Age-Dependent Efficacy of Ezetimibe for Low-Density Lipoprotein Cholesterol Reduction in Japanese Patients with or without Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Statistical Analysis
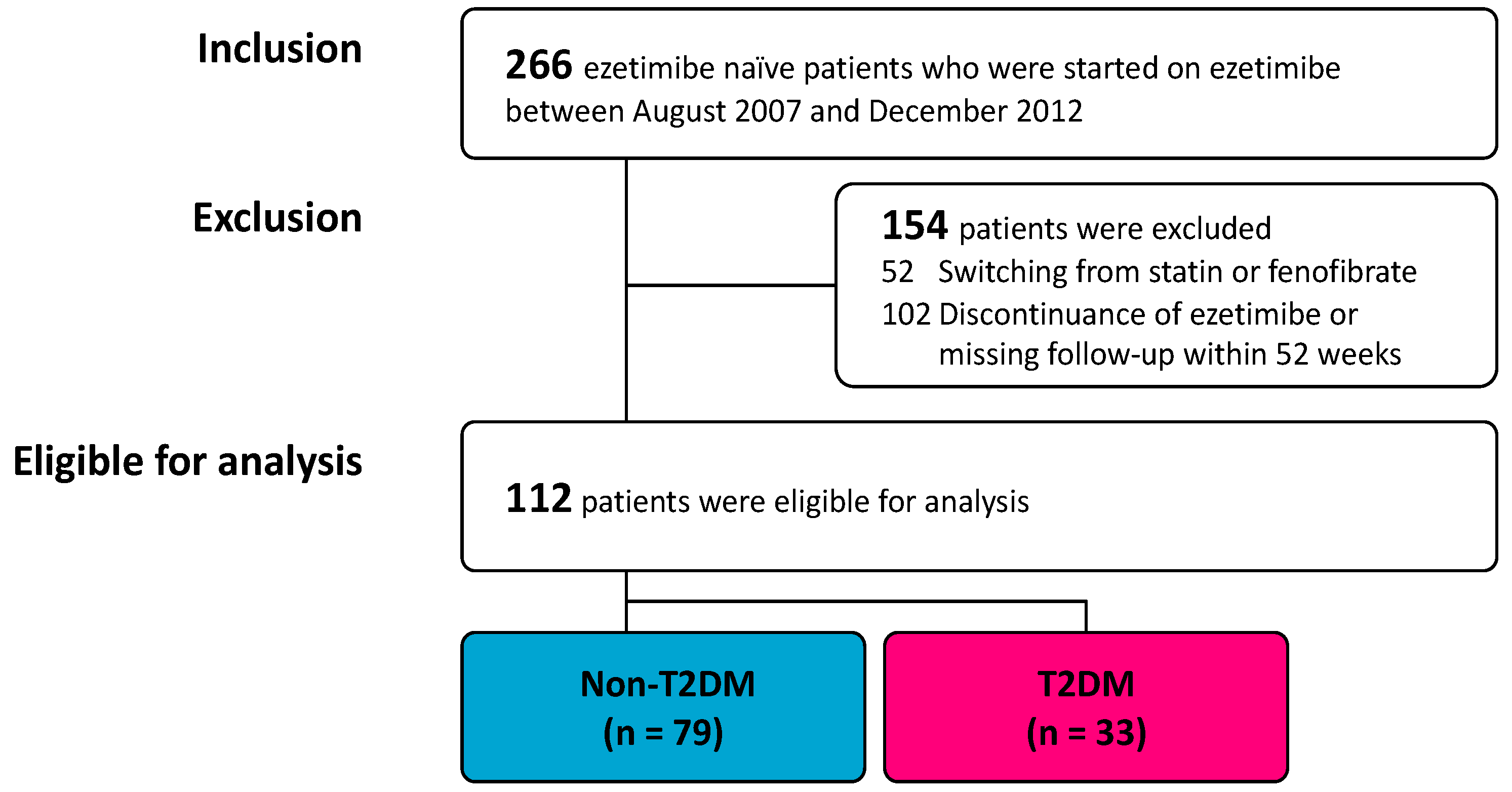
3. Results
3.1. Participants
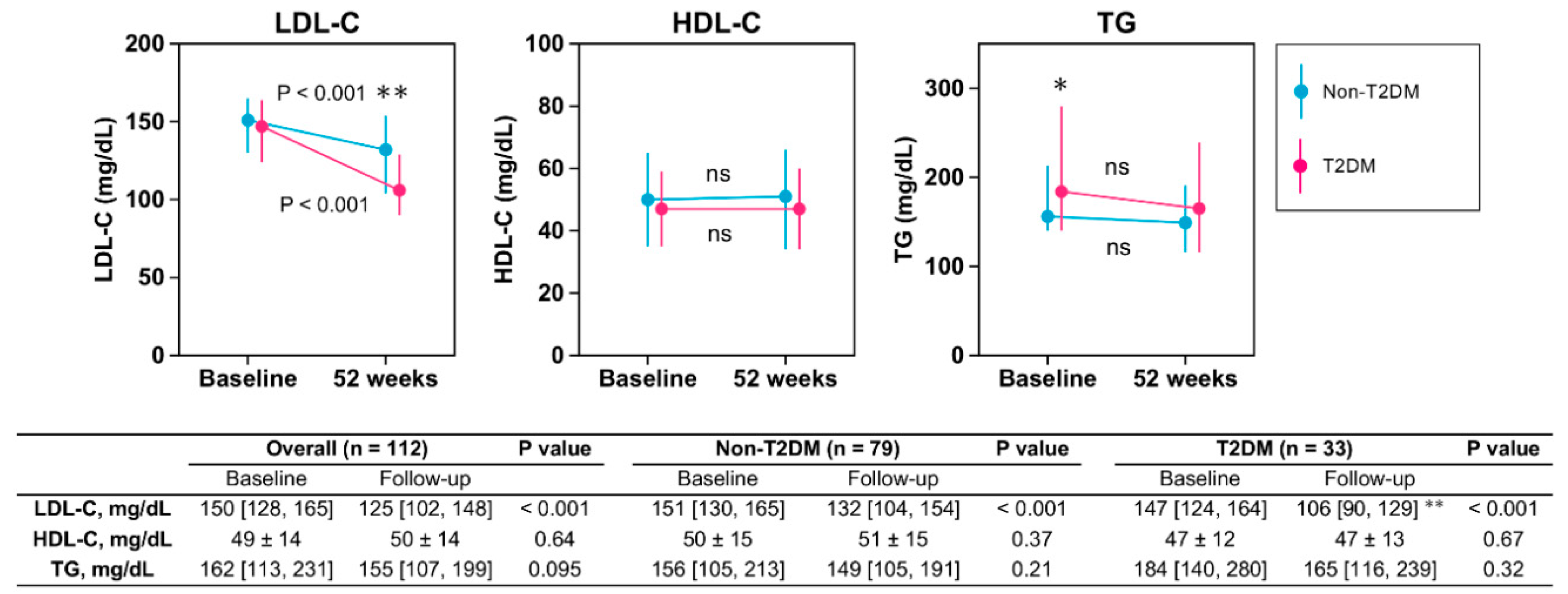
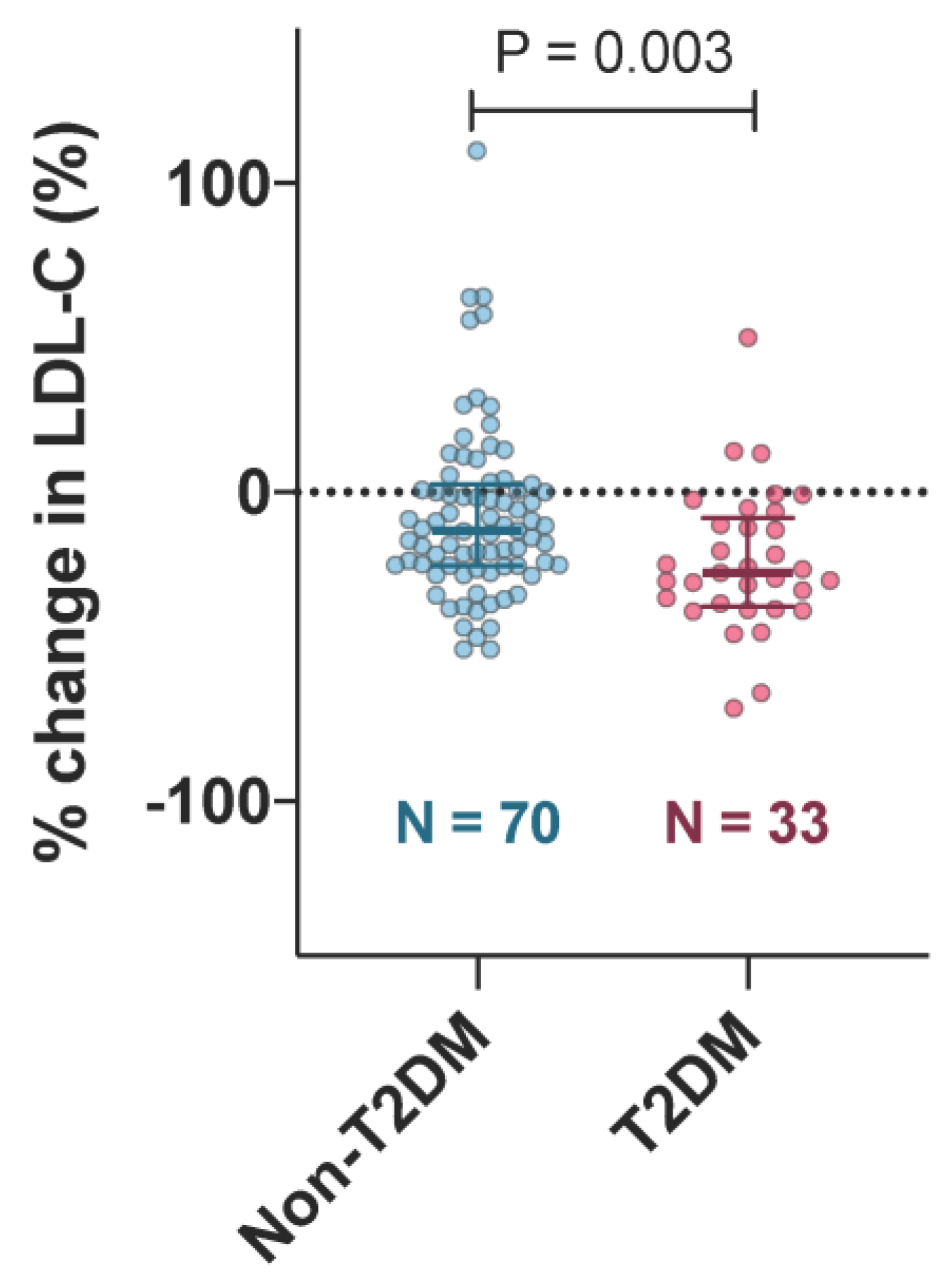
3.2. Lipid Profile
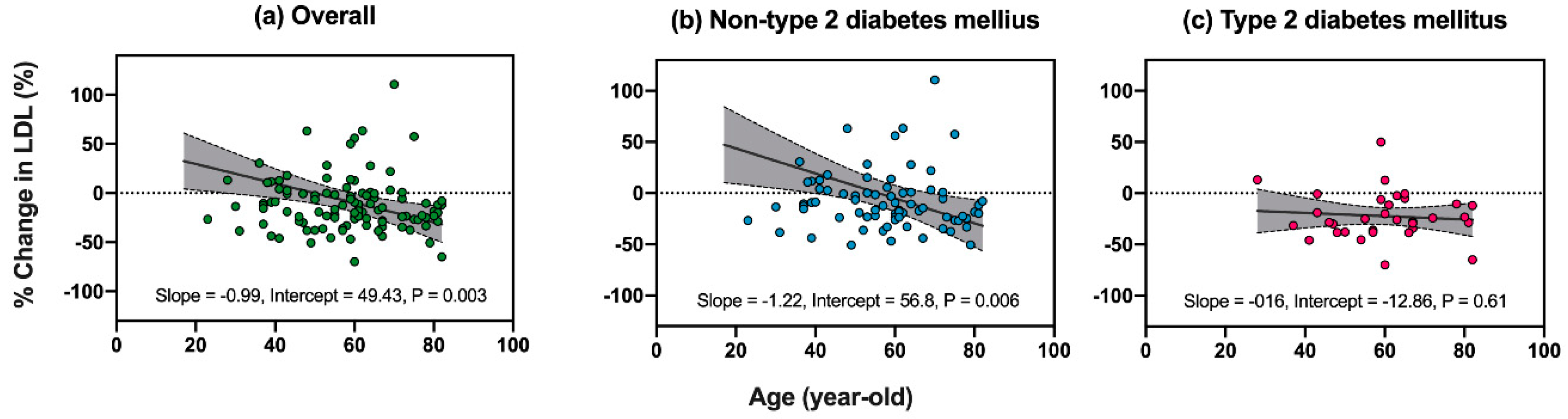
3.3. Factors Associated with Reductions in LDL-C after Ezetimibe Treatment
3.4. Post Hoc Power Calculation
4. Discussion
4.1. Factors Influencing the Efficacy of Ezetimibe
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nelson, R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care Clin. Off. Pract. 2012, 40, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar]
- Stone, n.J.; Robinson, J.G.; Lichtenstein, A.H.; Merz, C.N.B.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.; et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. J. Am. Coll. Cardiol. 2013, 129, S1–S45. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Tangney, C.C. Antiatherothrombotic properties of statins: Implications for cardiovascular event reduction. JAMA 1998, 279, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Reith, C.; Armitage, J. Management of residual risk after statin therapy. Atherosclerosis 2016, 245, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Steinberg, B.A.; Murphy, S.A.; Mega, J.L.; Braunwald, E. Meta-Analysis of Cardiovascular Outcomes Trials Comparing Intensive Versus Moderate Statin Therapy. J. Am. Coll. Cardiol. 2006, 48, 438–445. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, F.H.; Patel, D.D.; Knight, B.L.; Neuwirth, C.K.Y.; Bourbon, M.; Soutar, A.K.; Taylor, G.W.; Thompson, G.R.; Naoumova, R.P. Determinants of Variable Response to Statin Treatment in Patients With Refractory Familial Hypercholesterolemia. Arter. Thromb. Vasc. Boil. 2001, 21, 832–837. [Google Scholar] [CrossRef][Green Version]
- Roberts, W.C. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am. J. Cardiol. 1997, 80, 106–107. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Théroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Toth, P.P.; Phan, B.A.P.; Dayspring, T.D. Ezetimibe therapy: Mechanism of action and clinical update. Vasc. Heal. Risk Manag. 2012, 8, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Knopp, R.H.; Gitter, H.; Truitt, T.; Bays, H.; Manion, C.; Lipka, L.; LeBeaut, A.; Suresh, R.; Yang, B.; Veltri, E. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur. Heart J. 2003, 24, 729–741. [Google Scholar] [CrossRef]
- Nutescu, E.A.; Shapiro, N.L. Ezetimibe: A Selective Cholesterol Absorption Inhibitor. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2003, 23, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Leiter, L.A.; Betteridge, D.J.; Farnier, M.; Guyton, J.R.; Lin, J.; Shah, A.; Johnson-Levonas, A.O.; Brudi, P. Lipid-altering efficacy and safety profile of combination therapy with ezetimibe/statin vs. statin monotherapy in patients with and without diabetes: An analysis of pooled data from 27 clinical trials. Diabetes Obes. Metab. 2011, 13, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Cannon, C.P.; Blazing, M.A.; Nicolau, J.C.; Corbalán, R.; Špinar, J.; Park, J.-G.; White, J.A.; Bohula, E.A.; Braunwald, E. Benefit of Adding Ezetimibe to Statin Therapy on Cardiovascular Outcomes and Safety in Patients With Versus Without Diabetes Mellitus. Circulation 2018, 137, 1571–1582. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Yagyu, H.; Kotani, K.; Miyamoto, M.; Osuga, J.-I.; Nagasaka, S.; Ishibashi, S. Lipid-lowering effects of ezetimibe for hypercholesterolemic patients with and without type 2 diabetes mellitus. Endocr. J. 2010, 57, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Fernández, J.M.; Bedarida, G.V.; Adgey, J.; Allen, C.; Johnson-Levonas, A.O.; Massaad, R. Efficacy and safety of ezetimibe co-administered with ongoing atorvastatin therapy in achieving low-density lipoprotein goal in patients with hypercholesterolemia and coronary heart disease. Int. J. Clin. Pract. 2005, 59, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Shieh, G.; Jan, S.-L.; Randles, R.H. On power and sample size determinations for the Wilcoxon–Mann–Whitney test. J. Nonparametric Stat. 2006, 18, 33–43. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Goldberg, R.B.; Guyton, J.R.; Mazzone, T.; Weinstock, R.S.; Polis, A.B.; Tipping, D.; Tomassini, J.E.; Tershakovec, A.M. Relationships Between Metabolic Syndrome and Other Baseline Factors and the Efficacy of Ezetimibe/Simvastatin and Atorvastatin in Patients With Type 2 Diabetes and Hypercholesterolemia. Diabetes Care 2010, 33, 1021–1024. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hammersley, D.; Signy, M. Ezetimibe: An update on its clinical usefulness in specific patient groups. Ther. Adv. Chronic Dis. 2016, 8, 4–11. [Google Scholar] [CrossRef] [PubMed]
| Overall | Non-T2DM | T2DM | p Value | |
|---|---|---|---|---|
| n = 112 | n = 79 | n = 33 | ||
| Age, years | 60 (49, 67) | 59 (49, 68) | 60 (50, 67) | 0.5 |
| Male gender, n (%) | 64/112 (56) | 41/79 (52) | 22/33 (67) | 0.21 |
| Body weight, kg | 66 (57, 74) | 67 (55, 72) | 65 (57, 76) | 0.6 |
| Body mass index, kg/m2 | 25.5 (23.4, 28.1) | 25.4 (22.9, 28) | 25.9 (23.8, 28.1) | 0.61 |
| Past medical history, n (%) | ||||
| Hypertension | 46/112 (41) | 20/79 (25) | 26/33 (79) | <0.001 |
| Coronary artery disease | 15/112 (13) | 4/79 (5.1) | 11/33 (33) | <0.001 |
| Chronic kidney disease | 26/112 (23) | 14/79 (18) | 12/33 (36) | <0.001 |
| Hemodialysis | 16/112 (14) | 4/79 (5.1) | 12/33 (36) | <0.001 |
| Overall (n = 112) | ||||||||
| Univariate | Multivariate | |||||||
| Adj R2 = 0.19, p < 0.001 | ||||||||
| Coefficient | 95% CI | p Value | Coefficient | 95% CI | p Value | |||
| Age, 1 year | −0.99 | −1.64 | −0.34 | 0.003 | −0.75 | −1.37 | −0.14 | 0.016 |
| Male gender | −12.94 | −32.07 | 6.19 | 0.19 | ||||
| Type 2 diabetes mellitus | −20.3 | −40.93 | 0.34 | 0.056 | −14.51 | −36.22 | 7.19 | 0.19 |
| Hypertension | −17.36 | −36.53 | 1.81 | 0.079 | −10.45 | −30.5 | 9.6 | 0.31 |
| Coronary artery disease | −11.06 | −39.06 | 16.95 | 0.44 | ||||
| Statin pretreatment | 5.36 | −15.25 | 25.97 | 0.61 | ||||
| Baseline LDL-C, mg/dL | −0.52 | −0.77 | −0.27 | <0.001 | −0.48 | −0.72 | −0.24 | <0.001 |
| Non-Type 2 Diabetes Mellitus (n = 79) | ||||||||
| Univariate | Multivariate | |||||||
| Adj R2 = 0.17, p < 0.001 | ||||||||
| Coefficient | p Value | Coefficient | 95% CI | p Value | ||||
| Age, 1 year | −1.22 | −2.07 | −0.37 | 0.006 | −0.97 | −1.79 | −0.14 | 0.024 |
| Male gender | −11.97 | −37.82 | 13.88 | 0.37 | ||||
| Hypertension | −14.20 | −43.89 | 15.49 | 0.35 | ||||
| Coronary artery disease | −0.17 | −59.39 | 59.06 | >0.99 | ||||
| Statin pretreatment | 13.1 | −14.98 | 41.18 | 0.36 | ||||
| Baseline LDL-C, mg/dL | −0.58 | −0.9 | −0.25 | <0.001 | −0.5 | −0.82 | −0.18 | 0.003 |
| Type 2 Diabetes mellitus (n = 33) | ||||||||
| Univariate | Multivariate | |||||||
| Coefficient | p Value | |||||||
| Age, 1 year | −0.16 | −0.76 | 0.44 | 0.61 | ||||
| Male gender | −6.92 | −23.6 | 9.76 | 0.42 | ||||
| Hypertension | −1.07 | −20.51 | 18.36 | 0.91 | Univariate linear regression models did not reveal any significant factor | |||
| Coronary artery disease | −1.29 | −18.14 | 15.57 | 0.88 | ||||
| Statin pretreatment | −10.37 | −26.83 | 6.09 | 0.23 | ||||
| Baseline LDL-C, mg/dL | −0.39 | −0.59 | −0.18 | <0.001 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, S.; Oba, K.; Higa, M.; Arasaki, O.; Shimabukuro, M. Age-Dependent Efficacy of Ezetimibe for Low-Density Lipoprotein Cholesterol Reduction in Japanese Patients with or without Type 2 Diabetes Mellitus. J. Clin. Med. 2020, 9, 1675. https://doi.org/10.3390/jcm9061675
Yamaguchi S, Oba K, Higa M, Arasaki O, Shimabukuro M. Age-Dependent Efficacy of Ezetimibe for Low-Density Lipoprotein Cholesterol Reduction in Japanese Patients with or without Type 2 Diabetes Mellitus. Journal of Clinical Medicine. 2020; 9(6):1675. https://doi.org/10.3390/jcm9061675
Chicago/Turabian StyleYamaguchi, Satoshi, Kageyuki Oba, Moritake Higa, Osamu Arasaki, and Michio Shimabukuro. 2020. "Age-Dependent Efficacy of Ezetimibe for Low-Density Lipoprotein Cholesterol Reduction in Japanese Patients with or without Type 2 Diabetes Mellitus" Journal of Clinical Medicine 9, no. 6: 1675. https://doi.org/10.3390/jcm9061675
APA StyleYamaguchi, S., Oba, K., Higa, M., Arasaki, O., & Shimabukuro, M. (2020). Age-Dependent Efficacy of Ezetimibe for Low-Density Lipoprotein Cholesterol Reduction in Japanese Patients with or without Type 2 Diabetes Mellitus. Journal of Clinical Medicine, 9(6), 1675. https://doi.org/10.3390/jcm9061675





