An Integrative Neuro-Psychotherapy Treatment to Foster the Adjustment in Acquired Brain Injury Patients—A Randomized Controlled Study
Abstract
1. Introduction
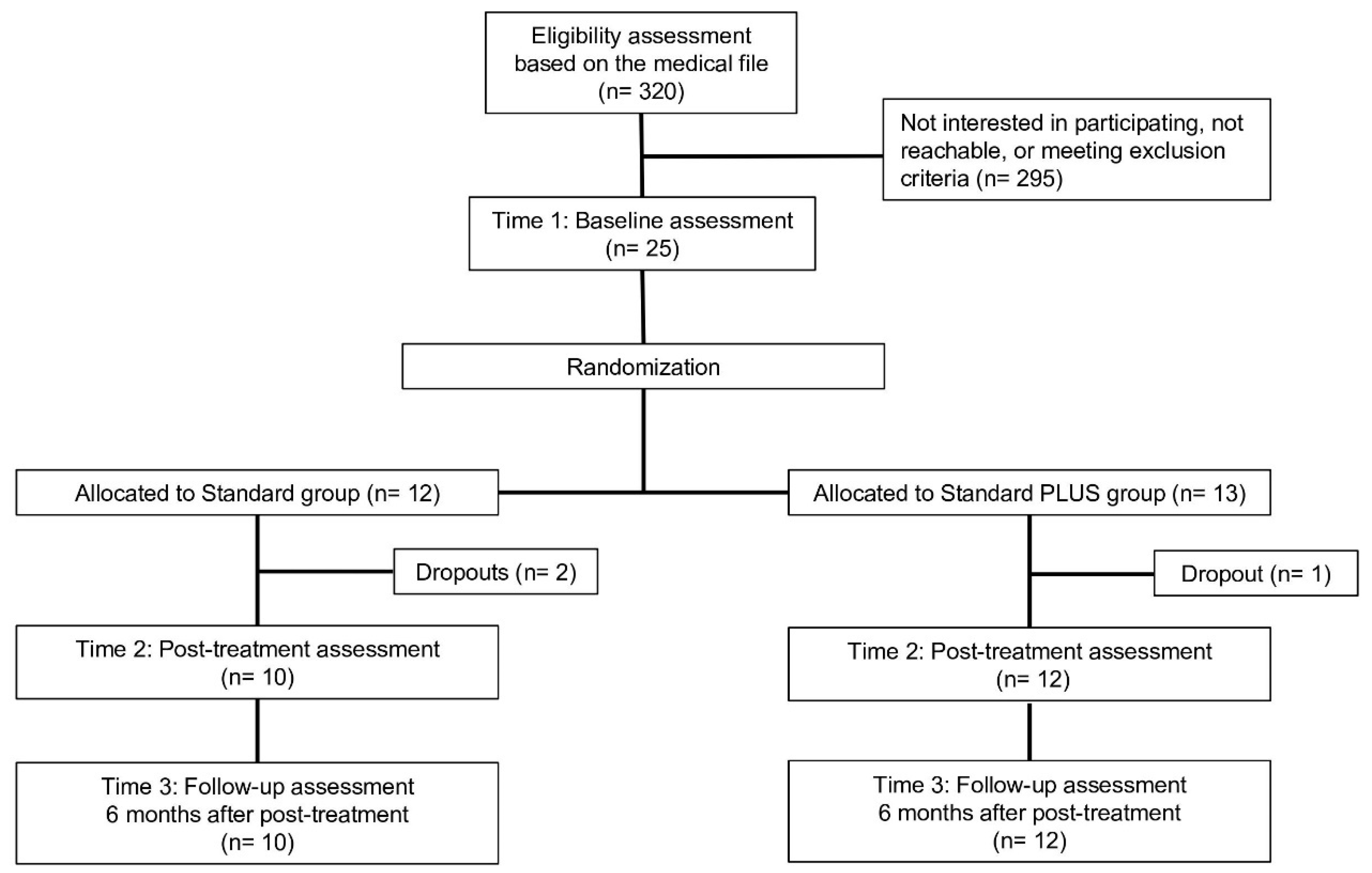
2. Materials and Methods
2.1. Patients
2.2. Measures
2.3. Primary Outcome
2.4. Secondary Outcomes
2.4.1. Acceptance of Disability
2.4.2. Awareness
2.4.3. Illness Coping
2.4.4. Emotion Regulation Skills
2.4.5. Relationship Quality
2.4.6. Mental Fatigue
3. Treatments
4. Power Analysis
5. Statistical Analysis
6. Results
6.1. Preliminary analyses
6.2. Intervention Effects at Post-Treatment
6.3. Intervention Effects at Follow-Up Assessment
7. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Norrving, B.; Davis, S.M.; Feigin, V.L.; Mensah, G.A.; Sacco, R.L.; Varghese, C. Stroke prevention worldwide-what could make it work. Neuroepidemiology 2015, 45, 215–220. [Google Scholar] [CrossRef]
- Thiele, I.; Linseisen, J.; Heier, M.; Holle, R.; Kirchberger, I.; Peters, A.; Meisinger, C. Time trends in stroke incidence and in prevalence of risk factors in Southern Germany, 1989 to 2008/09. Sci. Rep. 2018, 8, 11981. [Google Scholar] [CrossRef]
- Lee, S.; Shafe, A.C.; Cowie, M.R. UK stroke incidence, mortality and cardiovascular risk management 1999–2008: Time-trend analysis from the General Practice Research Database. BMJ Open 2011, 1, e000269. [Google Scholar] [CrossRef] [PubMed]
- Prigatano, G.P. Principles of Neuropsychological Rehabilitation; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Wilson, B.A.; Winegardner, J.; Van Heugten, C.M.; Ownsworth, T. Neuropsychological Rehabilitation: The International Handbook; Routledge: London, UK, 2017. [Google Scholar]
- Hill, K.; House, A.; Knapp, P.; Wardhaugh, C.; Bamford, J.; Vail, A. Prevention of mood disorder after stroke: A randomised controlled trial of problem solving therapy versus volunteer support. BMC Neurol. 2019, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Osborn, A.; Mathias, J.; Fairweather-Schmidt, A. Depression following adult, non-penetrating traumatic brain injury: A meta-analysis examining methodological variables and sample characteristics. Neurosci. Biobehav. Rev. 2014, 47, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Sheth, B.; Gill, J.; Yadegarfar, M.; Stubbs, B.; Yadegarfar, M.; Meader, N. Prevalence and predictors of post-stroke mood disorders: A meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen. Hosp. Psychiatry 2017, 47, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; Xiao, J. Mortality associated with incident mental health disorders after stroke. Aust. N. Z. J. Psychiatry 2007, 41, 274–281. [Google Scholar] [CrossRef]
- Ferro, J.M.; Caeiro, L.; Figueira, M.L. Neuropsychiatric sequelae of stroke. Nat. Rev. Neurol. 2016, 12, 269. [Google Scholar] [CrossRef]
- Shenal, B.V.; Harrison, D.W.; Demaree, H.A. The neuropsychology of depression: A literature review and preliminary model. Neuropsychol. Rev. 2003, 13, 33–42. [Google Scholar] [CrossRef]
- Fayed, N.; Morales, H.; Torres, C.; Viguera, L. Neuroimaging of Post-Stroke Depression Psychiatry and Neuroscience Update; Springer: Berlin, Germany, 2019; pp. 379–386. [Google Scholar]
- Farner, L.; Wagle, J.; Engedal, K.; Flekkøy, K.M.; Wyller, T.B.; Fure, B. Depressive symptoms in stroke patients: A 13 month follow-up study of patients referred to a rehabilitation unit. J. Affect. Disord. 2010, 127, 211–218. [Google Scholar] [CrossRef]
- Carota, A.; Berney, A.; Aybek, S.; Iaria, G.; Staub, F.; Ghika-Schmid, F.; Bogousslavsky, J. A prospective study of predictors of poststroke depression. Neurology 2005, 64, 428–433. [Google Scholar] [CrossRef]
- Hackett, M.L.; Anderson, C.S.; House, A.; Halteh, C. Interventions for preventing depression after stroke. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- Spalletta, G.; Bossu, P.; Ciaramella, A.; Bria, P.; Caltagirone, C.; Robinson, R. The etiology of poststroke depression: A review of the literature and a new hypothesis involving inflammatory cytokines. Mol. Psychiatry 2006, 11, 984. [Google Scholar] [CrossRef]
- Loubinoux, I.; Kronenberg, G.; Endres, M.; Schumann-Bard, P.; Freret, T.; Filipkowski, R.K.; Popa-Wagner, A. Post-stroke depression: Mechanisms, translation and therapy. J. Cell. Mol. Med. 2012, 16, 1961–1969. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, D.; Zeng, Y.; Wu, W. Risk factors for post-stroke depression: A meta-analysis. Front. Aging Neurosci. 2017, 9, 218. [Google Scholar] [CrossRef]
- Sjöholm, L.; Lavebratt, C.; Forsell, Y. A multifactorial developmental model for the etiology of major depression in a population-based sample. J. Affect. Disord. 2009, 113, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.P.; Biering, K.; Johnsen, S.P.; Andersen, G.; Hjollund, N.H. Self-rated health and return to work after first-time stroke. J. Rehabil. Med. 2016, 48, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Singam, A.; Ytterberg, C.; Tham, K.; von Koch, L. Participation in complex and social everyday activities six years after stroke: Predictors for return to pre-stroke level. PLoS ONE 2015, 10, e0144344. [Google Scholar] [CrossRef] [PubMed]
- Wilz, G. Predictors of subjective impairment after stroke: Influence of depression, gender and severity of stroke. Brain Injury 2007, 21, 39–45. [Google Scholar] [CrossRef]
- Casey, P. Adult adjustment disorder: A review of its current diagnostic status. J. Psychiatr. Pract. 2001, 7, 32–40. [Google Scholar] [CrossRef]
- Evans, S.C.; Reed, G.M.; Roberts, M.C.; Esparza, P.; Watts, A.D.; Correia, J.M.; Saxena, S. Psychologists’ perspectives on the diagnostic classification of mental disorders: Results from the WHO-IUPsyS Global Survey. Int. J. Psychol. 2013, 48, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Beblo, T.; Herrmann, M. Pathophysiologische und neuropsychologische Aspekte depressiver Störungen. Z. Neuropsychol. 2001, 12, 264–275. [Google Scholar] [CrossRef]
- Casey, P.; Maracy, M.; Kelly, B.D.; Lehtinen, V.; Ayuso-Mateos, J.-L.; Dalgard, O.S.; Dowrick, C. Can adjustment disorder and depressive episode be distinguished? Results from ODIN. J. Affect. Disord. 2006, 92, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Curio, N.; Petz, T.; Synowitz, H.; Wagner, S.; Bartels, C.; Wallesch, C.-W. Coping with illness after brain diseasesa comparison between patients with malignant brain tumors, stroke, Parkinson’s disease and traumatic brain injury. Disabil. Rehabil. 2000, 22, 539–546. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; France, A.; Pincus, H.A. DSM-IV-TR Guidebook; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2004. [Google Scholar]
- Schmid, A.A.; Kroenke, K.; Hendrie, H.; Bakas, T.; Sutherland, J.; Williams, L. Poststroke depression and treatment effects on functional outcomes. Neurology 2011, 76, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.; Domingo, J.; Rodríguez-Garcia, E.; Castro, M.-D.; del Ser, T. Frequency of cognitive impairment without dementia in patients with stroke: A two-year follow-up study. Stroke 2007, 38, 105–110. [Google Scholar] [CrossRef]
- Ahn, D.-H.; Lee, Y.-J.; Jeong, J.-H.; Kim, Y.-R.; Park, J.-B. The effect of post-stroke depression on rehabilitation outcome and the impact of caregiver type as a factor of post-stroke depression. Ann. Rehabil. Med. 2015, 39, 74. [Google Scholar] [CrossRef]
- Anson, K.; Ponsford, J. Coping and emotional adjustment following traumatic brain injury. J. Head Trauma Rehabil. 2006, 21, 248–259. [Google Scholar] [CrossRef]
- Bartoli, F.; Di Brita, C.; Crocamo, C.; Clerici, M.; Carrà, G. Early post-stroke depression and mortality: Meta-analysis and meta-regression. Front. Psychiatry 2018, 9, 530. [Google Scholar] [CrossRef]
- Ashworth, F.; Clarke, A.; Jones, L.; Jennings, C.; Longworth, C. An exploration of compassion focused therapy following acquired brain injury. Psychol. Psychother. Theory Res. Pract. 2015, 88, 143–162. [Google Scholar] [CrossRef]
- Salas, C.E.; Prigatano, G.P. From meaning to symptom reduction: Contemporary approaches to psychotherapy after traumatic brain injury. Rev. Chil. Neuropsicol. 2018, 13, 22–29. [Google Scholar]
- Klonoff, P.S. Psychotherapy after Brain Injury: Principles and Techniques; Guilford Press: New York, USA, 2010. [Google Scholar]
- Ownsworth, T.; Gracey, F. Cognitive behavioural therapy for people with brain injury. Assoc. Behav. Cogn. Ther. 2017, 313–326. [Google Scholar]
- Mead, G.E.; Hsieh, C.F.; Lee, R.; Kutlubaev, M.A.; Claxton, A.; Hankey, G.J.; Hackett, M.L. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst. Rev. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, S. Epidemiology and treatment of post-stroke depression. Neuropsychiatr. Dis. Treat. 2008, 4, 145. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.G.; Jorge, R.E. Post-stroke depression: A review. Am. J. Psychiatry 2015, 173, 221–231. [Google Scholar] [CrossRef]
- Fann, J.R.; Hart, T.; Schomer, K.G. Treatment for depression after traumatic brain injury: A systematic review. J. Neurotrauma 2009, 26, 2383–2402. [Google Scholar] [CrossRef]
- Stalder-Lüthy, F.; Messerli-Bürgy, N.; Hofer, H.; Frischknecht, E.; Znoj, H.; Barth, J. Effect of psychological interventions on depressive symptoms in long-term rehabilitation after an acquired brain injury: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2013, 94, 1386–1397. [Google Scholar] [CrossRef]
- Gertler, P.; Tate, R.L.; Cameron, I.D. Non-pharmacological interventions for depression in adults and children with traumatic brain injury. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Ashman, T.; Cantor, J.B.; Tsaousides, T.; Spielman, L.; Gordon, W. Comparison of cognitive behavioral therapy and supportive psychotherapy for the treatment of depression following traumatic brain injury: A randomized controlled trial. J. Head Trauma Rehabil. 2014, 29, 467–478. [Google Scholar] [CrossRef]
- Whiting, D.; Deane, F.; McLeod, H.; Ciarrochi, J.; Simpson, G. Can acceptance and commitment therapy facilitate psychological adjustment after a severe traumatic brain injury? A pilot randomized controlled trial. Neuropsychol. Rehabil. 2019, 1–24. [Google Scholar] [CrossRef]
- Hofer, H.; grosse Holtforth, M.; Frischknecht, E.; Znoj, H.-J. Fostering adjustment to acquired brain injury by psychotherapeutic interventions: A preliminary study. Appl. Neuropsychol. 2010, 17, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Doering, B.; Exner, C. Combining neuropsychological and cognitive–behavioral approaches for treating psychological sequelae of acquired brain injury. Curr. Opin. Psychiatry 2011, 24, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Wiart, L.; Luauté, J.; Stefan, A.; Plantier, D.; Hamonet, J. Non pharmacological treatments for psychological and behavioural disorders following traumatic brain injury (TBI). A systematic literature review and expert opinion leading to recommendations. Ann. Phys. Rehabil. Med. 2016, 59, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Zelviene, P.; Kazlauskas, E. Adjustment disorder: Current perspectives. Neuropsychiatr. Dis. Treat. 2018, 14, 375. [Google Scholar] [CrossRef]
- Dobkin, B.; Carmichael, T. Principles of recovery after stroke. Recovery Stroke 2005, 47–66. [Google Scholar] [CrossRef]
- Wittchen, H.; Zaudig, M.; Fydrich, T. Structured clinical interview for DSM-IV; Hogrefe: Göttingen, Germany, 1997. [Google Scholar]
- Hautzinger, M.; Keller, F.; Kühner, C. Das Beck Depressionsinventar II. Deutsche Bearbeitung und Handbuch zum BDI II [The Beck Depression Inventory II. German Adaptation and Manual of the BDI II]; Harcourt Test Services: Frankfurt, Germany, 2006. [Google Scholar]
- Green, A.; Felmingham, K.; Baguley, I.J.; Slewa-Younan, S.; Simpson, S. The clinical utility of the Beck Depression Inventory after traumatic brain injury. Brain Inj. 2001, 15, 1021–1028. [Google Scholar] [CrossRef]
- The WHOQOL Group. The World Health Organization quality of life assessment (WHOQOL): Development and general psychometric properties. Soc. Sci. Med. 1998, 46, 1569–1585. [Google Scholar] [CrossRef]
- Linkowski, D.C. A scale to measure acceptance of disability. Rehabil. Couns. Bull. 1971, 14, 236–245. [Google Scholar]
- Groomes, D.A.G.; Linkowski, D.C. Examining the structure of the revised Acceptance Disability Scale. J. Rehabil. 2007, 73, 3–9. [Google Scholar]
- Sherer, M.; Bergloff, P.; Boake, C.; High, W., Jr.; Levin, E. The Awareness Questionnaire: Factor structure and internal consistency. Brain Inj. 1998, 12, 63–68. [Google Scholar] [CrossRef]
- Sherer, M. The Awareness Questionnaire. 2004. Available online: http://www.tbims.org/combi/aq (accessed on 2 September 2007).
- Klauer, T.; Filipp, S. Trier Illness Coping Scales (TSK); Hogrefe: Göttingen, Germany, 1993. [Google Scholar]
- Berking, M.; Znoj, H. Entwicklung und Validierung eines Fragebogens zur standardisierten Selbsteinschätzung emotionaler Kompetenzen (SEK-27). Z. Psychiatr. Psychol. Psychother. 2008, 56, 141–153. [Google Scholar] [CrossRef]
- Hendrick, S.S. A generic measure of relationship satisfaction. J. Marriage Fam. 1988, 50, 93–98. [Google Scholar] [CrossRef]
- Johansson, B.; Starmark, A.; Berglund, P.; Rödholm, M.; Rönnbäck, L. A self-assessment questionnaire for mental fatigue and related symptoms after neurological disorders and injuries. Brain Inj. 2010, 24, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kiresuk, T.; Lund, S. Goal attainment scaling: Research, evaluation and utilization. In Program Evaluation in Health Fields; Schulberger, H.C., Parker, F., Eds.; Human Science Press: New York, NY, USA, 1979; Volume 2, pp. 214–237. [Google Scholar]
- Cicerone, K.D.; Dahlberg, C.; Malec, J.F.; Langenbahn, D.M.; Felicetti, T.; Kneipp, S.; Harley, J.P. Evidence-based cognitive rehabilitation: Updated review of the literature from 1998 through 2002. Arch. Phys. Med. Rehabil. 2005, 86, 1681–1692. [Google Scholar] [CrossRef]
- Gauggel, S. Grundlagen und Empirie der Neuropsychologischen Therapie: Neuropsychotherapie oder Hirnjogging? Z. Neuropsychol. 2003, 14, 217–246. [Google Scholar] [CrossRef]
- Sohlberg, M.M.; Mateer, C.A. Cognitive Rehabilitation: An Integrative Neuropsychological Approach; Guilford Press: New York, NY, USA, 2001. [Google Scholar]
- Hofer, H.; Holtforth, M.G.; Lüthy, F.; Frischknecht, E.; Znoj, H.; Müri, R.M. The potential of a mindfulness-enhanced, integrative neuro-psychotherapy program for treating fatigue following stroke: A preliminary study. Mindfulness 2014, 5, 192–199. [Google Scholar] [CrossRef]
- Weissman, M.M.; Markowitz, J.C.; Klerman, G. Comprehensive Guide to Interpersonal Psychotherapy; Basic Books: New York, NY, USA, 2008. [Google Scholar]
- Greenberg, L.S. Integrating an emotion-focused approach to treatment into psychotherapy integration. J. Psychother. Integr. 2002, 12, 154. [Google Scholar] [CrossRef]
- Dobson, K.S. Handbook of Cognitive-Behavioral Therapies, 2nd ed.; Guilford: New York, NY, USA, 2001. [Google Scholar]
- Lincoln, N.; Flannaghan, T. Cognitive behavioral psychotherapy for depression following stroke: A randomized controlled trial. Stroke 2003, 34, 111–115. [Google Scholar] [CrossRef]
- Vickers, A.J. Analysis of variance is easily misapplied in the analysis of randomized trials: A critique and discussion of alternative statistical approaches. Psychosom. Med. 2005, 67, 652–655. [Google Scholar] [CrossRef]
- Westbrook, D.; Kirk, J. The clinical effectiveness of cognitive behaviour therapy: Outcome for a large sample of adults treated in routine practice. Behav. Res. Ther. 2005, 43, 1243–1261. [Google Scholar] [CrossRef]
- Barth, J.; Munder, T.; Gerger, H.; Nüesch, E.; Trelle, S.; Znoj, H.; Cuijpers, P. Comparative efficacy of seven psychotherapeutic interventions for patients with depression: A network meta-analysis. Focus 2016, 14, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Wampold, B.E.; Serlin, R.C.; Kircher, J.C.; Brown, G.S.J. Benchmarks for psychotherapy efficacy in adult major depression. J. Consult. Clin. Psychol. 2007, 75, 232. [Google Scholar] [CrossRef] [PubMed]
- Coetzer, R. A clinical pathway including psychotherapy approaches for managing emotional difficulties after acquired brain injury. CNS Spectr. 2009, 14, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Fazel Nabavi, S.; Turner, A.; Dean, O.; Sureda, A.; Mohammad, S. Post-stroke depression therapy: Where are we now? Curr. Neurovasc. Res. 2014, 11, 279–289. [Google Scholar] [CrossRef]
- House, A. Depression after stroke. Br. Med J. (Clin. Res. Ed.) 1987, 294, 76. [Google Scholar] [CrossRef]
- Towfighi, A.; Ovbiagele, B.; El Husseini, N.; Hackett, M.L.; Jorge, R.E.; Kissela, B.M.; Mitchell, P.H.; Skolarus, L.E.; Whooley, M.A.; Williams, L.S.; et al. Poststroke depression: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017, 48, e30–e43. [Google Scholar] [CrossRef]
- Cicerone, K.D.; Goldin, Y.; Ganci, K.; Rosenbaum, A.; Wethe, J.V.; Langenbahn, D.M.; Nagele, D. Evidence-Based cognitive rehabilitation: Systematic review of the literature from 2009 through 2014. Arch. Phys. Med. Rehabil. 2019, 100, 1515–1533. [Google Scholar] [CrossRef]
- Coetzer, R.; Daisley, A.; Newby, G.; Weatherhead, S. Practical Neuropsychological Rehabilitation in Acquired Brain Injury: A Guide for Working Clinicians; Routledge: London, UK, 2018. [Google Scholar]
- Ponsford, J.; Lee, N.K.; Wong, D.; McKay, A.; Haines, K.; Alway, Y.; O′Donnell, M.L. Efficacy of motivational interviewing and cognitive behavioral therapy for anxiety and depression symptoms following traumatic brain injury. Psychol. Med. 2016, 46, 1079–1090. [Google Scholar] [CrossRef]
- Hart, T.; Hoffman, J.M.; Pretz, C.; Kennedy, R.; Clark, A.N.; Brenner, L.A. A longitudinal study of major and minor depression following traumatic brain injury. Arch. Phys. Med. Rehabil. 2012, 93, 1343–1349. [Google Scholar] [CrossRef]
- Nakling, A.E.; Aarsland, D.; Næss, H.; Wollschlaeger, D.; Fladby, T.; Hofstad, H.; Wehling, E. Cognitive deficits in chronic stroke patients: Neuropsychological assessment, depression, and self-reports. Dement. Geriatr. Cogn. Disord. Extra 2017, 7, 283–296. [Google Scholar] [CrossRef]
- Anderson, C.; Hackett, M.L.; House, A.O. Interventions for preventing depression after stroke. Cochrane Database Syst. Rev. 2004. [Google Scholar] [CrossRef]
- Robinson, R.G.; Jorge, R.E.; Moser, D.J.; Acion, L.; Solodkin, A.; Small, S.L.; Arndt, S. Escitalopram and problem-solving therapy for prevention of poststroke depression: A randomized controlled trial. Jama 2008, 299, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, M.; Müri, R.M. Neurorehabilitation of traumatic brain injury (TBI): A clinical review. Med Sci. 2019, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Motter, J.N.; Pimontel, M.A.; Rindskopf, D.; Devanand, D.P.; Doraiswamy, P.M.; Sneed, J.R. Computerized cognitive training and functional recovery in major depressive disorder: A meta-analysis. J. Affect. Disord. 2016, 189, 184–191. [Google Scholar] [CrossRef] [PubMed]
| Phase | Standard Treatment | Standard PLUS Treatment |
|---|---|---|
| Preparatory (Week 1) | Establishing a sustainable working alliance, information about the aims and procedures of the therapy. Formulation of individual therapy goals and introduction of a tiredness diary. | Analogs to the standard treatment procedure week 1 |
| Intervention (Week 1–18) | Implementation of the standard therapy, with the aim of achieving individual therapy goals by the 18th session. | Implementation of the standard PLUS Therapy, with the aim of achieving individual therapy goals by the 18th session. |
Important topics:
| Important topics:
| |
Therapeutic interventions:
| Therapeutic interventions:
| |
| PLUS: Psychotherapeutic techniques (e.g., emotional-focus therapy and clarification d, activating resources e, generating, positive expectancies, normalization) The principle of mindfulness based on “Mindfulness-Based Cognitive Therapy” f | ||
| Maintenance (Week 18–20) | Not intended as an actual therapy session. Overall review, consolidation of what has been achieved, and reiterating the individual strategies involved. Discussion potentially difficult situations and planning of coping methods. | Analogs to the standard treatment procedure week 18–20. |
| PLUS (n = 13) | Standard (n = 12) | Statistic | |
|---|---|---|---|
| Age, years (SD) | 50.8 (7.8) | 45.6 (12.6) | t(23) = −1.24, p = 0.22 |
| Gender, n (%) | χ2(1) = 0.05, p = 0.82 | ||
| Male | 6 (46.1) | 5 (41.6) | |
| Female | 7 (53.9) | 7 (58.4) | |
| Marital status, n (%) | χ2(2) = 1.22, p = 0.54 | ||
| Single/living alone | 2 (15.4) | 4 (33.3) | |
| Married/living together | 9 (69.2) | 6 (50.0) | |
| Divorced | 2 (15.4) | 2 (16.7) | |
| Education in years (SD) | 12.4 (1.1) | 12.6 (2.9) | t(23) = 0.17, p = 0.86 |
| Employment, n (%) | χ2(2) = 1.10, p = 0.56 | ||
| Self-Employed / Partner | 5 (38.4) | 6 (50.0) | |
| Student | 1 (7.8) | 0 | |
| Sick pay/unfit for work | 7 (53.8) | 6 (50.0) | |
| Neurological disorder, n (%) | χ2(4) = 3.10, p = 0.54 | ||
| Ischemic stroke | 6 (46.1) | 9 (75.0) | |
| Hemorrhagic stroke | 3 (23.1) | 2 (16.7) | |
| Traumatic brain Injury | 2 (15.4) | 1 (8.3) | |
| Tumor | 1 (7.7) | 0 (0.0) | |
| Encephalitis | 1 (7.7) | 0 (0.0) | |
| Time since injury, months (SD) | 14.1 (13.4) | 19.6 (25.7) | t(23) = 0.67, p = 0.50 |
| Baseline | Post | Follow-up | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | M | SD | Between-Group 1 | M | SD | M | SD | ||
| df | T a | U b | |||||||
| BDI-II | 23 | −1.07 | 58.00 | ||||||
| PLUS | 21.77 | 9.56 | 11.15 | 5.13 | 10.92 | 4.11 | |||
| Standard | 17.92 | 8.24 | 10.50 | 5.50 | 8.00 | 4.59 | |||
| MFS | 22 | −0.81 | 57.00 | ||||||
| PLUS | 9.92 | 2.67 | 7.50 | 2.68 | 7.00 | 2.82 | |||
| Standard | 8.83 | 3.74 | 6.58 | 2.74 | 6.91 | 3.31 | |||
| WHOQol | 23 | −1.02 | 54.00 | ||||||
| PLUS | 91.00 | 12.75 | 95.53 | 13.08 | 96.77 | 11.00 | |||
| Standard | 86.00 | 11.42 | 94.41 | 9.34 | 95.16 | 10.33 | |||
| ADS | 23 | −0.31 | 72.00 | ||||||
| PLUS | 78.15 | 8.07 | 73.38 | 10.94 | 72.83 | 8.70 | |||
| Standard | 76.92 | 11.34 | 72.50 | 9.42 | 72.84 | 10.63 | |||
| TSK_RU | 23 | 1.08 | 61.00 | ||||||
| PLUS | 25.69 | 7.79 | 25.23 | 7.99 | 23.15 | 7.38 | |||
| Standard | 28.83 | 7.38 | 23.83 | 5.65 | 23.91 | 6.51 | |||
| TSK_SS | 23 | −0.41 | 71.50 | ||||||
| PLUS | 36.00 | 7.38 | 38.07 | 4.87 | 38.69 | 4.99 | |||
| Standard | 34.83 | 6.49 | 38.33 | 6.28 | 38.08 | 5.93 | |||
| TSK_BA | 23 | −1.21 | 59.50 | ||||||
| PLUS | 37.08 | 5.48 | 36.38 | 4.85 | 36.23 | 6.71 | |||
| Standard | 33.75 | 8.09 | 35.50 | 6.72 | 34.25 | 7.24 | |||
| TSK_SI | 23 | 0.57 | 71.00 | ||||||
| PLUS | 25.77 | 6.07 | 25.61 | 6.55 | 24.38 | 7.38 | |||
| Standard | 27.33 | 7.63 | 28.58 | 6.93 | 26.33 | 8.56 | |||
| TSK_SR | 23 | −0.28 | 71.50 | ||||||
| PLUS | 8.54 | 4.01 | 8.15 | 3.60 | 7.92 | 4.55 | |||
| Standard | 8.08 | 4.23 | 8.83 | 3.29 | 7.33 | 2.87 | |||
| AQ_R | 20 | 2.30 * | 28.00 * | ||||||
| PLUS | 33.92 | 5.01 | 40.08 | 5.90 | 38.25 | 6.09 | |||
| Standard | 39.20 | 5.75 | 42.30 | 6.03 | 42.20 | 5.78 | |||
| AQ_C | 23 | −1.39 | 54.50 | ||||||
| PLUS | 46.00 | 3.13 | 50.23 | 3.32 | ------ | ------ | |||
| Standard | 43.75 | 4.86 | 48.16 | 6.13 | ------ | ------ | |||
| AQ_P | 23 | −0.08 | 72.50 | ||||||
| PLUS | 37.77 | 5.70 | 40.92 | 6.73 | 39.61 | 6.64 | |||
| Standard | 37.58 | 6.24 | 40.75 | 5.92 | 40.91 | 6.18 | |||
| RAS | 20 | −1.19 | 44.50 | ||||||
| PLUS | 25.17 | 2.16 | 24.91 | 1.83 | 26.79 | 4.26 | |||
| Standard | 23.90 | 2.80 | 25.50 | 3.34 | 24.90 | 2.88 | |||
| ERSQ | 23 | −1.18 | 58.00 | ||||||
| PLUS | 70.92 | 14.54 | 85.00 | 13.69 | 78.46 | 13.72 | |||
| Standard | 52.50 | 20.80 | 66.91 | 15.29 | 71.00 | 17.29 | |||
| Measure | Group | Mean Difference (T1-T2) | 95% CI | Within-Group | Between-Group | ||||
|---|---|---|---|---|---|---|---|---|---|
| df | T | Effect Size | df | F | Effect Size 1 | ||||
| BDI-II | PLUS | 10.70 | (2.01–19.38) | 12 | 3.53 ** | 1.32 | 1,22 | <0.01 | 0.30 |
| Standard | 7.33 | (1.51–13.14) | 11 | 3.51 ** | 1.02 | ||||
| MFS | PLUS | 2.90 | (0.51–5.29) | 11 | 2.59 * | 0.87 | 1,21 | 0.15 | 0.22 |
| Standard | 2.33 | (0.84–3.82) | 11 | 4.18 ** | 0.65 | ||||
| WHOQol | PLUS | −6.20 | (−13.76–1.36) | 12 | −1.61 | −0.34 | 1,22 | 0.35 | −0.43 |
| Standard | −7.78 | (−13.87–−1.67) | 11 | −3.38 ** | −0.77 | ||||
| ADS | PLUS | 6.10 | (−1.97–14.17) | 12 | 1.64 | 0.48 | 1,22 | <0.01 | 0.07 |
| Standard | 3.11 | (−1.95–8.17) | 11 | 2.41 * | 0.41 | ||||
| TSK_RU | PLUS | 2.01 | (−2.68–6.68) | 12 | 0.22 | 0.06 | 1,22 | 2.19 | −0.68 |
| Standard | 4.89 | (1.90–7.87) | 11 | 4.10 ** | 0.74 | ||||
| TSK_SS | PLUS | −3.00 | (−7.66–1.66) | 12 | −1.23 | −0.32 | 1,22 | 0.24 | −0.21 |
| Standard | −2.89 | (−6.44–0.66) | 11 | −2.63 * | −0.53 | ||||
| TSK_BA | PLUS | 1.20 | (−2.56–4.96) | 12 | 0.53 | 0.13 | 1,22 | 0.22 | 0.10 |
| Standard | −2.89 | (−8.11–2.33) | 11 | −0.96 | −0.23 | ||||
| TSK_SI | PLUS | −0.10 | (−6.00–5.80) | 12 | 0.07 | 0.02 | 1,22 | 0.84 | −0.14 |
| Standard | −2.22 | (−7.91–3.47) | 11 | −0.65 | −0.16 | ||||
| TSK_SR | PLUS | 0.40 | (−0.99–1.79) | 12 | 0.77 | 0.08 | 1,22 | 1.18 | −0.10 |
| Standard | −1.33 | (−3.67–1.01) | 11 | −0.79 | −0.18 | ||||
| AQ_R | PLUS | −6.40 | (−9.65–−3.14) | 11 | −4.86 *** | −1.09 | 1,19 | 0.56 | 0.59 |
| Standard | −3.67 | (−7.43–0.09) | 9 | −1.97 | −0.50 | ||||
| AQ_C | PLUS | −4.80 | (−7.27–−2.32) | 12 | −4.25 *** | −1.30 | 1,22 | 0.03 | 0.51 |
| Standard | −4.11 | (−7.07–−1.14) | 11 | −3.92 ** | −0.79 | ||||
| AQ_P | PLUS | −2.50 | (−5.22–0.22) | 12 | −2.91 * | −0.50 | 1,22 | <0.01 | 0.01 |
| Standard | −3.22 | (−6.74–0.29) | 11 | −2.74 * | −0.49 | ||||
| RAS | PLUS | 0.00 | (−1.01–1.01) | 11 | 0.61 | 0.14 | 1,19 | 2.23 | −0.36 |
| Standard | −1.78 | (−4.14–0.58 | 9 | −1.71 | −0.50 | ||||
| ERSQ | PLUS | −14.50 | (−28.40–−0.59) | 12 | −2.84 * | −0.97 | 1,22 | 7.56 * | 0.21 |
| Standard | −9.89 | (−20.82–1.04) | 11 | −0.93 | −0.76 | ||||
| Measure | Group | Mean Difference (T1-T2) | 95% CI | Within-Group | Between-Group | ||||
|---|---|---|---|---|---|---|---|---|---|
| df | T | Effect Size | df | F | Effect Size 1 | ||||
| BDI-II | PLUS | 10.30 | (1.76–18.83) | 12 | 3.65 ** | 1.42 | 1,22 | 1.91 | −0.02 |
| Standard | 10.11 | (5.06–15.15) | 11 | 5.46 *** | 1.44 | ||||
| MFS | PLUS | 3.30 | (1.06–5.53) | 11 | 3.41 ** | 1.02 | 1,21 | 0.32 | 0.50 |
| Standard | 1.78 | (−0.24–3.80) | 11 | 2.73 * | 0.52 | ||||
| WHOQol | PLUS | −8.20 | (−16.46–0.06) | 12 | −1.83 | −0.46 | 1,22 | 0.35 | −0.34 |
| Standard | −9.55 | (−17.65–−1.45) | 11 | −3.17 ** | −0.80 | ||||
| ADS | PLUS | 6.70 | (−1.22–14.62) | 12 | 1.90 | 0.62 | 1,22 | 0.05 | 0.26 |
| Standard | 3.67 | (−0.94–8.28) | 11 | 2.12 | 0.36 | ||||
| TSK_RU | PLUS | 2.54 | (2.95–6.87) | 12 | 0.22 | 0.32 | 1,22 | 2.19 | −0.36 |
| Standard | 4.91 | (−2.00–7.07) | 11 | 4.11 ** | 0.68 | ||||
| TSK_SS | PLUS | −2.72 | (−6.39–1.01) | 12 | −1.23 | −0.42 | 1,22 | 0.24 | −0.09 |
| Standard | −3.33 | (−6.75–0.25) | 11 | −2.63 * | −0.51 | ||||
| TSK_BA | PLUS | −0.81 | (−2.73–4.42) | 12 | 0.53 | 0.14 | 1,22 | 0.22 | 0.06 |
| Standard | −0.50 | (−5.48–4.48) | 11 | −0.96 | −0.08 | ||||
| TSK_SI | PLUS | 1.43 | (−4.01–6.78) | 12 | 0.07 | 0.19 | 1,22 | 0.84 | 0.07 |
| Standard | 1.01 | (−4.51–6.51) | 11 | −0.65 | 0.12 | ||||
| TSK_SR | PLUS | 0.67 | (−0.74–1.97) | 12 | 0.77 | 0.13 | 1,22 | 1.18 | −0.08 |
| Standard | 0.81 | (−0.96–2.46) | 11 | −0.79 | 0.21 | ||||
| AQ_R | PLUS | −5.10 | (−7.65–−2.54) | 11 | −3.76 ** | −0.76 | 1,19 | 0.08 | 0.26 |
| Standard | −3.22 | (−6.29–−0.15) | 9 | −1.15 | −0.50 | ||||
| AQ_P | PLUS | −1.60 | (−4.97–1.77) | 12 | −1.52 | −0.30 | 1,22 | 0.73 | −0.21 |
| Standard | −2.67 | (−6.29–0.95) | 11 | −2.74 * | −0.51 | ||||
| RAS | PLUS | −0.40 | (−2.15–1.35) | 11 | −0.64 | −0.42 | 1,19 | 0.03 | 0.08 |
| Standard | −1.11 | (−3.33–1.11) | 9 | −1.15 | −0.34 | ||||
| ERSQ | PLUS | −7.30 | (−23.23–8.63) | 12 | −1.39 | −0.51 | 1,22 | 0.52 | −0.42 |
| Standard | −13.89 | (−24.86–−2.91) | 11 | −1.87 | −0.93 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urech, A.; Krieger, T.; Frischknecht, E.; Stalder-Lüthy, F.; grosse Holtforth, M.; Müri, R.M.; Znoj, H.; Hofer, H. An Integrative Neuro-Psychotherapy Treatment to Foster the Adjustment in Acquired Brain Injury Patients—A Randomized Controlled Study. J. Clin. Med. 2020, 9, 1684. https://doi.org/10.3390/jcm9061684
Urech A, Krieger T, Frischknecht E, Stalder-Lüthy F, grosse Holtforth M, Müri RM, Znoj H, Hofer H. An Integrative Neuro-Psychotherapy Treatment to Foster the Adjustment in Acquired Brain Injury Patients—A Randomized Controlled Study. Journal of Clinical Medicine. 2020; 9(6):1684. https://doi.org/10.3390/jcm9061684
Chicago/Turabian StyleUrech, Antoine, Tobias Krieger, Eveline Frischknecht, Franziska Stalder-Lüthy, Martin grosse Holtforth, René Martin Müri, Hansjörg Znoj, and Helene Hofer. 2020. "An Integrative Neuro-Psychotherapy Treatment to Foster the Adjustment in Acquired Brain Injury Patients—A Randomized Controlled Study" Journal of Clinical Medicine 9, no. 6: 1684. https://doi.org/10.3390/jcm9061684
APA StyleUrech, A., Krieger, T., Frischknecht, E., Stalder-Lüthy, F., grosse Holtforth, M., Müri, R. M., Znoj, H., & Hofer, H. (2020). An Integrative Neuro-Psychotherapy Treatment to Foster the Adjustment in Acquired Brain Injury Patients—A Randomized Controlled Study. Journal of Clinical Medicine, 9(6), 1684. https://doi.org/10.3390/jcm9061684





