Gamma Knife Radiosurgery Followed by Flow-Reductive Embolization for Ruptured Arteriovenous Malformation
Abstract
1. Introduction
2. Methods
2.1. Patient Characteristics
2.2. Gamma Knife Radiosurgery
2.3. Endovascular Embolization
2.4. Neuroimaging Follow-up and Outcomes Assessment
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics, AVM Characteristics, and Radiosurgical Parameters
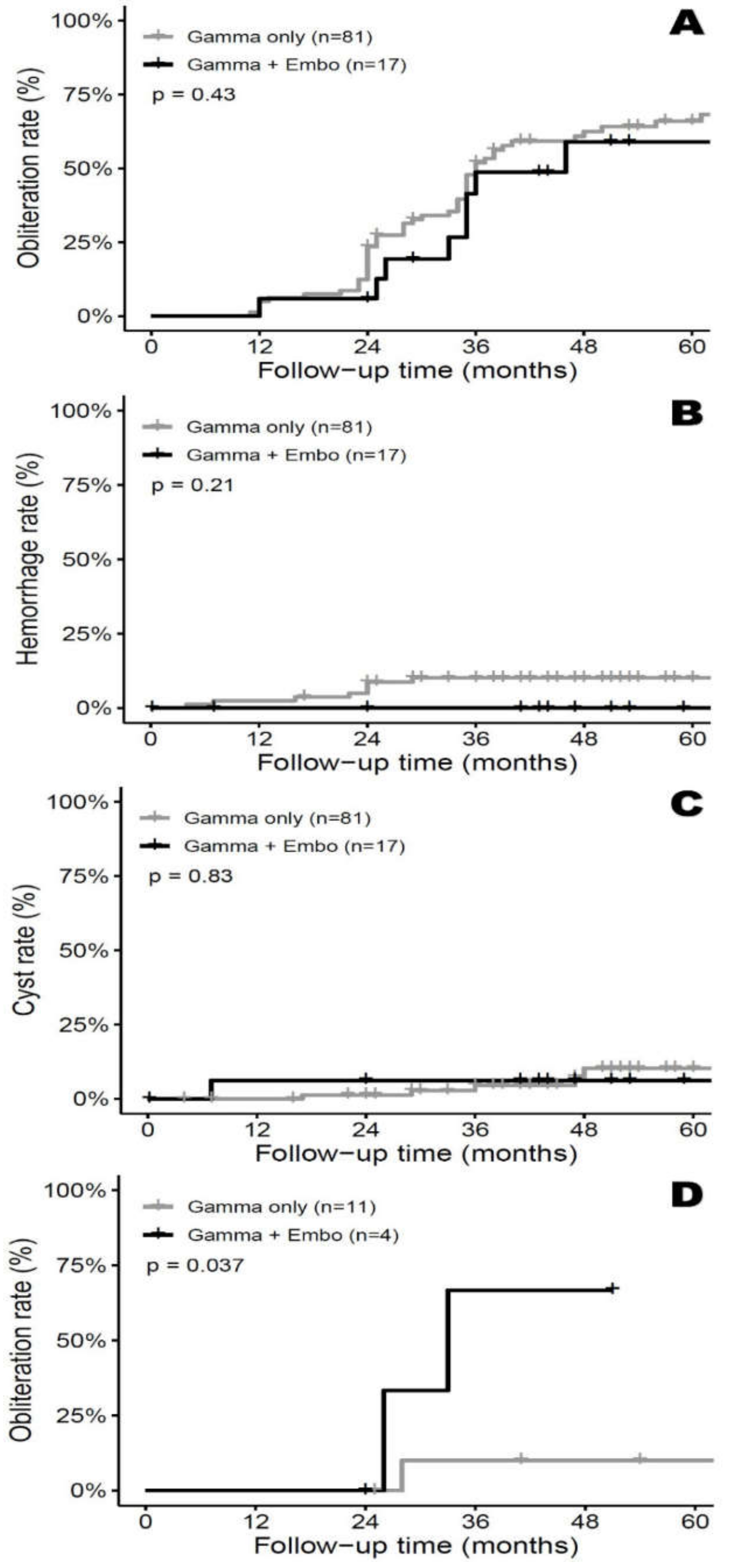
3.2. Imaging Outcome—AVM Obliteration
3.3. Complications – Repeat Hemorrhage/Delayed Cyst Formation
3.4. Subgroup Analysis—SM Grade III&IV
4. Case Illustration
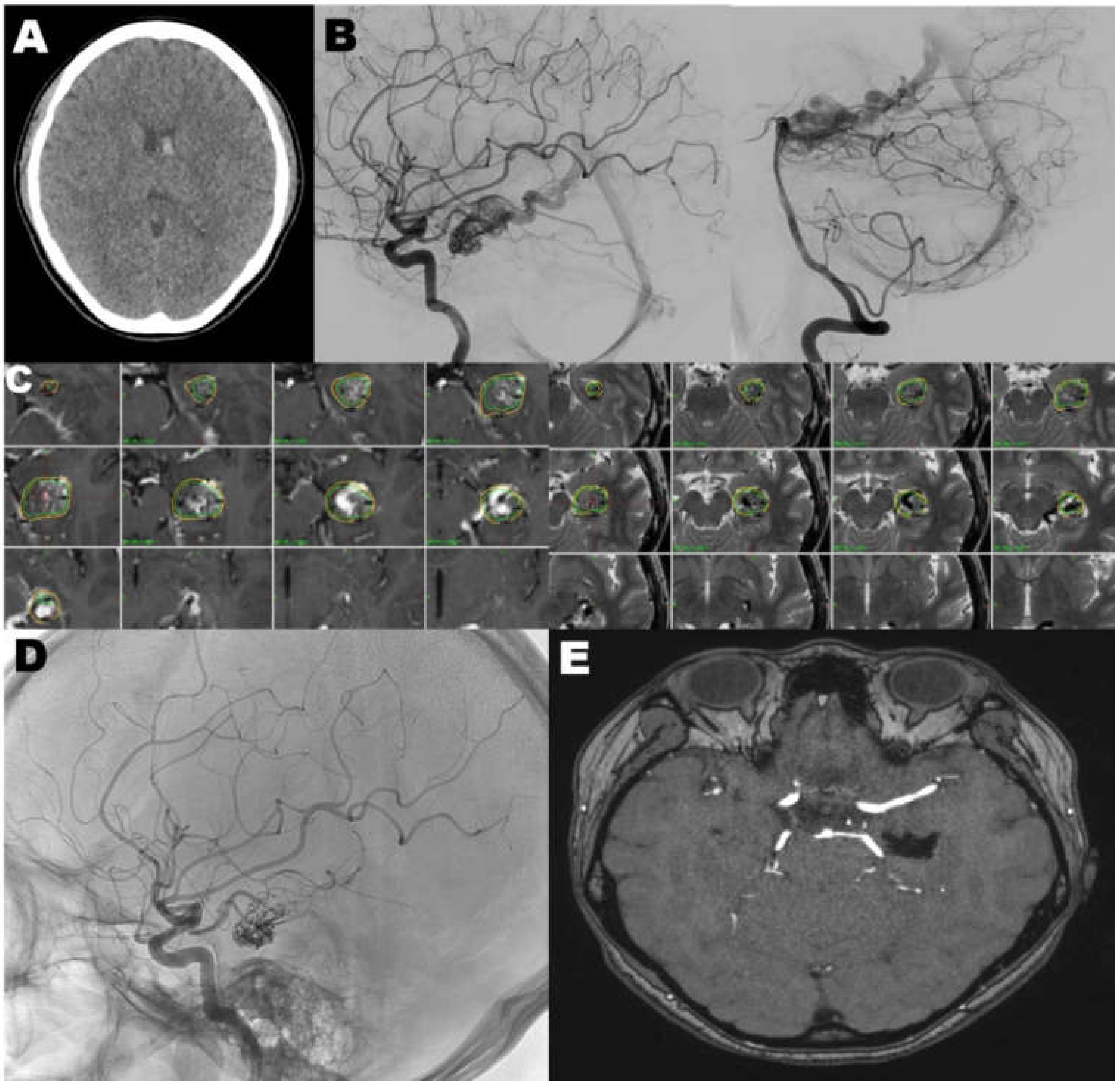
4.1. Case 1
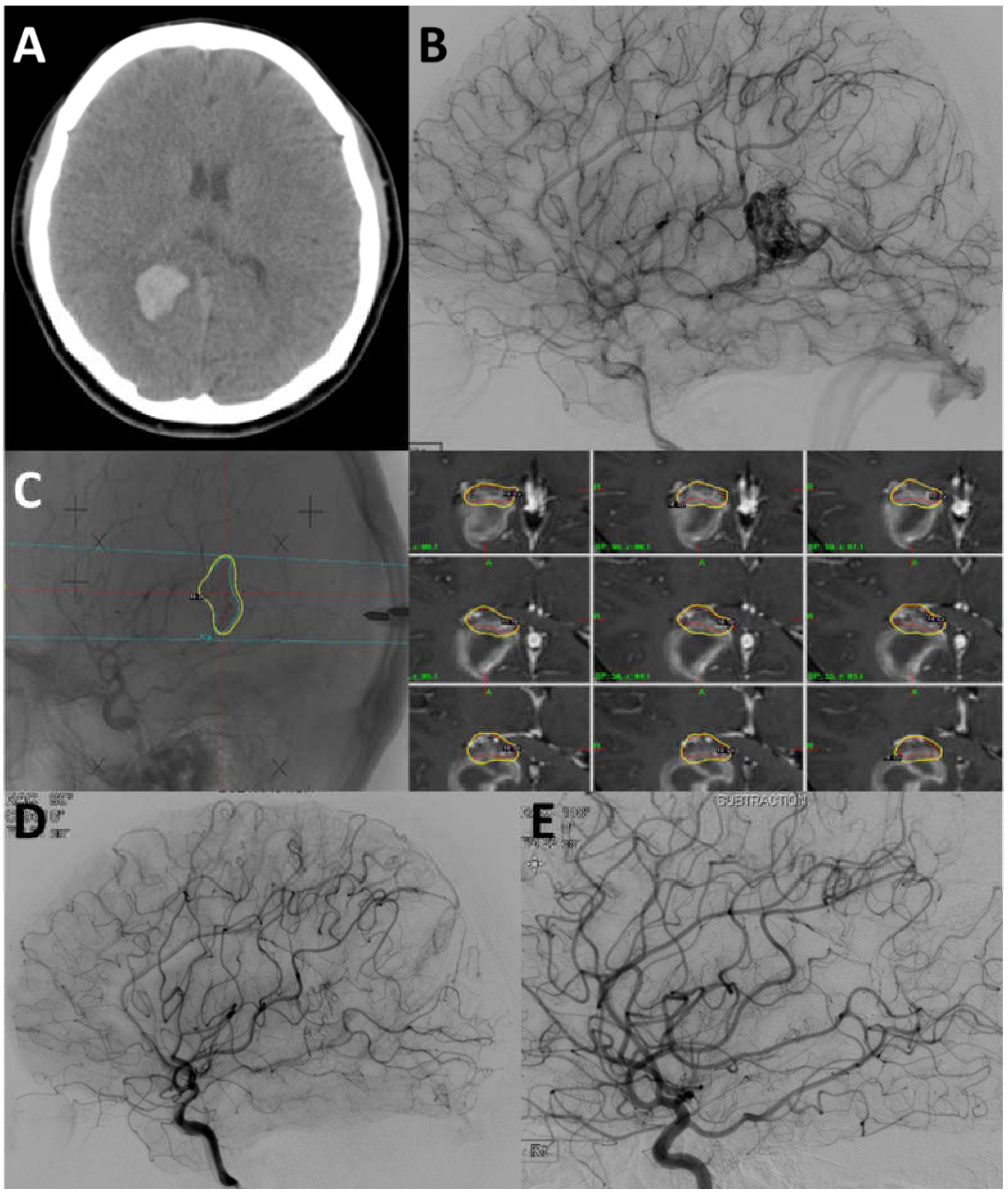
4.2. Case 2
5. Discussion
Study Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kano, H.; Kondziolka, D.; Flickinger, J.C.; Yang, H.C.; Flannery, T.J.; Awan, N.R.; Niranjan, A.; Novotny, J., Jr.; Lunsford, L.D. Stereotactic radiosurgery for arteriovenous malformations, part 3: Outcome predictors and risks after repeat radiosurgery. J. Neurosurg. 2012, 116, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Auger, R.G.; Wiebers, D.O. Management of unruptured intracranial arteriovenous malformations: A decision analysis. Neurosurgery 1992, 30, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.D., Jr.; Wiebers, D.O.; Forbes, G.; O’Fallon, W.M.; Piepgras, D.G.; Marsh, W.R.; Maciunas, R.J. The natural history of unruptured intracranial arteriovenous malformations. J. Neurosurg. 1988, 68, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.E.; Flickinger, J.C.; Lunsford, L.D.; Bissonette, D.J.; Kondziolka, D. Factors that predict the bleeding risk of cerebral arteriovenous malformations. Stroke 1996, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.E.; Flickinger, J.C.; Lunsford, L.D.; Bissonette, D.J.; Kondziolka, D. Hemorrhage risk after stereotactic radiosurgery of cerebral arteriovenous malformations. Neurosurgery 1996, 38, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Izawa, M.; Chernov, M.; Hayashi, M.; Iseki, H.; Hori, T.; Takakura, K. Combined management of intracranial arteriovenous malformations with embolization and gamma knife radiosurgery: Comparative evaluation of the long-term results. Surg. Neurol. 2009, 71, 43–52. [Google Scholar] [CrossRef]
- Solomon, R.A.; Connolly, E.S., Jr. Arteriovenous malformations of the brain. N. Engl. J. Med. 2017, 377, 498. [Google Scholar] [CrossRef]
- Da Costa, L.; Wallace, M.C.; Ter Brugge, K.G.; O’Kelly, C.; Willinsky, R.A.; Tymianski, M. The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke 2009, 40, 100–105. [Google Scholar] [CrossRef]
- Todnem, N.; Ward, A.; Nahhas, M.; Vender, J.R.; Alleyne, C.H.; Rahimi, S.Y. A retrospective cohort analysis of hemorrhagic arteriovenous malformations treated with combined endovascular embolization and gamma knife stereotactic radiosurgery. World Neurosurg. 2019, 122, e713–e722. [Google Scholar] [CrossRef]
- Kano, H.; Kondziolka, D.; Flickinger, J.C.; Park, K.J.; Iyer, A.; Yang, H.C.; Liu, X.; Monaco, E.A., III; Niranjan, A.; Lunsford, L.D. Stereotactic radiosurgery for arteriovenous malformations after embolization: A case-control study. J. Neurosurg. 2012, 117, 265–275. [Google Scholar] [CrossRef]
- Friedman, W.A.; Bova, F.J. Linear accelerator radiosurgery for arteriovenous malformations. J. Neurosurg. 1992, 77, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Jimbo, M.; Hara, M.; Saito, I.; Mori, K. Gamma knife radiosurgery for arteriovenous malformations: Long-term follow-up results focusing on complications occurring more than 5 years after irradiation. Neurosurgery 1996, 38, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Van Beijnum, J.; van der Worp, H.B.; Buis, D.R.; Al-Shahi Salman, R.; Kappelle, L.J.; Rinkel, G.J.; van der Sprenkel, J.W.; Vandertop, W.P.; Algra, A.; Klijn, C.J. Treatment of brain arteriovenous malformations: A systematic review and meta-analysis. JAMA 2011, 306, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Jiang, Y.; Lv, X.; Yang, H.; Zhao, Y.; Li, Y. Targeted embolization reduces hemorrhage complications in partially embolized cerebral avm combined with gamma knife surgery. Interv. Neuroradiol. 2015, 21, 80–87. [Google Scholar]
- Ogilvy, C.S. Radiation therapy for arteriovenous malformations: A review. Neurosurgery 1990, 26, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Jeon, S.R.; Kim, J.H.; Lee, J.K.; Ra, D.S.; Lee, D.J.; Kwun, B.D. Analysis of the causes of treatment failure in gamma knife radiosurgery for intracranial arteriovenous malformations. J. Neurosurg. 2000, 93 (Suppl. 3), 104–106. [Google Scholar] [CrossRef]
- Andrade-Souza, Y.M.; Ramani, M.; Scora, D.; Tsao, M.N.; terBrugge, K.; Schwartz, M.L. Embolization before radiosurgery reduces the obliteration rate of arteriovenous malformations. Neurosurgery 2007, 60, 443–452. [Google Scholar] [CrossRef]
- Sure, U.; Battenberg, E.; Dempfle, A.; Tirakotai, W.; Bien, S.; Bertalanffy, H. Hypoxia-inducible factor and vascular endothelial growth factor are expressed more frequently in embolized than in nonembolized cerebral arteriovenous malformations. Neurosurgery 2004, 55, 663–669. [Google Scholar] [CrossRef]
- Hung, Y.C.; Mohammed, N.; Eluvathingal Muttikkal, T.J.; Kearns, K.N.; Li, C.E.; Narayan, A.; Schlesinger, D.; Xu, Z.; Sheehan, J.P. The impact of preradiosurgery embolization on intracranial arteriovenous malformations: A matched cohort analysis based on de novo lesion volume. J. Neurosurg. 2019, 1–12. [Google Scholar] [CrossRef]
- Lee, C.C.; Chen, C.J.; Ball, B.; Schlesinger, D.; Xu, Z.; Yen, C.P.; Sheehan, J. Stereotactic radiosurgery for arteriovenous malformations after onyx embolization: A case-control study. J. Neurosurg. 2015, 123, 126–135. [Google Scholar] [CrossRef]
- Oermann, E.K.; Ding, D.; Yen, C.P.; Starke, R.M.; Bederson, J.B.; Kondziolka, D.; Sheehan, J.P. Effect of prior embolization on cerebral arteriovenous malformation radiosurgery outcomes: A case-control study. Neurosurgery 2015, 77, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Yen, C.P.; Starke, R.M.; Xu, Z.; Sun, X.; Sheehan, J.P. Outcomes following single-session radiosurgery for high-grade intracranial arteriovenous malformations. Br. J. Neurosurg. 2014, 28, 666–674. [Google Scholar] [CrossRef]
- Huo, X.; Jiang, Y.; Lv, X.; Yang, H.; Zhao, Y.; Li, Y. Gamma knife surgical treatment for partially embolized cerebral arteriovenous malformations. J. Neurosurg. 2016, 124, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Starke, R.M.; Kano, H.; Ding, D.; Lee, J.Y.; Mathieu, D.; Whitesell, J.; Pierce, J.T.; Huang, P.P.; Kondziolka, D.; Yen, C.P.; et al. Stereotactic radiosurgery for cerebral arteriovenous malformations: Evaluation of long-term outcomes in a multicenter cohort. J. Neurosurg. 2017, 126, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Lunsford, L.D.; Kondziolka, D.; Flickinger, J.C.; Bissonette, D.J.; Jungreis, C.A.; Maitz, A.H.; Horton, J.A.; Coffey, R.J. Stereotactic radiosurgery for arteriovenous malformations of the brain. J. Neurosurg. 1991, 75, 512–524. [Google Scholar] [CrossRef]
- Thompson, R.C.; Steinberg, G.K.; Levy, R.P.; Marks, M.P. The management of patients with arteriovenous malformations and associated intracranial aneurysms. Neurosurgery 1998, 43, 202–211. [Google Scholar] [CrossRef]
- Wilkins, R.H. Natural history of intracranial vascular malformations: A review. Neurosurgery 1985, 16, 421–430. [Google Scholar] [CrossRef]
- Batjer, H.; Suss, R.A.; Samson, D. Intracranial arteriovenous malformations associated with aneurysms. Neurosurgery 1986, 18, 29–35. [Google Scholar] [CrossRef]
- Hodgson, T.J.; Kemeny, A.A.; Gholkar, A.; Deasy, N. Embolization of residual fistula following stereotactic radiosurgery in cerebral arteriovenous malformations. Ajnr. Am. J. Neuroradiol. 2009, 30, 109–110. [Google Scholar] [CrossRef]
- Ogilvy, C.S.; Stieg, P.E.; Awad, I.; Brown, R.D., Jr.; Kondziolka, D.; Rosenwasser, R.; Young, W.L.; Hademenos, G. Aha scientific statement: Recommendations for the management of intracranial arteriovenous malformations: A statement for healthcare professionals from a special writing group of the stroke council, american stroke association. Stroke 2001, 32, 1458–1471. [Google Scholar] [CrossRef]
- Friedman, W.A.; Bova, F.J.; Mendenhall, W.M. Linear accelerator radiosurgery for arteriovenous malformations: The relationship of size to outcome. J. Neurosurg. 1995, 82, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.E.; Flickinger, J.C.; Lunsford, L.D.; Maitz, A.; Kondziolka, D. Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery 1998, 42, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, R.F.; Martin, N.A. A proposed grading system for arteriovenous malformations. J. Neurosurg. 1986, 65, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, A.; Chen, C.J.; Ding, D.; Mastorakos, P.; Taylor, D.G.; Pomeraniec, I.J.; Lee, C.C.; Sheehan, J. Cyst formation after stereotactic radiosurgery for brain arteriovenous malformations: A systematic review. J. Neurosurg. 2018, 128, 1354–1363. [Google Scholar] [CrossRef]
- Bir, S.C.; Ambekar, S.; Maiti, T.K.; Nanda, A. Clinical outcome and complications of gamma knife radiosurgery for intracranial arteriovenous malformations. J. Clin. Neurosci. 2015, 22, 1117–1122. [Google Scholar] [CrossRef]
- Shuto, T.; Yagishita, S.; Matsunaga, S. Pathological characteristics of cyst formation following gamma knife surgery for arteriovenous malformation. Acta Neurochir. 2015, 157, 293–298. [Google Scholar] [CrossRef]
- Bowden, G.; Kano, H.; Tonetti, D.; Niranjan, A.; Flickinger, J.; Arai, Y.; Lunsford, L.D. Stereotactic radiosurgery for sylvian fissure arteriovenous malformations with emphasis on hemorrhage risks and seizure outcomes. J. Neurosurg. 2014, 121, 637–644. [Google Scholar] [CrossRef]
- Matsuo, T.; Kamada, K.; Izumo, T.; Hayashi, N.; Nagata, I. Cyst formation after linac-based radiosurgery for arteriovenous malformation: Examination of predictive factors using magnetic resonance imaging. Clin. Neurol. Neurosurg. 2014, 121, 10–16. [Google Scholar] [CrossRef]
- Kano, H.; Kondziolka, D.; Flickinger, J.C.; Yang, H.C.; Flannery, T.J.; Niranjan, A.; Novotny, J., Jr.; Lunsford, L.D. Stereotactic radiosurgery for arteriovenous malformations, part 5: Management of brainstem arteriovenous malformations. J. Neurosurg. 2012, 116, 44–53. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, S.I.; Sim, J.H. A case of very large cyst formation with gamma knife radiosurgery for an arteriovenous malformation. Stereotact. Funct. Neurosurg. 1999, 72 (Suppl. 1), 168–174. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kato, T.; Naito, T.; Tanei, T.; Torii, J.; Ishii, K.; Tsukamoto, E.; Hatanaka, K.C.; Sugiyama, T. Long-term outcomes for pediatric patients with brain arteriovenous malformations treated with gamma knife radiosurgery, part 2: The incidence of cyst formation, encapsulated hematoma, and radiation-induced tumor. World Neurosurg. 2019, 126, e1526–e1536. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Sheehan, J.; Stroila, M.; Steiner, M.; Steiner, L. Late cyst formation following gamma knife surgery of arteriovenous malformations. J. Neurosurg. 2005, 102, 124–127. [Google Scholar] [CrossRef] [PubMed]
| Non-Embolized (n = 81) | Embolized (n = 17) | p-Value | |
|---|---|---|---|
| Median age | 22 (14, 38) | 39 (29, 48) | 0.0045 |
| Sex | 0.3073 | ||
| Male | 49 (60.5%) | 8 (47.1%) | |
| Female | 32 (39.5%) | 9 (52.9%) | |
| Median FU time, month | 47 (36, 67) | 47 (41, 59) | 0.9738 |
| Median marginal dose, Gy | 16 (15, 18) | 16 (16, 17) | 0.6685 |
| Median isodense line | 50% (50.0%) | 50% (50.0%) | |
| Median nidus volume, cm3 | 2.14 (0.87, 5.41) | 3.30 (2.10, 5.70) | 0.4084 |
| Median remnant nidus volume, cm3 | 0 (0, 0.40) | 0.29 (0, 0.90) | 0.1792 |
| Median delta nidus volume, % | 100 (89.2, 100) | 89.2 (81.8, 100) | 0.1221 |
| VRAS | 0.1161 | ||
| 0 | 0 (0%) | 0 (0%) | |
| 1 | 34 (42.0%) | 3 (17.6%) | |
| 2 | 13 (16.0%) | 3 (17.6%) | |
| 3 | 20 (24.7%) | 8 (47.2%) | |
| 4 | 14 (17.3%) | 3 (17.6%) | |
| Pollock-Flickinger score | 0.95 (0.59, 1.37) | 1.30 (1.05, 1.95) | 0.0316 |
| <1 | 44 (54.3%) | 4 (23.5%) | |
| 1.01–1.50 | 20 (24.7%) | 5 (29.4%) | |
| 1.51–2.00 | 6 (7.4%) | 5 (29.4%) | |
| >2 | 11 (13.6%) | 3 (17.7%) | |
| SM grade | 0.0023 | ||
| I | 42 (51.8%) | 2 (11.8%) | |
| II | 28 (34.6%) | 11 (64.7%) | |
| III | 11 (13.6%) | 3 (17.6%) | |
| IV | 0 (0%) | 1 (5.9%) | |
| Eloquence | 0.029 | ||
| Noneloquent | 67 (82.7%) | 10 (58.8%) | |
| Eloquent | 14 (17.3%) | 7 (41.2%) | |
| Venous drainage | |||
| Superficial only | 62 (76.5%) | 10 (58.8%) | 0.132 |
| Deep | 19 (23.5%) | 7 (41.2%) | |
| Presence of Aneurysm | 12 (14.8%) | 8 (47.0%) | 0.0060 |
| Intranidal | 12 (14.8%) | 6 (35.3%) | |
| Flow-related | 0 (0%) | 2 (11.7%) |
| Obliteration | Hemorrhage | Cyst Formation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Univariate Analysis | Univariate Analysis | |||||||
| Factors | HR | CI | p-Value | HR | CI | p-Value | HR | CI | p-Value |
| Post GKRS Embo | 0.746 | (0.354, 1.574) | 0.4418 | 0.302 | (0.015, 6.220) | 0.4377 | 0.798 | (0.096, 6.633) | 0.8346 |
| Age | 0.992 | (0.977, 1.008) | 0.3486 | 1.010 | (0.971, 1.049) | 0.6305 | 0.990 | (0.942, 1.040) | 0.6785 |
| Female sex | 1.268 | (0.757, 2.122) | 0.3671 | 0.462 | (0.093, 2.291) | 0.3448 | 1.097 | (0.245, 4.921) | 0.9033 |
| Marginal dose, Gy | 1.010 | (0.983, 1.039) | 0.4621 | 0.438 | (0.259, 0.741) | 0.0021 | 0.605 | (0.340, 1.075) | 0.0865 |
| Nidus volume, cm3 | 0.981 | (0.971, 0.991) | 0.0003 | 1.010 | (1.006, 1.015) | <0.0001 | 1.009 | (1.002, 1.015) | 0.0119 |
| Remnant nidus volume, cm3 | 0.940 | (0.737, 1.199) | 0.6187 | 1.013 | (1.006, 1.021) | 0.0003 | 1.013 | (0.998, 1.027) | 0.0855 |
| Delta nidus volume, % | 20.168 | (0.132, 3085.252) | 0.2418 | 0.956 | (0.933, 0.979) | 0.0002 | 0.959 | (0.924, 0.996) | 0.0304 |
| Higher SM grade | 0.480 | (0.319, 0.721) | 0.0004 | 3.082 | (1.359, 6.986) | 0.0070 | 1.216 | (0.438, 3.380) | 0.7073 |
| Higher VRAS | 0.579 | (0.449, 0.747) | <0.0001 | 3.759 | (1.448, 9.757) | 0.0065 | 1.192 | (0.597, 2.378) | 0.6185 |
| Higher Pollock–Flickinger score | 0.390 | (0.246, 0.619) | <0.0001 | 2.819 | (1.674, 4.746) | <0.0001 | 2.066 | (0.992, 4.304) | 0.0525 |
| Aneurysm | 1.031 | (0.546, 1.947) | 0.9255 | 1.276 | (0.257, 6.321) | 0.7655 | 0.670 | (0.080, 5.595) | 0.7113 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Park, S.H.; Park, K.Y.; Jung, H.H.; Chang, J.H.; Chang, J.W.; Lee, J.W.; Chang, W.S. Gamma Knife Radiosurgery Followed by Flow-Reductive Embolization for Ruptured Arteriovenous Malformation. J. Clin. Med. 2020, 9, 1318. https://doi.org/10.3390/jcm9051318
Kim MJ, Park SH, Park KY, Jung HH, Chang JH, Chang JW, Lee JW, Chang WS. Gamma Knife Radiosurgery Followed by Flow-Reductive Embolization for Ruptured Arteriovenous Malformation. Journal of Clinical Medicine. 2020; 9(5):1318. https://doi.org/10.3390/jcm9051318
Chicago/Turabian StyleKim, Myung Ji, So Hee Park, Keun Young Park, Hyun Ho Jung, Jong Hee Chang, Jin Woo Chang, Jae Whan Lee, and Won Seok Chang. 2020. "Gamma Knife Radiosurgery Followed by Flow-Reductive Embolization for Ruptured Arteriovenous Malformation" Journal of Clinical Medicine 9, no. 5: 1318. https://doi.org/10.3390/jcm9051318
APA StyleKim, M. J., Park, S. H., Park, K. Y., Jung, H. H., Chang, J. H., Chang, J. W., Lee, J. W., & Chang, W. S. (2020). Gamma Knife Radiosurgery Followed by Flow-Reductive Embolization for Ruptured Arteriovenous Malformation. Journal of Clinical Medicine, 9(5), 1318. https://doi.org/10.3390/jcm9051318





