Sleep in the Supine Position During Pregnancy is Associated with Fetal Cerebral Redistribution
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Izci-Balserak, B.; Keenan, B.T.; Corbitt, C.; Staley, B.; Perlis, M.; Pien, G.W. Changes in Sleep Characteristics and Breathing Parameters During Sleep in Early and Late Pregnancy. J. Clin. Sleep Med. 2018, 14, 1161–1168. [Google Scholar]
- O’Brien, L.M.; Warland, J. Typical sleep positions in pregnant women. Early Hum. Dev. 2014, 90, 315–317. [Google Scholar] [CrossRef]
- McCowan, L.M.E.; Thompson, J.M.D.; Cronin, R.S.; Li, M.; Stacey, T.; Stone, P.R.; Lawton, B.; Ekeroma, A.; Mitchell, E.A. Going to sleep in the supine position is a modifiable risk factor for late pregnancy stillbirth; Findings from the New Zealand multicentre stillbirth case-control study. PLoS ONE 2017, 12, e0179396. [Google Scholar]
- Heazell, A.E.P.; Li, M.; Budd, J.; Thompson, J.; Stacey, T.; Cronin, R.S.; Martin, B.; Roberts, D.; Mitchell, E.; McCowan, L. Association between maternal sleep practices and late stillbirth—Findings from a stillbirth case-control study. BJOG Int. J. Obstet. Gynaecol. 2017, 125, 254–262. [Google Scholar]
- Stacey, T.; Thompson, J.M.; Mitchell, E.A.; Ekeroma, A.J.; Zuccollo, J.M.; McCowan, L.M. Association between maternal sleep practices and risk of late stillbirth: A case-control study. BMJ 2011, 342, d3403. [Google Scholar]
- Owusu, J.T.; Anderson, F.J.; Coleman, J.; Oppong, S.; Seffah, J.D.; Aikins, A.; O’Brien, L.M. Association of maternal sleep practices with pre-eclampsia, low birth weight, and stillbirth among Ghanaian women. Int. J. Gynecol. Obstet. 2013, 121, 261–265. [Google Scholar]
- Gordon, A.; Greenow, C.H.R.; Bond, D.; Morris, J.; Rawlinson, W.D.; Jeffery, H. Sleep Position, Fetal Growth Restriction, and Late-Pregnancy Stillbirth. Obstet. Gynecol. 2015, 125, 347–355. [Google Scholar] [CrossRef]
- Cronin, R.S.; Li, M.; Thompson, J.M.; Gordon, A.; Raynes-Greenow, C.H.; Heazell, A.E.; Stacey, T.; Culling, V.M.; Bowring, V.; Anderson, N.H.; et al. An Individual Participant Data Meta-analysis of Maternal Going-to-Sleep Position, Interactions with Fetal Vulnerability, and the Risk of Late Stillbirth. EClinicalMedicine 2019, 10, 49–57. [Google Scholar]
- Warland, J.; O’Brien, L.M.; Heazell, A.E.P.; Mitchell, E. STARS Consortium An international internet survey of the experiences of 1,714 mothers with a late stillbirth: The STARS cohort study. BMC Pregnancy Childbirth 2015, 15, 172. [Google Scholar] [CrossRef]
- Warland, J.; Dorrian, J.; Morrison, J.L.; O’Brien, L.M. Maternal sleep during pregnancy and poor fetal outcomes: A scoping review of the literature with meta-analysis. Sleep Med. Rev. 2018, 41, 197–219. [Google Scholar]
- Leppänen, T.; Töyräs, J.; Muraja-Murro, A.; Kupari, S.; Tiihonen, P.; Mervaala, E.; Kulkas, A. Length of Individual Apnea Events Is Increased by Supine Position and Modulated by Severity of Obstructive Sleep Apnea. Sleep Disord. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Brown, N.T.; Turner, J.; Kumar, S. The intrapartum and perinatal risks of sleep-disordered breathing in pregnancy: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2018, 219, 147–161.e1. [Google Scholar]
- Robertson, N.; Flatley, C.; Kumar, S. An Epworth Sleep Score >/=11 is associated with emergency operative birth and poor neonatal composite outcome at term. Aust. N. Z. J. Obstet. Gynaecol. 2020, 60, 49–54. [Google Scholar]
- Milsom, I.; Forssman, L. Factors influencing aortocaval compression in late pregnancy. Am. J. Obstet. Gynecol. 1984, 148, 764–771. [Google Scholar]
- Ryo, E.; Okai, T.; Kozuma, S.; Kobayashi, K.; Kikuchi, A.; Taketani, Y. Influence of compression of the inferior vena cava in the late second trimester on uterine and umbilical artery blood flow. Int. J. Gynecol. Obstet. 1996, 55, 213–218. [Google Scholar]
- Humphries, A.; Mirjalili, S.A.; Tarr, G.P.; Thompson, J.M.D.; Stone, P. The effect of supine positioning on maternal hemodynamics during late pregnancy. J. Matern. Neonatal Med. 2018, 32, 3923–3930. [Google Scholar]
- Jeffreys, R.; Stepanchak, W.; Lopez, B.; Hardis, J.; Clapp, J.F. Uterine blood flow during supine rest and exercise after 28 weeks of gestation. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 1239–1247. [Google Scholar]
- Kauppila, A.; Koskinen, M.; Puolakka, J.; Tuimala, R.; Kuikka, J. Decreased intervillous and unchanged myometrial blood flow in supine recumbency. Obstet. Gynecol. 1980, 55, 203–205. [Google Scholar]
- Abitbol, M.M. Aortic compression and uterine blood flow during pregnancy. Obstet. Gynecol. 1977, 50, 562–570. [Google Scholar]
- Khatib, N.; Winer, Z.; Beloosesky, R.; Vitner, D.; Thaler, I. The effect of maternal supine position on umbilical and cerebral blood flow indices. Eur. J. Obstet. Gynecol. Reprod. Boil. 2014, 175, 112–114. [Google Scholar]
- Hadlock, F.P.; Harrist, R.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar]
- Prior, T.; Mullins, E.; Bennett, P.; Kumar, S. Umbilical venous flow rate in term fetuses: Can variations in flow predict intrapartum compromise? Am. J. Obstet. Gynecol. 2014, 210, 610. [Google Scholar] [CrossRef]
- Alsolai, A.A.; Bligh, L.N.; Greer, R.M.; Gooi, A.; Kumar, S. Prelabour myocardial deformation and cardiac output in fetuses that develop intrapartum compromise at term: A prospective observational study. J. Matern. Neonatal Med. 2018, 32, 3618–3626. [Google Scholar]
- Hernandez-Andrade, E.; Benavides-Serralde, J.A.; Cruz-Martinez, R.; Welsh, A.; Mancilla-Ramirez, J. Evaluation of Conventional Doppler Fetal Cardiac Function Parameters: E/A Ratios, Outflow Tracts, and Myocardial Performance Index. Fetal Diagn. Ther. 2012, 32, 22–29. [Google Scholar]
- Dobbins, T.; Sullivan, E.A.; Roberts, C.L.; Simpson, J.M. Australian national birthweight percentiles by sex and gestational age, 1998–2007. Med. J. Aust. 2012, 197, 291–294. [Google Scholar] [CrossRef]
- Flatley, C.; Kumar, S.; Greer, R.M. Reference centiles for the middle cerebral artery and umbilical artery pulsatility index and cerebro-placental ratio from a low-risk population–a Generalised Additive Model for Location, Shape and Scale (GAMLSS) approach. J. Matern. Neonatal Med. 2018, 32, 2338–2345. [Google Scholar]
- Dubiel, M.; Gunnarsson, G.O.; Gudmundsson, S. Blood redistribution in the fetal brain during chronic hypoxia. Ultrasound Obstet. Gynecol. 2002, 20, 117–121. [Google Scholar] [CrossRef]
- Oros, D.; Figueras, F.; Padilla, N.; Meler, E.; Gratacos, E.; Cruz-Martinez, R.; Hernandez-Andrade, E. Middle versus anterior cerebral artery Doppler for the prediction of perinatal outcome and neonatal neurobehavior in term small-for-gestational-age fetuses with normal umbilical artery Doppler. Ultrasound Obstet. Gynecol. 2010, 35, 456–461. [Google Scholar]
- Morales-Roselló, J.; Khalil, A.; Ferri-Folch, B.; Perales-Marín, A. Neonatal Acid-Base Status in Fetuses with Abnormal Vertebro- and Cerebro-Placental Ratios. Fetal Diagn. Ther. 2015, 38, 103–112. [Google Scholar]
- Morales-Roselló, J.; Khalil, A.; Fornés, V.; Hervás, D.; Peralta-Llorens, N.; Rubio-Moll, J.; Perales-Marín, A. The vertebroplacental ratio as an alternative to the cerebroplacental ratio in the evaluation of the fetus at the end of pregnancy. J. Matern. Neonatal Med. 2017, 31, 70–79. [Google Scholar]
- Morales-Roselló, J.; Peralta-Llorens, N. Doppler study of the fetal vertebral artery in small for gestational age fetuses with intrauterine growth restriction. J. Ultrasound Med. 2012, 31, 1003–1010. [Google Scholar] [CrossRef]
- Antony, K.M.; Agrawal, A.; Arndt, M.; Murphy, A.M.; Alapat, P.M.; Guntupalli, K.K.; Aagaard, K.M. Association of adverse perinatal outcomes with screening measures of obstructive sleep apnea. J. Perinatol. 2014, 34, 441–448. [Google Scholar] [CrossRef]
- Bin, Y.S.; Cistulli, P.A.; Ford, J.B. Population-Based Study of Sleep Apnea in Pregnancy and Maternal and Infant Outcomes. J. Clin. Sleep Med. 2016, 12, 871–877. [Google Scholar]
- Telerant, A.; Dunietz, G.L.; Many, A.; Tauman, R. Mild Maternal Obstructive Sleep Apnea in Non-obese Pregnant Women and Accelerated Fetal Growth. Sci. Rep. 2018, 8, 10768. [Google Scholar] [CrossRef]
- Higgins, N.; Leong, E.; Park, C.; Facco, F.; McCarthy, R.J.; Wong, C.A. The Berlin Questionnaire for assessment of sleep disordered breathing risk in parturients and non-pregnant women. Int. J. Obstet. Anesthesia 2011, 20, 22–25. [Google Scholar]
- Luria, O.; Bar, J.; Kovo, M.; Malinger, G.; Golan, A.; Barnea, O. The role of blood flow distribution in the regulation of cerebral oxygen availability in fetal growth restriction. Med. Eng. Phys. 2012, 34, 364–369. [Google Scholar]
- Kiserud, T.; Ebbing, C.; Kessler, J.; Rasmussen, S. Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound Obstet. Gynecol. 2006, 28, 126–136. [Google Scholar] [CrossRef]
- Crispi, F.; Hernandez-Andrade, E.; Pelsers, M.M.A.L.; Plasencia, W.; Benavides-Serralde, J.A.; Eixarch, E.; Le Noble, F.; Ahmed, A.; Glatz, J.F.C.; Nicolaides, K.H.; et al. Cardiac dysfunction and cell damage across clinical stages of severity in growth-restricted fetuses. Am. J. Obstet. Gynecol. 2008, 199, 254.e1–254.e8. [Google Scholar]
- Alsolai, A.A.; Bligh, L.N.; Greer, R.M.; Kumar, S. Relationship of prelabor fetal cardiac function with intrapartum fetal compromise and neonatal status at term. Ultrasound Obstet. Gynecol. 2018, 51, 799–805. [Google Scholar]
- Alsolai, A.A.; Bligh, L.N.; Greer, R.M.; Kumar, S. Correlation between fetoplacental Doppler indices and measurements of cardiac function in term fetuses. Ultrasound Obstet. Gynecol. 2019, 53, 358–366. [Google Scholar]
- Warland, J.; Mitchell, E. A triple risk model for unexplained late stillbirth. BMC Pregnancy Childbirth 2014, 14, 1422. [Google Scholar]
- Stone, P.; Burgess, W.; McIntyre, J.; Gunn, A.J.; Lear, C.A.; Bennet, L.; Mitchell, E.A.; Thompson, J.M.D. Effect of maternal position on fetal behavioural state and heart rate variability in healthy late gestation pregnancy. J. Physiol. 2016, 595, 1213–1221. [Google Scholar]
- Warland, J.; Dorrian, J.; Kember, A.J.; Phillips, C.; Borazjani, A.; Morrison, J.L.; O’Brien, L.M. Modifying Maternal Sleep Position in Late Pregnancy Through Positional Therapy: A Feasibility Study. J. Clin. Sleep Med. 2018, 14, 1387–1397. [Google Scholar]
- Silver, R.M.; Hunter, S.; Reddy, U.M.; Facco, F.; Gibbins, K.J.; Grobman, W.A.; Mercer, B.M.; Haas, D.M.; Simhan, H.N.; Parry, S.; et al. Prospective Evaluation of Maternal Sleep Position through 30 Weeks of Gestation and Adverse Pregnancy Outcomes. Obstet. Gynecol. 2019, 134, 667–676. [Google Scholar]
- McCowan, L.M.E.; Cronin, R.S.; Gordon, A.; O’brien, L.; Heazell, A.E.P. Prospective Evaluation of Maternal Sleep Position through 30 Weeks of Gestation and Adverse Pregnancy Outcomes. Obstet. Gynecol. 2020, 135, 218. [Google Scholar]
- Warland, J.; Dorrian, J. Accuracy of Self-Reported Sleep Position in Late Pregnancy. PLoS ONE 2014, 9, e115760. [Google Scholar]
- O’Brien, L.M.; Warland, J.; Stacey, T.; Heazell, A.E.P.; Mitchell, E.A.; Collins, J.; Huberty, J.; Kliman, H.; McGregor, J.; Parast, M.; et al. Maternal sleep practices and stillbirth: Findings from an international case-control study. Birth 2019, 46, 344–354. [Google Scholar]
- McIntyre, J.; Ingham, C.M.; Hutchinson, B.L.; Thompson, J.M.D.; McCowan, L.M.E.; Stone, P.R.; Veale, A.G.; Cronin, R.S.; Stewart, A.W.; Ellyett, K.M.; et al. A description of sleep behaviour in healthy late pregnancy, and the accuracy of self-reports. BMC Pregnancy Childbirth 2016, 16, 115. [Google Scholar]
- Dunietz, G.L.; Sever, O.; DeRowe, A.; Tauman, R. Sleep Position and Breathing in Late Pregnancy and Perinatal Outcomes. J. Clin. Sleep Med. 2020. [Google Scholar] [CrossRef]
- Morales-Rosello, J.; Khalil, A.; Morlando, M.; Papageorghiou, A.; Bhide, A.; Thilaganathan, B. Changes in fetal Doppler indices as a marker of failure to reach growth potential at term. Ultrasound Obstet. Gynecol. 2014, 43, 303–310. [Google Scholar] [CrossRef]
- Prior, T.; Paramasivam, G.; Bennett, P.; Kumar, S. Are fetuses that fail to achieve their growth potential at increased risk of intrapartum compromise? Ultrasound Obstet. Gynecol. 2015, 46, 460–464. [Google Scholar]
- Dunn, L.; Sherrell, H.; Kumar, S. Review: Systematic review of the utility of the fetal cerebroplacental ratio measured at term for the prediction of adverse perinatal outcome. Placenta 2017, 54, 68–75. [Google Scholar]
- Khalil, A.; Thilaganathan, B. Role of uteroplacental and fetal Doppler in identifying fetal growth restriction at term. Best Pr. Res. Clin. Obstet. Gynaecol. 2017, 38, 38–47. [Google Scholar]
- Khalil, A.; Morales-Roselló, J.; Elsaddig, M.; Khan, N.; Papageorghiou, A.T.; Bhide, A.; Thilaganathan, B. The association between fetal Doppler and admission to neonatal unit at term. Am. J. Obstet. Gynecol. 2015, 213, 57.e1–57.e7. [Google Scholar]
- Khalil, A.; Morales-Roselló, J.; Morlando, M.; Hannan, H.; Bhide, A.; Papageorghiou, A.T.; Thilaganathan, B. Is fetal cerebroplacental ratio an independent predictor of intrapartum fetal compromise and neonatal unit admission? Am. J. Obstet. Gynecol. 2015, 213, 54.e1–54.e10. [Google Scholar]
- Khalil, A.; Morales-Roselló, J.; Townsend, R.; Morlando, M.; Papageorghiou, A.; Bhidé, A.; Thilaganathan, B. Value of third-trimester cerebroplacental ratio and uterine artery Doppler indices as predictors of stillbirth and perinatal loss. Ultrasound Obstet. Gynecol. 2016, 47, 74–80. [Google Scholar]
- Schiffner, R.; Bischoff, S.J.; Lehmann, T.; Rakers, F.; Rupprecht, S.; Reiche, J.; Matziolis, G.; Schubert, H.; Schwab, M.; Huber, O.; et al. Redistribution of Cerebral Blood Flow during Severe Hypovolemia and Reperfusion in a Sheep Model: Critical Role of α1-Adrenergic Signaling. Int. J. Mol. Sci. 2017, 18, 1031. [Google Scholar]
- Schiffner, R.; Bischoff, S.J.; Lehmann, T.; Rakers, F.; Rupprecht, S.; Matziolis, G.; Schubert, H.; Schwab, M.; Huber, O.; Lemke, C.; et al. Underlying mechanism of subcortical brain protection during hypoxia and reoxygenation in a sheep model-Influence of α1-adrenergic signalling. PLoS ONE 2018, 13, e0196363. [Google Scholar]
- Low, J.A. Cerebral perfusion, metabolism, and outcome. Curr. Opin. Pediatr. 1995, 7, 132–139. [Google Scholar]
- Greisen, G. Effect of Cerebral Blood Flow and Cerebrovascular Autoregulation on the Distribution, Type and Extent of Cerebral Injury. Brain Pathol. 1992, 2, 223–228. [Google Scholar]
- Cahill, L.S.; Zhou, Y.-Q.; Seed, M.; MacGowan, C.K.; Sled, J.G. Brain sparing in fetal mice: BOLD MRI and Doppler ultrasound show blood redistribution during hypoxia. Br. J. Pharmacol. 2014, 34, 1082–1088. [Google Scholar]
- Cahill, L.S.; Hoggarth, J.; Lerch, J.P.; Seed, M.; MacGowan, C.K.; Sled, J.G. Fetal brain sparing in a mouse model of chronic maternal hypoxia. Br. J. Pharmacol. 2017, 39, 1172–1184. [Google Scholar] [CrossRef]
- Silva, K.P.; Hamamoto, T.E.N.; Nomura, R. Transient fetal blood redistribution associated with maternal supine position. J. Périnat. Med. 2017, 45, 343–347. [Google Scholar]
- Robertson, N.; Turner, J.M.; Kumar, S. Pathophysiological changes associated with sleep disordered breathing and supine sleep position in pregnancy. Sleep Med. Rev. 2019, 46, 1–8. [Google Scholar] [CrossRef]
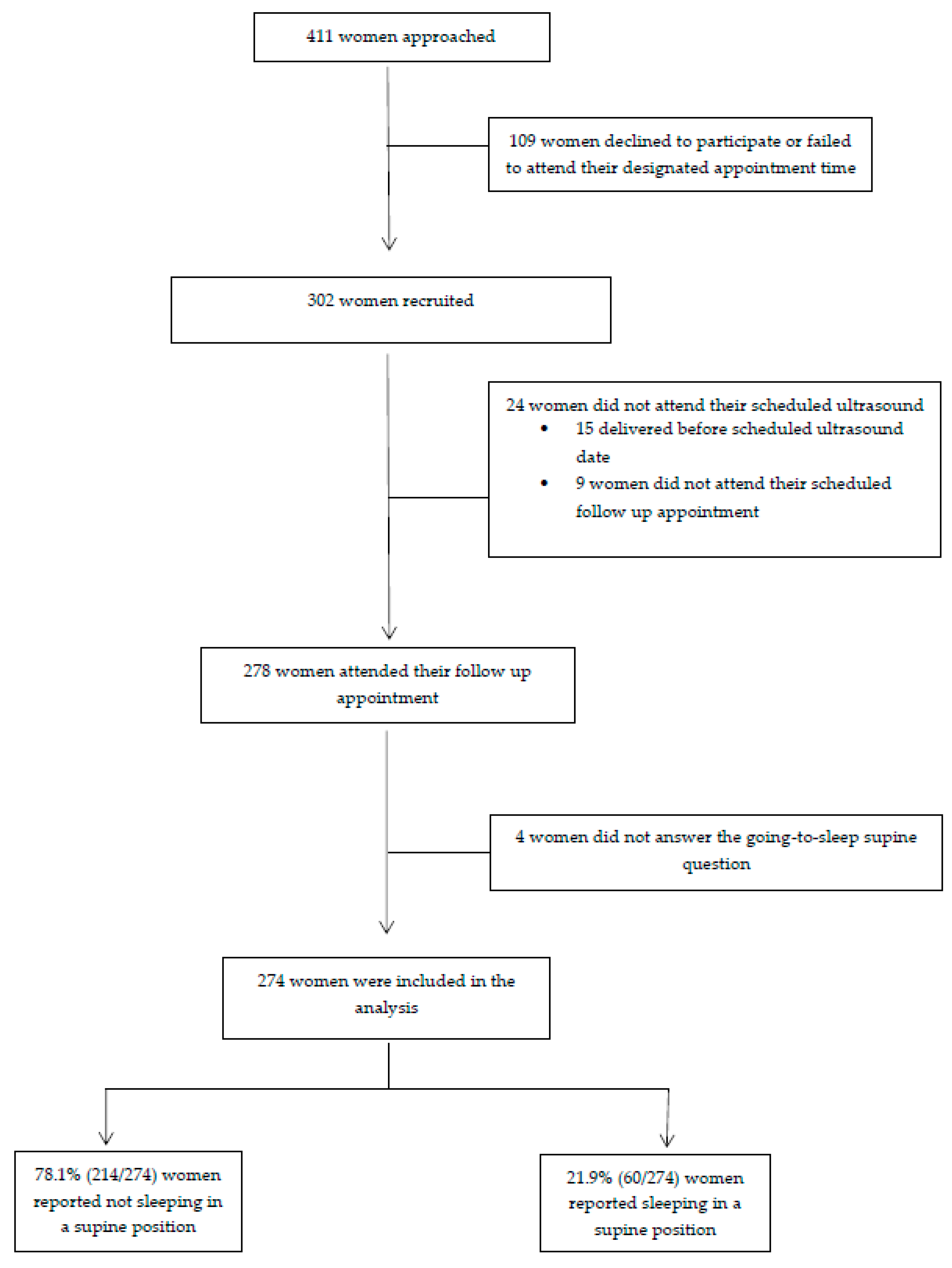
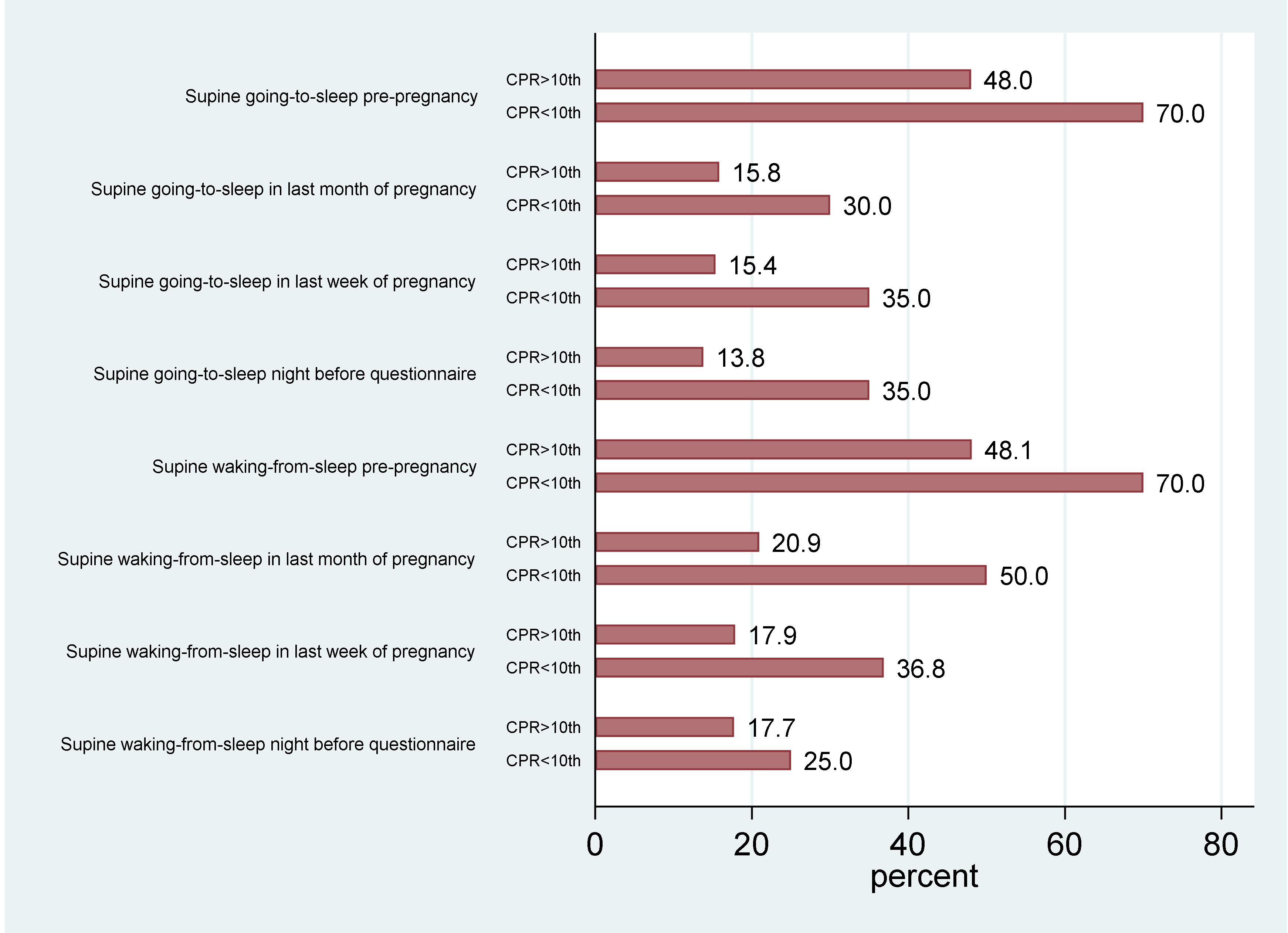
| Variable | No Supine Sleep n = 214 (78.1%) | Supine Sleep n = 60 (21.9%) | p Value |
|---|---|---|---|
| Maternal age (mean, SD) † | 31.5 (4.2) | 31.0 (3.8) | 0.43 |
| Gestational age at ultrasound assessment (weeks) (mean, SD) † | 36.4 (0.8) | 36.2(0.6) | 0.06 |
| Maternal booking BMI (median, IQR) ‡ | 23.3 (21.1–27.1) | 23.5 (20.9–28.3) | 0.99 |
| Ethnicity § | 0.11 | ||
| Caucasian | 64.5% (138/214) | 53.3% (32/60) | |
| ATSI | 0.9% (2/214) | 1.7% (1/60) | |
| Asian | 15.9% (34/214) | 13.3% (8/60) | |
| Indian | 8.4% (18/214) | 20.0% (12/60) | |
| Other | 10.3% (22/214) | 11.7% (7/60) | |
| Parity § | 0.93 | ||
| Nulliparous | 40.6% (87/214) | 40.0% (24/60) | |
| Multiparous | 59.4% (127/214) | 60.0% (36/60) | |
| Smoking § | 27.6% (59/214) | 25.0% (15/60) | 0.69 |
| Hypertension ∆ | 3.8% (8/213) | 5.1% (3/59) | 0.71 |
| Diabetes § | 15.0% (32/213) | 13.6% (8/59) | 0.78 |
| Parameter | No Supine Sleep n = 214 (78.1%) | Supine Sleep n = 60 (21.9%) | p Value * |
|---|---|---|---|
| MCA PI | 1.74 (0.29) | 1.62 (0.25) | 0.001 |
| UA PI | 0.83 (0.12) | 0.83 (0.12) | 0.81 |
| CPR | 2.13 (0.42) | 1.98 (0.37) | 0.008 |
| CPR < 10th centile | 6.1% (13/214) | 11.7% (7/60) | 0.16 |
| ACA PI | 1.59 (0.30) | 1.52 (0.27) | 0.046 |
| PCA PI | 1.48 (0.28) | 1.42 (0.23) | 0.055 |
| VertA PI | 1.68 (0.35) | 1.57 (0.27) | 0.019 |
| ACA/UA ratio | 1.94 (0.43) | 1.87 (0.41) | 0.19 |
| PCA/UA ratio | 1.80 (0.38) | 1.74 (0.34) | 0.15 |
| VertA/UA ratio | 2.04 (0.48) | 1.89 (0.42) | 0.06 |
| LCO | 492.50 (147.02) | 465.00 (118.61) | 0.30 |
| # LCO (mL/min/kg) | 167.03 (44.38) | 161.82 (38.68) | 0.39 |
| RCO | 778.67 (189.22) | 758.93 (178.37) | 0.68 |
| # RCO (mL/min/kg) | 264.50 (57.56) | 264.58 (60.52) | 0.92 |
| LCO/RCO ratio | 0.64 (0.16) | 0.62 (0.13) | 0.38 |
| CCO | 1271.15 (297.86) | 1223.93 (266.50) | 0.44 |
| # CCO (mL/min/kg) | 431.59 (87.29) | 426.39 (88.66) | 0.62 |
| UtA PI | 0.68 (0.17) | 0.71 (0.22) | 0.23 |
| UV flow (mL/min/kg) | 84.61 (25.94) | 81.89 (27.96) | 0.31 |
| EFW (grams) | 2935.46 (332.75) | 2877.58 (287.51) | 0.79 |
| EFW centile | 52.73 (23.91) | 50.83 (24.49) | 0.65 |
| Outcome | No Supine Sleep n = 214 (78.1%) | Supine Sleep n = 60 (21.9%) | p Value * |
|---|---|---|---|
| Gestation at delivery (weeks) (median, IQR) | 39.3(38.6–40.3) | 39.6 (38.9–40.4) | 0.21 |
| Mode of birth | |||
| SVD | 56.3% (120/213) | 66.7% (40/60) | 0.20 |
| Elective CS | 8.9% (19/213) | 5.0% (3/60) | 0.35 |
| Instrumental all | 18.3% (39/213) | 15.0% (9/60) | 0.64 |
| Em CS all | 16.4% (35/213) | 13.3% (8/60) | 0.59 |
| Em CS IFC | 7.5% (16/213) | 5.0% (3/60) | 0.51 |
| Em CS Other | 8.9% (19/213) | 8.3% (5/60) | 0.92 |
| Perinatal outcomes | |||
| BW grams (median, IQR) | 3384 (3084–3680) | 3419 (3124–3745) | 0.66 |
| BW <10th centile | 12.2% (26/213) | 13.3% (8/60) | 0.93 |
| BW >90th centile | 5.2% (11/213) | 13.3% (8/60) | 0.04 |
| 5-min Apgar < 7 | 1.4% (3/211) | 3.4% (2/59) | 0.24 |
| Acidosis (pH < 7 or BE <−12) | 1.9% (4/213) | 1.7% (1/60) | 0.95 |
| NICU admission | 5.2% (11/211) | 5.0% (3/60) | 0.95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robertson, N.; Okano, S.; Kumar, S. Sleep in the Supine Position During Pregnancy is Associated with Fetal Cerebral Redistribution. J. Clin. Med. 2020, 9, 1773. https://doi.org/10.3390/jcm9061773
Robertson N, Okano S, Kumar S. Sleep in the Supine Position During Pregnancy is Associated with Fetal Cerebral Redistribution. Journal of Clinical Medicine. 2020; 9(6):1773. https://doi.org/10.3390/jcm9061773
Chicago/Turabian StyleRobertson, Nicole, Satomi Okano, and Sailesh Kumar. 2020. "Sleep in the Supine Position During Pregnancy is Associated with Fetal Cerebral Redistribution" Journal of Clinical Medicine 9, no. 6: 1773. https://doi.org/10.3390/jcm9061773
APA StyleRobertson, N., Okano, S., & Kumar, S. (2020). Sleep in the Supine Position During Pregnancy is Associated with Fetal Cerebral Redistribution. Journal of Clinical Medicine, 9(6), 1773. https://doi.org/10.3390/jcm9061773





