestinal well-being and immunoinflammatory status | 3 | Tablets/L. Acidophilus La5, B. animalis Ssp. Lactis Bb-12, Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, and Lactobacillus paracasei ssp. paracasei and FOS | Daily: 2.4 × 109 | 42/placebo tablets with no probiotics) | 37/36 | Range 25–45 | 16/44.4 | 23.74 (±2.19) | 23.06(±2.32) |
| 43 | Ostan et al./2015/Multicenter/Non-industry [71] | ND/N/Y: from 4 arms only probiotic and control arm were analysed | Inflammageing, oxidative stress, and gut microbiota composition | 3 | Capsules/Lactobacillus paracasei, L. plantarum, L. acidophilus, L. delbrueckii subsp bulgaricus, bifidobacterium longum, B. breve, B. infantis, Streptococcus thermophilus | Daily: 2.24 × 1011 | 56/Ristomed diet alone | 69/59 | 70.4 (±3.9) | 58/46.4 | 26.7(±3.8) | 26.9(±3.4) |
| 44 | Osterberg et al./2015/USA/Non-industry [72] | DB/N/N | Body and fat mass, insulin sensitivity, and skeletal muscle substrate oxidation | 3 | Powder/Streptococcus thermophilus DSM24731, Lactobacillus acidophilus DSM24735, Lactobacillus delbrueckii ssp. Bulgaricus DSM24734, Lactobacillus paracasei DSM24733, Lactobacillus plantarum DSM24730, Bifidobacterium longum DSM24736, Bifidobacterium infantis DSM24737, and Bifidobacterium breve DSM24732 | Daily: 9 × 1011 | 28/placebo powder | 20/20 | 22.6 (±3.59) | 20/100 | 23.9 (±2.7) | 23.2 (±1.99) |
| 45a | Rajkumar et al./2014/india/Non-industry [73] | SB/N/Y: 4 arms: placebo, probiotic, omega-3 fatty acid, omega-3 fatty acid + probiotic (probiotics and placebo arms were analyzed) | Insulin sensitivity, blood lipids, and inflammation | 6 | Capsules/Bifidobacterium longum, B. infantis, B. breve, Lactobacillus acidophilus, L. paracasei, L. delbrueckii subsp. bulgaricus, L. Plantarum, Streptococcus salivarius subsp. thermophilus; | Daily: 1.13 × 1011 | 42/placebo capsules | 30/30 | 49 (40-60) | 30/50 | 28.79 (Range: 27–30) | |
| 45b | Capsules/Bifidobacterium longum, B. infantis, B. breve, Lactobacillus acidophilus, L. paracasei, L. delbrueckii subsp. bulgaricus, L. plantarum, Streptococcus salivarius subsp. thermophilus, Omega 3 fatty acids | Daily: 1.13 × 1011 + Omega 3 (360 mg EPA and 240 mg DHA) | ||||||||||
| 46 | Sadrzadeh-Yeganeh et al./2010/Multicenter/industry [74] | TB/N/Y: 3 arms: placebo yoghurt, probiotic yoghurt, and no intervention (probiotic and placebo arms were analysed) | Lipid profile | 2 | Yoghurt/Placebo: Lactobacillus bulgaricus and Streptococcus thermophilus. Probiotic: Placebo + Lactobacillus acidophilus La5, Bifidobacterium lactis Bb12 | ND | 42/placebo yoghurt, | 60/59 | 34.06 (±5.74) | ND | Placebo yoghurt 23 (±2.4) Probiotic yoghurt 24 (±2.4) | |
| 47 | Sanchez et al./2014/Multicenter/industry [75] | DB/N/N | Weight loss and weight maintenance | 4 | Capsules/Lactobacillus rhamnosus CGMCC1.3724 and mix of oligofructose and inulin | 2 Daily: 3.24 × 108 and 600 mg of a mix of oligofructose and inulin (70:30, v/v) | 168/placebo capsules | 125/93 | 36 (±79.06) | 48/38.4 | 33.8 (±25.98) | 33.3 (±25.39) |
| 48a | Savard et al./2011/Canada/industry [76] | DB/N/Y: 3 arms: lower dose of probiotics and green tea extract, higher dose of probiotic and green tea extract, and placebo | Fecal bacterial counts of Lactobacillus acidophilus LA-5 and Bifidobacterium animalis subsp. Lactis BB-12 and lipid profile | 3 | Yoghurt/Starters: Lactobacillus delbrueckii subsp. Bulgaricus and Streptococcus thermophilus; Probiotics: Bifidobacterium animalis subsp. Lactis BB-12, Lactobacillus acidophilus LA-5 and green tea extract | 1 arm, daily: Bifidobacterium animalis subsp. Lactis BB-12 1 × 109, Lactobacillus acidophilus LA-5 1 × 109 and 40 mg of green tea extract | 28/placebo yoghurt containing no starter culture, no probiotic, and no green tea extract | 40/38 | 32 (±11.9) | 12/30 | 22.8 (±3.8) | 23.8 (±4.1) |
| 48b | 2 arm, daily: Bifidobacterium animalis subsp. Lactis BB-12 1 × 1010, Lactobacillus acidophilus LA-5 1 × 109 and 40 mg of green tea extract | 38/36 | 33.27 (±12.37) | 12/31.6 | 23.7 (±2.7) | 23.8 (±4.1) | ||||||
| 49 | Simon et al./2015/Multicenter/Non-industry [77] | DB/N/Y: 4 arms: lean group: 1) probiotics, 2) placebo; obese group: 1) probiotics, 2) placebo | Insulin sensitivity | 4 | Capsules/Lactobacillus reuteri SD5865 | Daily: 2 × 1010 | 28/placebo capsules | Lean: 11/11 Obese:10/10 | 50 (±7) | Lean: 5/45 Obese:5/50 | Lean: 23.6 (±6 1.7) Obese: 35.5±4.9 | |
| 50 | Simons et al./2006/Australia/Industry [78] | DB/N/N | LDL cholesterol and other lipid fractions level | 3 | Capsules/Lactobacillus fermentum | Daily: 8 × 109 | 70/placebo capsules | 46/44 | 51.5 (±11.5) | 16/36.36 | 27 (±5.7) | 24.4 (±3.7) |
| 51a | Stenman et al./2016/Multicenter/industry [79] | DB/N/Y: 4 arms: (1) placebo, (2) prebiotic, (3) probiotic (4) synbiotic (probiotic, synbiotic vs. Placebo arms were analyzed) | Body fat mass and other obesity-related parameters | 4 | Powder/Probiotic: Bifidobacterium animalis ssp. Lactis 420 (B420); Prebiotic: polydextrose; Synbiotic: combination of above | Daily: B420 1 × 1010; | 186/placebo powder, | 112/61 | 48.67 (±10.23) | 17/27.86 | 30.9 (±1.9) 31.2 (±2) | 31 (±2.2) 31 (±2.2) |
| 51b | Synbiotic: B420 1 × 1010 +; polydextrose 12g | 113/73 | 47.75 (±9.75) | 16/21.92 | ||||||||
| 52a | Szulińska et al./2018/Poland/Non-industry [80] | DB DB/N/Y: 3 arms: (1) lower dose probiotic, (2) higher dose probiotic, and (3) placebo | Functional (primary endpoint) and biochemical parameters (secondary endpoint) of endothelial dysfunction | 5 | Powder/Bifidobacterium bifidum W23, Bifidobacterium lactis W51, B. Lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19, Lactococcus lactis W58 | Daily: 1 × 109 | 84/placebo powder | 54/48 | 57.55 (±7) | 0/0 | 36 (±5.2) | 36.1 (±4.37) |
| 52b | Daily: 2.5 × 1010 | 54/47 | 56.94 (±7.28) | 36.57 (±5.95 | 36.1 (±4.37) | |||||||
| 53a | Szulińska et al./2018a/Poland/Non-industry [81] | DB/N/Y: 3 arms: (1) lower dose probiotic, (2) higher dose probiotic, (3) placebo | Cardiometabolic biochemical parameters, and lipopolysaccharide levels | 5 | Powder/Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19, and Lactococcus lactis W58. | Daily: 1 × 109 | 84/placebo powder | 54/48 | 57.55 (±7) | 0/0 | 36 (±5.2) | 36.1 (±4.37) |
| 53b | Daily: 2.5 × 1010 | 54/47 | 56.94 (±7.28) | 36.57 (±5.95) | 36.1 (±4.37) | |||||||
| 54a | Tenore et al./2019/Italy/Non-industry [82] | DB/N/Y: 3 arms: lactofermented Annurca apple puree, probiotic, unfermented apple puree | Lipid profile and oxidative metabolites level | 6 | Capsules/Lactobacillus rhamnosus LRH11, Lactobacillus plantarum SGL07 | 1 arm, daily: 3 × 108 | 112/Annurca apple puree with no probiotics | 54/42 | 46.65 (±10.36) | 30/71.43 | ≤30 | |
| 54b | Annurca apple puree fermeted with Lactobacillus rhamnosus LRH11, Lactobacillus plantarum SGL07 | Daily: probiotics 3.0 × 108 + Annurca apple puree 125 2 arm, Daily: probiotics 3.0 × 108 + Annurca apple puree 125 g | 53/41 | 45.64 (±10.51) | 31/75.61 | |||||||
| 55 | Trautvetter et al./2012/Germany/industry [83] | DB/Y/N | Intestinal colonisation of L. Paracasei and blood cholesterol level | 3 | Yoghurt/Lactobacillus paracasei LPC37 and bread containing pentacalcium hydroxy-triphosphate | Daily: probiotic 1 × 1012 and calcium 1 g | 28-28 washout-28/placebo yoghurt and bread) | 32/32 | 25 (±5) | ND | 22 (±3) | |
| 56a | Usinger et al./2010/Denmark/Industry [84] | DB/N/Y:3 arms: 150mL probiotic milk, 300 mL of probiotic milk, chemically acidifies milk | Blood pressure | 5 | Milk/Lactobacillus helveticus Cardi-04 | Daily 150 mL of milk fermented with probiotic strain (dose not available) and contains 1.25 mg Val–Pro–Pro (VPP) and 0.55 mg Ile–Pro–Pro (IPP) | 56/chemically acidified milk | 47/45 | 53.3 (±7.41) | 36/60 | 26 (±4) | 26 (±4) |
| 56b | Daily 300 mL of milk fermentem with probiotic strain (dose not available) and contains 2.5 mg Val–Pro–Pro (VPP) and 1.1 mg Ile–Pro–Pro (IPP) | 47/44 | 28/46.67 | 27 (±4) | 26 (±4) | |||||||
| 57 | Valentini et al./2015/Multicenter/Non-industry [85] | SB/N/N | Biomarkers of inflammation, nutrition, oxidative stress and intestinal microbiota | 4 | Capsules/Bifidobacterium infantis DSM24737, Bifidobacterium longum DSM24736, Bifidobacterium breve DSM24732, Lactobacillus acidophilus DSM24735, Lactobacillus delbruckii ssp.bulgaricus DSM 27734, Lacctobacillus paracasei DSM 24733, Lactobacillus plantarum DSM24730, Streptococcus thermophilus DSM 24731 | Daily: 2.24 × 1011 | 56/Ristomed diet | 69/62 | 70.1(±3.9) | 29/46.77 | 26.8 (±3.59) | |
| 58 | Välimäki et al./2012/finland/Non-industry [86] | DB/N/N | Oxidized LDL lipids, serum antioxidant potential (s-TRAP) and serum antioxidants (s-α-tocopherol, s-γ-tocopherol, s-retinol, s-β-carotene, and s-ubiquinone-10) | 4 | Milk drink or capsules/Lactobacillus rhamnosus GG | Daily: drink 4x × 1010 or capsules 1 × 1010 | 84/placebo drink or capsules | 141/119 | 40 (23–69) | 105/88.2 | 22 (Range: 18–26) | |
| 59 | Venkataraman et al./2018/India/ND [87] | SB/N/N | Blood glycemic markers concentration | 2 | Capsules/Lactobacillus salivarius UBLS22, Lactobacillus casei UBLC 42, Lactobacillus plantarum UBLP 40, Lactobacillus acidophilus UBLA 34, Bifidobacteriu breve UBBR 01, Bacillus coagulans Unique-IS2/FOS | 3.0 × 108 cfu/30 × 109 CFU/capsule | 84/placebo capsule | 80/80 | ND | ND | ND | ND |
| 60 | Xiao et al./2003/Japan/industry/Non-industry [88] | SB/N/N | Blood lipids level | 2 | Yoghurt/Bifidobacterium longum BL1 | Daily: 3 × 1010 | 28/placebo yoghurt | 32/32 | 43.85 (±8.05) | 32/100 | ND | ND |
| 61a | Zarrati et al./2014/Iran/Non-industry [89] | DB/N/Y: 3 arms: probiotic yoghurt with low calorie diet (LCD), probiotic yoghurt without LCD, regular yoghurt with LCD | Body fat percentage, blood proinflammatory markers and cytokines content | 3 | Yoghurt/Lactobacillus acidophilus LA5, Lactobacillus casei DN001, Bifidobacterium lactis BB12 without LCD | Daily: 2 × 1010 | 56/regular yoghurt with LCD | 50/50 | 35.5 (±9.27) | 24/32 | 32 (±3.62) | 33.9 (±6.73) |
| 61b | Yoghurt/Lactobacillus acidophilus LA5, Lactobacillus casei DN001, Bifidobacterium lactis BB12 with lcd | 50/50 | 36 (±9.07) | 33.8 (±6.35) | 33.9 (±6.73) | |||||||
| No. | Reference/Year/Country/Sponsorship | Microbiota | Microbiota Related (Metabolites) | Gut Barrier and Inflammatory Markers | Methods |
|---|---|---|---|---|---|
| 1 | Boesmans et al./2018/Belgium/Non-industry [35] | No impact on the microbiota richness and single genera abundances, only transcient gut colonization by probiotic strain used in the study | No influence on microbiota metabolic activity as well as on saccharolytic and proteolytic fermentation processes markers (SCFAs and dimethyl sulfide, p-cresol, indole, and the branched-chain fatty acids) | No influence on faecal calprotectin concentrations | NGS, GC-MS |
| 2 | Brahe et al./2015/Multicenter/Academic/Industry [36] | Probiotc group: alterations in faecal abundance of 2493 bacterial genes ≥ ↑ Eubacterium rectale and ↑Ruminococcus torques. Placebo group: altered faecal abundance of 7436 genes ≥ ↑Roseburia hominis, ↑two Clostridiales, ↑ one unknown species, ↓ Eubacterium ventriosum, and ↓ one unknown species. | No impact on faecal total SCFAs and butyric acid | No impact on lipopolysaccharide-(LPS)-binding protein and inflammatory markers (plasma high-sensitivity C-reactive protein (CRP), serum tumor necrosis factor-α (TNFα), and plasma interleukin (IL)-6 | metagenomics, ethyl chloroformate NEFA method and GC |
| 3 | de Roos et al./2017/Netherlands/Non-industry [40] | No impact on zonulin concentration and intestinal permeability measured by means of lactulose-mannitol test; no changes of IL -6, IL-10, TNFα, and CRP | ELISA, GC | ||
| 4 | Hibberd et al./2019/Multicenter/Industry [48] | Probiotic group ↑Akkermansia muciniphila, ↑Lactobacillus, ↑ Bifidobacterium OTU, ↑Akkermansia, ↑ Streptococcus, ↑ S24-7, ↑Methanobrevibacter, ↑Clostridiaceae spp., ↑Clostridium, ↑Phascolarctobacterium, ↑Dialister; ↓Bacteroides, ↓ Erysipelotrichaceae spp., ↓ Enterobacteriaceae spp., ↓ RF39 spp. Bifidobacterium was positively correlated to lean body mass (total, arms, legs, trunk, and android). Paraprevotella negatively correlated with fat mass. Synbiotic group The most pronounced changes of microbiota alterations in clustering analysis (long-term effect). Phylum Bacteroidetes: ↑ taxa: S24-7, Barnesiellaceae spp., Parabacteroides, and Rickenellaceae spp.; ↓ taxa: Paraprevotella. Phylum Firmicutes: ↑ taxa: Christensenellaceae, Ruminococcaceae spp., Oscillospira, Phascolarctobacterium, Erysipelotrichaceae spp. ↓ taxa: Lactobacillus, Lactococcus, Turicibacter Streptococcus, Clostridiales spp., Lachnospira Phylum Actinobacteria: ↓ taxa: Adlercreutzia, Collinsella, Eggerthella Others: ↑Methanobrevibacter, ↑ Akkermansia; ↓ Enterobacteriaceae spp., and ↓ RF39 spp. Christensenellaceae spp. abundance was correlated negatively to WHR and energy intake at baseline, and waist-area body fat and cholesterol markers were correlated to android fat, trunk fat, and lipid parameters Christensenellaceae spp. was positively correlated to the faecal branched-chain fatty acids (BCFAs), isobutyric acid, isovaleric acid, 2-methyl-butyric acid, and 3-methyl-2-oxovalerate, and to the plasma bile acids. | Probiotic group ↓ propionate Synbiotic group Metabolites: ↓ primary conjugated plasma bile acid glycocholic acid (GCA) ↓ secondary conjugated bile acids glycoursodeoxycholic acid (GUDCA) and taurohyodeoxycholic acid and tauroursodeoxycholic acid (THDCA + TUDCA). ↑ carbohydrates/polysaccharides PICRUSt: “Cellular Processes” and “Metabolism”-KEGG pathways differentially abundant from placebo group No significant changes in short-chained fatty acids (SCFA) or amino acids for any treatment group | NGS, NMR | |
| 5 | Jones et al./2016/Canada/Industry [55] | Highly sensitive (hs) CRP was unchanged. | LC-MS, GC-MS | ||
| 6 | Klein et al./2008/Germany/Non-industry [61] | L. acidophilus and B. lactis elevation | No impact on SCFAs | ↑ phagocytic activity as a marker for the unspecific cellular immune response | EUB-positive, DAPI-staining, FISH-based quantification, GC |
| 7 | Lee et al./2017/USA/Non-industry [63] | ↑fecal acetate in control yoghurt group and in probiotic added before fermentation group; other SCFAs were unchanged. | CRP level was unchanged. | GC-MS | |
| 8 | Macfarlane et al./2013/United Kingdom/Non-industry [65] | ↑Actinobacteria, ↑ some species of Firmicutes,↑ total bifidobacterial population, ↑ B. angulatum, ↑ B. longum, ↑ B. adolescentis, ↑ B. bifidum. ↓Proteobacteria.↑ Firmicutes/Bacteroidetes ratio. | ↑ butyrate, succinate, total acetate, propionate. | ↓TNF-α | FISH, GC |
| 9 | Osterberg et al./2015/USA/Non-industry [72] | ↑Streptococcus thermophiles, ↑ Lactobacillus acidophilus | No changes of LPS Binding Protein (LBP), LBP/sCD14, IL6, TNFα, hsCRP | qPCR | |
| 10 | Rajkumar et al./2014/India/Non-industry [73] | Probiotic group and probiotic + omega-3 group ↑ total aerobes, ↑total anaerobes, ↑lactobacillus, ↑ bifidobacteria, ↑ streptococcus in the. Probiotic + omega-3 group significant effect on↑ Bacteroides, ↓coliforms, and ↓E. coli. | ↓hsCRP | culture-dependent | |
| 11 | Sanchez et al./2014/Multicenter/Industry [75] | Males: No changes in gut microbiota Females: ↓, Lachnospiraceae family↓ Subdoligranulum genus. | No change of β-hydroxybutyrate level. | No change of CRP and LPS level | NGS, ELISA |
| 12 | Savard et al./2011/Canada/Industry [76] | ↑B. animalis subsp. Lactis, ↑L. acidophilus LA-5, ↑Bifidobacteria, ↑ Lactobacilli, ↓Enterococci | qPCR | ||
| 13 | Simon et al./2015/Multicenter/Non-industry [77] | No impact on microbiota, only ↑L. reuterii-probiotic bacteria used in the study | No changes of LPS and cytokines | NGS | |
| 14 | Stenman et al./2016/Multicenter/Industry [79] | Probiotic: ↑propionic acid, butyric acid, and valeric acid, | Synbiotic: changes in zonulin and hsCRP were statistically significantly correlated with changes in trunk fat mass; ↑ LPS level, but no effect on inflammatory markers. | Limulus Amebocyte Lysate assay | |
| 15 | Szulińska et al./2018a/Poland/Non-industry [81] | ↓LPS level | kinetic assay | ||
| 16 | Tenore et al./2019/Italy/Non-industry [82] | ↑ Bifidobacterium and ↑Lactobacillus population, and ↓ Bacteroides and ↓Enterococcus genera but predominantly in the control group followed by probiotic one and lactofermented control meal | ↓TMAO blood level | culture-dependent | |
| 17 | Trautvetter et al./2012/Germany/Industry [83] | ↑L. paracasei and ↑ Lactobacilli | qPCR | ||
| 18 | Valentini et al./2015/Multicenter/Non-industry [85] | No significan influence on gut microbiota. | qPCR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skonieczna-Żydecka, K.; Kaźmierczak-Siedlecka, K.; Kaczmarczyk, M.; Śliwa-Dominiak, J.; Maciejewska, D.; Janda, K.; Stachowska, E.; Łoniewska, B.; Malinowski, D.; Borecki, K.; et al. The Effect of Probiotics and Synbiotics on Risk Factors Associated with Cardiometabolic Diseases in Healthy People—A Systematic Review and Meta-Analysis with Meta-Regression of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 1788. https://doi.org/10.3390/jcm9061788
Skonieczna-Żydecka K, Kaźmierczak-Siedlecka K, Kaczmarczyk M, Śliwa-Dominiak J, Maciejewska D, Janda K, Stachowska E, Łoniewska B, Malinowski D, Borecki K, et al. The Effect of Probiotics and Synbiotics on Risk Factors Associated with Cardiometabolic Diseases in Healthy People—A Systematic Review and Meta-Analysis with Meta-Regression of Randomized Controlled Trials. Journal of Clinical Medicine. 2020; 9(6):1788. https://doi.org/10.3390/jcm9061788
Chicago/Turabian StyleSkonieczna-Żydecka, Karolina, Karolina Kaźmierczak-Siedlecka, Mariusz Kaczmarczyk, Joanna Śliwa-Dominiak, Dominika Maciejewska, Katarzyna Janda, Ewa Stachowska, Beata Łoniewska, Damian Malinowski, Krzysztof Borecki, and et al. 2020. "The Effect of Probiotics and Synbiotics on Risk Factors Associated with Cardiometabolic Diseases in Healthy People—A Systematic Review and Meta-Analysis with Meta-Regression of Randomized Controlled Trials" Journal of Clinical Medicine 9, no. 6: 1788. https://doi.org/10.3390/jcm9061788
APA StyleSkonieczna-Żydecka, K., Kaźmierczak-Siedlecka, K., Kaczmarczyk, M., Śliwa-Dominiak, J., Maciejewska, D., Janda, K., Stachowska, E., Łoniewska, B., Malinowski, D., Borecki, K., Marlicz, W., & Łoniewski, I. (2020). The Effect of Probiotics and Synbiotics on Risk Factors Associated with Cardiometabolic Diseases in Healthy People—A Systematic Review and Meta-Analysis with Meta-Regression of Randomized Controlled Trials. Journal of Clinical Medicine, 9(6), 1788. https://doi.org/10.3390/jcm9061788









