The Neurokinin-1 Receptor Antagonist Aprepitant, a New Drug for the Treatment of Hematological Malignancies: Focus on Acute Myeloid Leukemia
Abstract
:1. Introduction
2. The SP/NK-1R System as a Predictive Factor in Cancer and the Use of the NK-1R as a Potential Tumor Biomarker
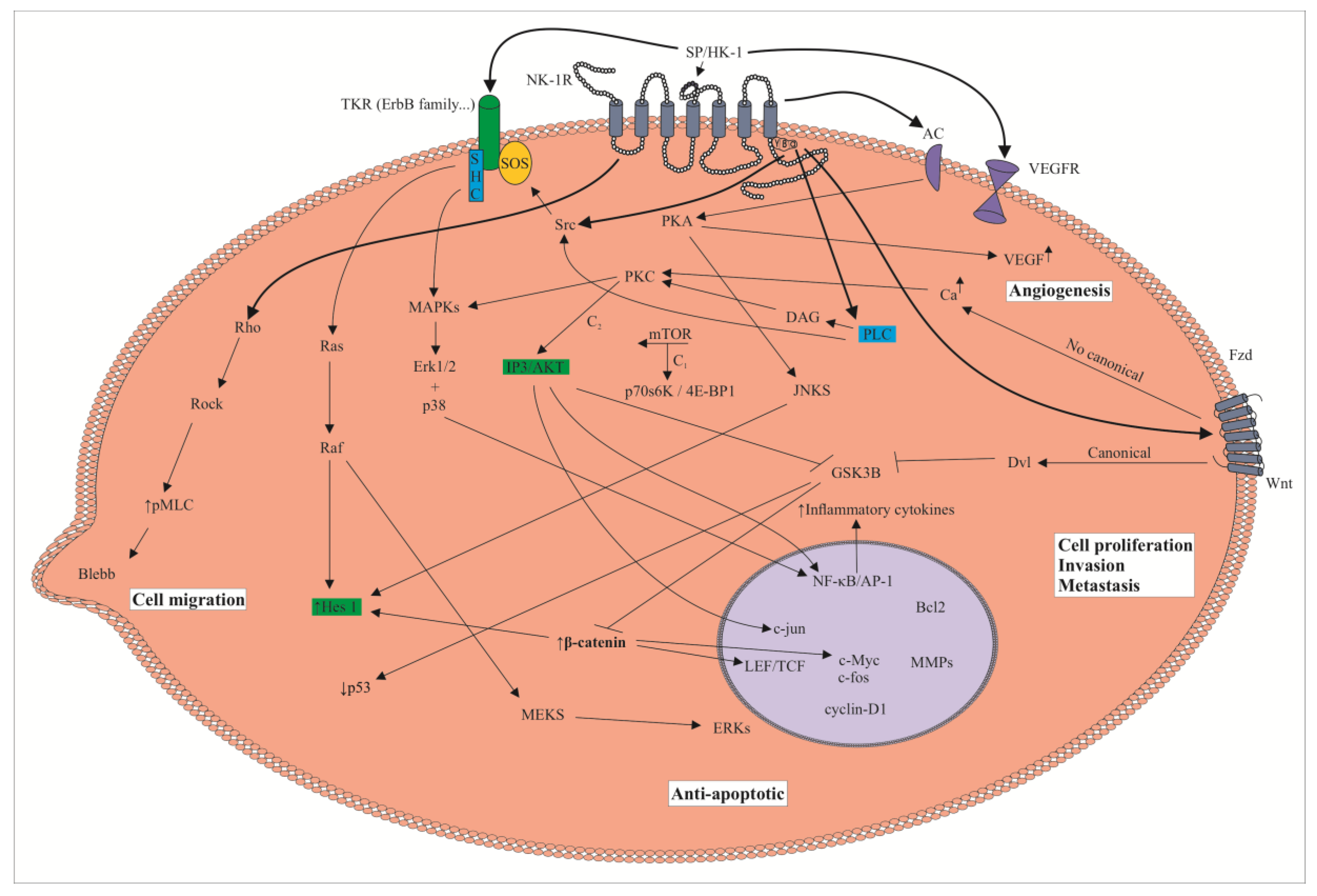
3. The SP/NK-1R System and Cancer: Cell Signaling Pathways, Overexpression of the NK-1R, Metastasis and NK-1R Antagonists
4. The NK-1R Is Essential for the Viability of Tumor Cells
5. Acute Myeloid Leukemia: SP/NK-1R System and the NK-1R Antagonist Aprepitant
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ge, C.; Huang, H.; Huang, F.; Yang, T.; Zhang, T.; Wu, H.; Zhou, H.; Chen, Q.; Shi, Y.; Sun, Y.; et al. Neurokinin-1 receptor is an effective oxidative stress through mitochondrial calcium overload. Proc. Natl. Acad. Sci. USA 2019, 116, 19635–19645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokharel, M. Leukemia: A review article. IJARPB 2012, 2, 397–407. [Google Scholar]
- Belson, M.; Kingsley, B.; Holme, A. Risk factors for acute leukemia in children: A review. Environ. Health Perspect. 2007, 115, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikmen, M.; Gökhaner, G.; Cantürk, Z. Evaluation of the antileukemic effects of neurokinin-1 receptor antagonists, aprepitant, and L-733,060, in chronic and acute myeloid leukemic cells. Anticancer Drugs 2019, 30, e0769. [Google Scholar] [CrossRef] [PubMed]
- Deschler, B.; Lübbert, M. Acute myeloid leukemia: Epidemiology and etiology. Cancer 2006, 107, 2099–2107. [Google Scholar] [CrossRef]
- Shah, A.; Andersson, T.M.; Rachet, B.; Björkholm, M.; Lambert, P.C. Survival and cure of acute myeloid leukaemia in England, 1971–2006: A population-based study. Br. J. Haematol. 2013, 162, 509–516. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. Substance P. In Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Martini, L., Eds.; Academic Press: Oxford, UK, 2019; Volume 1, pp. 571–578. [Google Scholar]
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids 2014, 46, 1727–1750. [Google Scholar] [CrossRef]
- Steinhoff, M.S.; von Mentzer, B.; Geppetti, P.; Pothoulakis, C.; Bunnett, N.W. Tachykinins and their receptors: Contributions to physiological control and the mechanisms of disease. Physiol. Rev. 2014, 94, 265–301. [Google Scholar] [CrossRef] [Green Version]
- Ebner, K.; Sartori, S.B. Singewald. Tachykinin receptors as therapeutic targets in stress-related disorders. Curr. Pharm. Des. 2009, 15, 1647–1674. [Google Scholar] [CrossRef]
- Pennefather, J.N.; Lecci, A.; Candenas, M.L.; Patak, E.; Pinto, F.M.; Maggi, C.A. Tachykinins and tachykinin receptors: A growing family. Life Sci. 2004, 74, 1445–1463. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosso, M.; Coveñas, R. Neurokinin-1 receptor. In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer: Cham, Switzerland, 2018; pp. 3437–3445. [Google Scholar]
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in cancer progression. Peptides 2013, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hennig, I.M.; Laissue, J.A.; Horisberger, U.; Reubi, J.C. Substance-P receptors in human primary neoplasms: Tumoral and vascular localization. Int. J. Cancer 1995, 61, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Tsuchida, K.; Negishis, M.; Itos, S.; Nakanishie, S. Direct linkage of three tachykinin receptors to stimulation of both phosphatidylinositol hydrolysis and cyclic AMP cascades in transfected Chinese hamster ovary cells. J. Biol. Chem. 1992, 267, 2437–2442. [Google Scholar] [PubMed]
- Takeda, Y.; Blount, P.; Sachais, B.S.; Hershey, A.D.; Raddatz, R.; Krause, J.E. Ligand binding kinetics of substance P and neurokinin A receptors stably expressed in Chinese hamster ovary cells and evidence for differential stimulation of inositol 1,4,5-trisphosphate and cyclic AMP second messenger responses. J. Neurochem. 1992, 59, 740–745. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L.; Pacini, M.; Geppetti, P.; Alessandri, G.; Maggi, C.A. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc. Res. 1990, 40, 264–278. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. Glioma and neurokinin-1 receptor antagonists: A new therapeutic approach. Anticancer Agents Med. Chem. 2020, 19, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yin, W.; Zeng, Q.; Jia, H.; Lin, L.; Liu, X.; Mu, L.; Wang, R. Hemokinins modulate endothelium function and promote angiogenesis through neurokinin-1 receptor. Int. J. Biochem. Cell Biol. 2012, 44, 1410–1421. [Google Scholar] [CrossRef]
- Javid, H.; Mohammadi, F.; Zahiri, E.; Hashemy, S.I. The emerging role of substance P/neurokinin-1 receptor signaling pathways in growth and development of tumor cells. J. Physiol. Biochem. 2019, 75, 415–421. [Google Scholar] [CrossRef]
- Molinos-Quintana, A.; Trujillo-Hacha, P.; Piruat, J.I.; Bejarano-García, J.A.; García-Guerrero, E.; Pérez-Simón, J.A.; Muñoz, M. Human acute myeloid leukemia cells express neurokinin-1 receptor, which is involved in the antileukemic effect of neurokinin-1 receptor antagonists. Investig. New Drugs 2019, 37, 17–26. [Google Scholar] [CrossRef]
- Muñoz, M.; González-Ortega, A.; Coveñas, R. The NK-1 receptor is expressed in human leukemia and is involved in the antitumor action of aprepitant and other NK-1 receptor antagonists on acute lymphoblastic leukemia cell lines. Investig. New Drugs 2012, 30, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Erin, N. Role of sensory neurons, neuroimmune pathways, and transient receptor potential vanilloid 1 (TRPV1) channels in a murine model of breast cancer metastasis. Cancer Immunol. Immunother. 2020, 69, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Erin, N.; İpekçi, T.; Akkaya, B.; Özbudak, İ.H.; Baykara, M. Neuropeptide levels as well as neprilysin activity decrease in renal cell carcinoma. Cancer Microenviron. 2016, 9, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Sharif, T.R.; Sharif, M. Substance P-induced mitogenesis in human astrocytoma cells correlates with activation of the mitogen-activated protein kinase signaling pathway. Cancer Res. 1996, 56, 4983–4991. [Google Scholar] [PubMed]
- Walczak-Drzewiecka, A.; Ratajewski, M.; Wagner, W.; Dastych, J. HIF-1α is up-regulated in activated mast cells by a process that involves calcineurin and NFAT. J. Immunol. 2008, 181, 1665–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, M.; Coveñas, R.; Esteban, F.; Redondo, M. The substance P/NK-1 receptor system: NK-1 receptor antagonists as anti-cancer drugs. J. Biosci. 2015, 40, 441–463. [Google Scholar] [CrossRef] [PubMed]
- Davoodian, M.; Boroumand, N.; Mehrabi Bahar, M.; Jafarian, A.H.; Asadi, M.; Hashemy, S.I. Evaluation of serum level of substance P and tissue distribution of NK-1 receptor in breast cancer. Mol. Biol. Rep. 2019, 46, 1285–1293. [Google Scholar] [CrossRef]
- Gharaee, N.; Pourali, L.; Jafarian, A.H.; Hashemy, S.I. Evaluation of serum level of substance P and tissue distribution of NK-1 receptor in endometrial cancer. Mol. Biol. Rep. 2018, 45, 2257–2262. [Google Scholar] [CrossRef]
- Akazawa, T.; Kwatra, S.G.; Goldsmith, L.E.; Richardson, M.D.; Cox, E.A.; Sampson, J.H.; Kwatra, M.M. A constitutively active form of neurokinin 1 receptor and neurokinin 1 receptor-mediated apoptosis in glioblastomas. J. Neurochem. 2009, 109, 1079–1086. [Google Scholar] [CrossRef] [Green Version]
- García-Recio, S.; Fuster, G.; Fernández-Nogueira, P.; Pastor-Arroyo, E.M.; Park, S.Y.; Mayordomo, C.; Ametller, E.; Mancino, M.; González-Farré, X.; Russnes, H. Substance P autocrine signaling contributes to persistent HER2 activation that drives malignant progression and drug resistance in breast cancer. Cancer Res. 2013, 73, 6424–6434. [Google Scholar] [CrossRef] [Green Version]
- Mayordomo, C.; García-Recio, S.; Ametller, E.; Fernández-Nogueira, P.; Pastor-Arroyo, E.M.; Vinyals, L.; Casas, I.; Gascón, P.; Almendro, V. Targeting of substance P induces cancer cell death and decreases the steady state of EGFR and Her2. J. Cell. Physiol. 2012, 227, 1358–1366. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, M.; Tong, Y.; Liu, X.; Zhang, L.; Dong, D.; Shao, J.; Zhou, Y. miR-206 promotes cancer progression by targeting full-length neurokinin-1 receptor in breast cancer. Technol. Cancer Res. Treat. 2019, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Liu, S.; Liu, J.; Feng, F.; Guo, Y.; Zhang, W.; Zheng, G.; Wang, Q.; Cai, L.; Guo, M.; et al. SP promotes cell proliferation in esophageal squamous cell carcinoma through the NK1R/Hes1 axis. Biochem. Biophys. Res. Commun. 2019, 514, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Castro, T.A.; Cohen, M.C.; Rameshwar, P. The expression of neurokinin-1 and preprotachykinin-1 in breast cancer cells depends on the relative degree of invasive and metastatic potential. Clin. Exp. Metastasis 2005, 22, 621–628. [Google Scholar] [CrossRef]
- Deng, X.T.; Tang, S.M.; Wu, P.Y.; Li, Q.P.; Ge, X.X.; Xu, B.M.; Wang, H.S.; Miao, L. SP/NK-1R promotes gallbladder cancer cell proliferation and migration. J. Cell. Mol. Med. 2019, 23, 7961–7973. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E.; Ramiro, S.; Lladó, S.; Toro, S.; Coveñas, R.; Muñoz, M. Antitumor action of temozolomide, ritonavir and aprepitant against human glioma cells. J. Neurooncol. 2016, 126, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Brener, S.; González-Moles, M.A.; Tostes, D.; Esteban, F.; Gil-Montoya, J.A.; Ruiz-Ávila, I.; Bravo, M.; Muñoz, M. A role for the substance P/NK-1 receptor complex in cell proliferation in oral squamous cell carcinoma. Anticancer Res. 2009, 29, 2323–2329. [Google Scholar] [PubMed]
- González-Moles, M.A.; Mosqueda-Taylor, A.; Esteban, F.; Gil-Montoya, J.A.; Díaz-Franco, M.A.; Delgado, M.; Muñoz, M. Cell proliferation associated with actions of the substance P/NK-1 receptor complex in keratocystic odontogenic tumours. Oral Oncol. 2008, 44, 1127–1133. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. Neurokinin-1 receptor antagonists as antitumor drugs in gastrointestinal cancer: A new approach. Saudi J. Gastroenterol. 2016, 22, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Rosso, M.; Robles-Frías, M.J.; Salinas-Martín, M.V.; Rosso, R.; González-Ortega, A.; Coveñas, R. The NK-1 receptor is expressed in human melanoma and is involved in the antitumor action of the NK-1 receptor antagonist aprepitant on melanoma cell lines. Lab. Investig. 2010, 90, 1259–1269. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; González-Ortega, A.; Rosso, M.; Robles-Frías, M.J.; Carranza, A.; Salinas-Martín, M.V.; Coveñas, R. The substance P/neurokinin-1 receptor system in lung cancer: Focus on the antitumor action of neurokinin-1 receptor antagonists. Peptides 2012, 38, 318–325. [Google Scholar] [CrossRef]
- Muñoz, M.; González-Ortega, A.; Salinas-Martín, M.V.; Carranza, A.; García-Recio, S.; Almendro, V.; Coveñas, R. The neurokinin-1 receptor antagonist aprepitant is a promising candidate for the treatment of breast cancer. Int. J. Oncol. 2014, 45, 1658–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, M.; Muñoz, M.F.; Ayala, A. Immunolocalization of substance P and NK-1 receptor in adipose stem cells. J. Cell Biochem. 2017, 118, 4686–4696. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Richardson, M.D.; Bigner, D.D.; Kwatra, M.M. Signal transduction through substance P receptor in human glioblastoma cells: Roles for Src and PKCδ. Cancer Chemother. Pharmacol. 2005, 56, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Ilmer, M.; Garnier, A.; Vykoukal, J.; Alt, E.; von Schweinitz, D.; Kappler, R.; Berger, M. Targeting the neurokinin-1 receptor compromises canonical Wnt signaling in hepatoblastoma. Mol. Cancer Ther. 2015, 14, 2712–2721. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; Pavón, A.; Rosso, M.; Salinas, M.V.; Pérez, A.; Carranza, A.; González-Ortega, A. Immunolocalization of NK-1 receptor and substance P in human normal placenta. Placenta 2010, 31, 649–651. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosso, M. The NK-1 receptor antagonist aprepitant as a broad spectrum antitumor drug. Investig. New Drugs 2010, 28, 187–193. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Dong, D.; Wang, Z.; Ji, W.; Yu, M.; Zhang, F.; Niu, R.; Zhou, Y. miR-34b/c-5p and the neurokinin-1 receptor regulate breast cancer cell proliferation and apoptosis. Cell Prolif. 2019, 52, e12527. [Google Scholar] [CrossRef]
- Muñoz, M.; Crespo, J.C.; Crespo, J.P.; Coveñas, R. Neurokinin-1 receptor antagonist aprepitant and radiotherapy, a successful combination therapy in a patient with lung cancer: A case report. Mol. Clin. Oncol. 2019, 11, 50–54. [Google Scholar]
- Cordier, D.; Merlo, A. Long-Term results of targeted low-grade glioma treatment with 213Bi-DOTA-[Thi8, Met(O2)11]-Substance P. Cancer Biother. Radiopharm. 2019, 34, 413–416. [Google Scholar] [CrossRef]
- Królicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Safety and efficacy of targeted alpha therapy with 213Bi-DOTA-substance P in recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 614–622. [Google Scholar] [CrossRef]
- Majkowska-Pilip, A.; Koźmiński, P.; Wawrzynowska, A.; Budlewski, T.; Kostkiewicz, B.; Gniazdowska, E. Application of neurokinin-1 receptor in targeted strategies for glioma treatment. Part I: Synthesis and evaluation of substance P fragments labeled with 99mTc and 177Lu as potential receptor radiopharmaceuticals. Molecules 2018, 23, 2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majkowska-Pilip, A.; Rius, M.; Bruchertseifer, F.; Apostolidis, C.; Weis, M.; Bonelli, M.; Laurenza, M.; Królicki, L.; Morgenstern, A. In vitro evaluation of 225Ac-DOTA-substance P for targeted alpha therapy of glioblastoma multiforme. Chem. Biol. Drug Des. 2018, 92, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Majkowska-Pilip, A.; Halik, P.K.; Gniazdowska, E. The significance of NK1 receptor ligands and their application in targeted radionuclide tumour therapy. Pharmaceutics 2019, 11, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, S.; Boada, M.D. Neuropeptide-induced modulation of carcinogenesis in a metastatic breast cancer cell line (MDA-MB-231LUC+). Cancer Cell Int. 2018, 18, 216. [Google Scholar] [CrossRef]
- Fackler, O.T.; Grosse, R. Cell motility through plasma membrane blebbing. J. Cell Biol. 2008, 181, 879–884. [Google Scholar] [CrossRef] [Green Version]
- Mierke, C.T. Physical view on migration modes. Cell Adhes. Migr. 2015, 9, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Yuan, S.; Cheng, J.; Kang, S.; Zhao, W.; Zhang, J. Substance P promotes the progression of endometrial adenocarcinoma. Int. J. Gynecol. Cancer 2016, 26, 845–850. [Google Scholar] [CrossRef]
- Pohl, A.; Kappler, R.; Mühling, J.; von Schweinitz, D.; Berger, M. Expression of truncated neurokinin-1 receptor in childhood neuroblastoma is independent of tumor biology and stage. Anticancer Res. 2017, 37, 6079–6085. [Google Scholar]
- Muñoz, M.; Pérez, A.; Coveñas, R.; Rosso, M.; Castro, E. Antitumoral action of L-733,060 on neuroblastoma and glioma cell lines. Arch. Ital. Biol. 2004, 142, 105–112. [Google Scholar]
- Muñoz, M.; Rosso, M.; Pérez, A.; Coveñas, R.; Rosso, R.; Zamarriego, C.; Piruat, J.I. The NK receptor is involved in the antitumoural action of L-733,060 and in the mitogenic action of substance P on neuroblastoma and glioma cell lines. Neuropeptides 2005, 39, 427–432. [Google Scholar] [CrossRef]
- Bayati, S.; Bashash, D.; Ahmadian, S.; Safaroghli-Azar, A.; Alimoghaddam, K.; Ghavamzadeh, A.; Ghaffari, S.H. Inhibition of tachykinin NK 1 receptor using aprepitant induces apoptotic cell death and G1 arrest through Akt/p53 axis in pre-B acute lymphoblastic leukemia cells. Eur. J. Pharmacol. 2016, 791, 274–283. [Google Scholar] [CrossRef]
- Medrano, S.; Gruenstein, E.; Dimlich, R.V.W. Substance P receptors on human astrocytoma cells are linked to glycogen breakdown. Neurosci. Lett. 1994, 167, 14–18. [Google Scholar] [CrossRef]
- Feng, F.; Yang, J.; Tong, L.; Yuan, S.; Tian, Y.; Hong, L.; Wang, W.; Zhang, H. Substance P immunoreactive nerve fibres are related to gastric cancer differentiation status and could promote proliferation and migration of gastric cancer cells. Cell Biol. Int. 2011, 35, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Paige, C.J. Hemokinin-1 has substance P-like function in U-251 MG astrocytoma cells: A pharmacological and functional study. J. Neuroimmunol. 2005, 164, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Rameshwar, P.; Gascón, P. Induction of negative hematopoietic regulators by neurokinin-a in bone marrow stroma. Blood 1996, 88, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rameshwar, P.; Zhu, G.; Donnelly, R.J.; Qian, J.; Ge, H.; Goldstein, K.R.; Denny, T.N.; Gascón, P. The dynamics of bonemarrow stromal cells in the proliferation of multipotent Hematopoietic progenitors by substance P: An understanding of the effects of a neurotransmitter on the differentiating hematopoietic stem cell. J. Neuroimmunol. 2001, 121, 22–31. [Google Scholar] [CrossRef]
- Rameshwar, P.; Ganea, D.; Gascón, P. In vitro stimulatory effect of substance P on hematopoiesis. Blood 1993, 81, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Nowicki, M.; Mískowiak, B.; Ostalska-Nowicka, D. Detection of substance P and its mRNA in human blast cells in childhood lymphoblastic leukaemia using immunocytochemistry and in situ hybridization. Folia Histochem. Cytobiol. 2003, 41, 33–36. [Google Scholar]
- Rameshwar, P.; Oh, H.S.; Yook, C.; Gascón, P.; Chang, V.T. Substance p-fibronectin-cytokine interactions in myeloproliferative disorders with bone marrow fibrosis. Acta Haematol. 2003, 109, 1–10. [Google Scholar] [CrossRef]
- Nowicki, M.; Ostalska-Nowicka, D.; Kondraciuk, B.; Miskowiak, B. The significance of substance P in physiological and malignant haematopoiesis. J. Clin. Pathol. 2007, 60, 749–755. [Google Scholar] [CrossRef] [Green Version]
- Rameshwar, P.; Joshi, D.D.; Yadav, P.; Gascón, P.; Qian, J.; Chang, V.T.; Anjaria, A.; Harrison, J.S.; Xiaosong, S. Mimicry between neurokinin-1 and fibronectin may explain the transport and stability of increased substance P-immunoreactivity in patients with bone marrow fibrosis. Blood 2001, 97, 3025–3031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, R.; Furuno, T.; Okamoto, K.; Teshima, R.; Nakanishi, M. ATP plays a role in neurite stimulation with activated mast cells. J. Neuroimmunol. 2007, 192, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Padró, T.; Ruiz, S.; Bieker, R.; Bürger, H.; Steins, M.; Kienast, J.; Büchner, T.; Berdel, W.E.; Mesters, R.M. Increased angiogenesis in the bone marrow of patients with acute myeloid leukemia. Blood 2000, 95, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Yoshizawa, H.; Yoshimori, K.; Katakami, N.; Katsumata, N.; Kawahara, M.; Eguchi, K. Efficacy and safety of single-dose fosaprepitant in the prevention of chemotherapy-induced nausea and vomiting in patients receiving high-dose cisplatin: A multicentre, randomised, double-bind, placebo-controlled phase 3 trial. Ann. Oncol. 2013, 24, 1067–1073. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. Safety of neurokinin-1 receptor antagonists. Expert Opin. Drug Saf. 2013, 12, 673–685. [Google Scholar] [CrossRef]
- Kramer, M.S.; Cutler, N.; Feighner, J.; Shrivastava, R.; Carman, J.; Sramek, J.J.; Reines, S.A.; Liu, G.; Snavely, D.; Wyatt-Knowles, E.; et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 1998, 281, 1640–1645. [Google Scholar] [CrossRef]
- Okumura, L.M.; da Silva Ries, S.A.; Meneses, C.F.; Michalowski, M.B.; Ferreira, M.A.P.; Moreira, L.B. Adverse events associated with aprepitant pediatric bone cancer patients. J. Oncol. Pharm. Pract. 2019, 25, 735–738. [Google Scholar] [CrossRef]
- Ebner, K.; Singewald, N. The role of substance P in stress and anxiety responses. Amino Acids 2006, 31, 251–272. [Google Scholar] [CrossRef]
- Aziz, F. Neurokinin-1 receptor antagonists for chemotherapy-induced nausea and vomiting. Ann. Palliat. Med. 2012, 1, 130–136. [Google Scholar]
- Harford-Wright, E.; Lewis, K.M.; Vink, R.; Ghabriel, M.N. Evaluating the role of substance P in the growth of brain tumors. Neuroscience 2014, 261, 85–94. [Google Scholar] [CrossRef]
- Muñoz, M.; Berger, M.; Rosso, M.; González-Ortega, A.; Carranza, A.; Coveñas, R. Antitumor activity of neurokinin-1 receptor antagonists in MG-63 human osteosarcoma xenografts. Int. J. Oncol. 2014, 44, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Garnier, A.; Vykoukal, J.; Hubertus, J.; Alt, E.; von Schweinitz, D.; Kappler, R.; Berger, M.; Ilmer, M. Targeting the neurokinin-1 receptor inhibits growth of human colon cancer cells. Int. J. Oncol. 2015, 47, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harford-Wright, E.; Lewis, K.M.; Ghabriel, M.N.; Vink, R. Treatment with the NK1 antagonist emend reduces blood brain barrier dysfunction and edema formation in an experimental model of brain tumors. PLoS ONE 2014, 9, e97002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayati, S.; Razani, E.; Bashash, D.; Safaroghli-Azar, A.; Safa, M.; Ghaffari, S.H. Antileukemic effects of neurokinin-1 receptor inhibition on hematologic malignant cells: A novel therapeutic potential for aprepitant. Anticancer Drugs 2018, 29, 243–252. [Google Scholar] [CrossRef]
- Bashash, D.; Safaroghli-Azar, A.; Bayati, S.; Razani, E.; Pourbagheri-Sigaroodi, A.; Gharehbaghian, A.; Momeny, M.; Sanjadi, M.; Rezaie-Tavirani, M.; Ghaffari, S.H. Nueokinin-1 receptor (NK1R) inhibition sensitizes APL cells to anti-tumor effect of arsenic trioxide via restriction of NF-κB axis: Shedding new light on resistance to aprepitant. Int. J. Biochem. Cell Biol. 2018, 103, 105–114. [Google Scholar] [CrossRef]
- Vousden, K.H. Partners in death: A role for p73 and NF-κB in promoting apoptosis. Aging 2009, 1, 275. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Bai, L.; Chen, W.; Xu, S. The NF-κB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin. Ther. Targets 2010, 14, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Duyao, M.P.; Buckler, A.J.; Sonenshein, G.E. Interaction of an NF-kappa B-like factor with a site upstream of the c-myc promoter. Proc. Natl. Acad. Sci. USA 1990, 87, 4727–4731. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Matsumura, I.; Ezoe, S.; Satoh, Y.; Sakamaki, T.; Albanese, C.; Machii, T.; Pestell, R.G.; Kanakura, Y. E2F1 and c-Myc potentiate apoptosis through inhibition of NF-κB activity that facilitates MnSOD-mediated ROS elimination. Mol. Cell 2002, 9, 1017–1029. [Google Scholar] [CrossRef]
- Wang, X.; Douglas, S.D.; Lai, J.P.; Tuluc, F.; Tebas, P.; Ho, W.Z. Neurokinin-1 receptor antagonist (aprepitant) inhibits drugresistant HIV-1 infection of macrophages in vitro. J. Neuroimmune Pharmacol. 2007, 2, 42–48. [Google Scholar] [CrossRef]
- Berger, M.; Neth, O.; Ilmer, M.; Garnier, A.; Salinas-Martín, M.V.; de Agustín Asencio, J.C.; von Schweinitz, D.; Kappler, R.; Muñoz, M. Hepatoblastoma cells express truncated neurokinin-1 receptor and can be growth inhibited by aprepitant in vitro and in vivo. J. Hepatol. 2014, 60, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Burt, R.W. Colon cancer screening. Gastroenterology 2000, 119, 837–853. [Google Scholar] [CrossRef] [PubMed]
- Simeonidis, S.; Castagliuolo, I.; Pan, A.; Liu, J.; Wang, C.C.; Mykoniatis, A.; Pasha, A.; Valenick, L.; Sougioultzis, S.; Zhao, D.; et al. Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-kappa B site on its promoter. Proc. Natl. Acad. Sci. USA 2003, 100, 2957–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogo, H.; Kuroyanagi, N.; Inoue, A.; Nishio, H.; Hirai, Y.; Akiyama, M.; DiMaggio, D.A.; Krause, J.E.; Nakata, Y. Human astrocytoma cells (U-87 MG) exhibit a specific substance P binding site with the characteristics of an NK-1 receptor. J. Neurochem. 1996, 67, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Sorrell, M.; Berman, Z. Functional interplay between ATM/ATR-mediated DNA damage response and DNA repair pathways in oxidative stress. Cell. Mol. Life Sci. 2014, 71, 3951–3967. [Google Scholar] [CrossRef] [Green Version]
- Hwang, M.S.; Schwall, C.T.; Pazarentzos, E.; Datler, C.; Alder, N.N.; Grimm, S. Mitochondrial Ca(2+) influx targets cardiolipin to disintegrate respiratory chain complex II for cell death induction. Cell Death Differ. 2014, 21, 1733–1745. [Google Scholar] [CrossRef] [Green Version]
- Luger, N.M.; Mach, D.B.; Sevcik, M.A.; Mantyh, P.W. Bone cancer pain: From model to mechanism to therapy. J. Pain Symptom Manag. 2005, 29 (Suppl. 5), S32–S46. [Google Scholar] [CrossRef]
- Sakata, H.; Nakao, A.; Matsuda, K.; Yoshie, N.; Yamada, T.; Osako, T.; Iwano, M.; Kotani, J. Acute leukemia presenting as bone pain with normal white blood cell count. Acute Med. Surg. 2014, 1, 249. [Google Scholar] [CrossRef]
- Slosky, L.M.; Largent-Milnes, T.M.; Vanderah, T.W. Use of animal models in understanding cancer-induced bone pain. Cancer Growth Metastasis 2015, 8 (Suppl. 1), 47–62. [Google Scholar] [CrossRef] [Green Version]
- Suvas, S. Role of substance P neuropeptide in inflammation, wound healing, and tissue homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, G. Cytokines as modulators of malignant glioma progression. In Cytokines and the CNS; Ransohoff, R.M., Beneviste, E., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 269–286. [Google Scholar]
- Harrison, S.; Geppetti, P. Substance P. Int. J. Biochem. Cell Biol. 2001, 33, 555–576. [Google Scholar] [CrossRef]
- Lotz, M.; Vaughan, J.H.; Carson, D.A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 1988, 241, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Kumakura, S.; Murakami, T.; Someya, A.; Inada, E.; Nagaoka, I. Ketamine suppresses the substance P-induced production of IL-6 and IL-8 by human U373MG glioblastoma/astrocytoma cells. Int. J. Mol. Med. 2017, 39, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Estey, E.H. Treatment of acute myeloid leukemia. Haematologica 2009, 94, 10–16. [Google Scholar] [CrossRef]
- Robinson, P.; Kasembeli, M.; Bharadwaj, U.; Engineer, N.; Eckols, K.T.; Tweardy, D.J. Substance P receptor signaling mediates doxorubicin-induced cardiomyocyte apoptosis and triple-negative breast cancer chemoresistance. BioMed Res. Int. 2016, 2016, 195927. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; Parrilla, J.; Rosso, M.; Coveñas, R. Antipruritic vs. antitumour action of aprepitant: A question of dose. Acta Derm. Venereol. 2019, 99, 620–621. [Google Scholar] [CrossRef] [Green Version]
| Leukemia cells express SP but not neurokinin A: related to leukemia and bone marrow fibrosis |
| SP increases the proliferation of bone marrow progenitors; neurokinin A exerts an opposite effect |
| AML cells express the NK-1R which mediates the antileukemic action of NK-1R antagonists |
| SP induces the proliferation of AML cells |
| Two NK-1R isoforms in AML cells: the truncated form is higher expressed than the full-length |
| AML cells: full-length expression is higher than in healthy cells, in which is not expressed |
| Compared with lymphocytes, AML cells overexpress the truncated splice variant |
| AML patients/cells: a high NK-1R expression is found in white blood cells |
| AML patients show an up-regulation of the NK-1R mRNA expression |
| SP, via the NK-1R, promotes a weak mitochondrial and intracellular Ca++ flux |
| All AML patients express the NK-1R. Healthy subjects: expression observed in 8% of population |
| AML cells express SP and NK-1R. Healthy subjects: no immunoreactivity |
| In a dose-dependent manner exerts cytotoxic/antiproliferative effects and decreases AML cell survival/proliferative potential |
| Increases p73/p21 expression and alters cell cycle/DNA replication rate |
| Activates caspase-3-dependent apoptotic pathway |
| Aprepitant-ATO increase the number of AML apoptotic cells when compared to aprepitant alone |
| Aprepitant-ATO accumulate AML cells in sub-G1 phase and decrease DNA synthesis |
| Sensitizes AML cells to ATO via suppression of anti-apoptotic target genes of NF-κB |
| Aprepitant-ATO increase mRNA expression of pro-apoptotic targets (Bax, Bid, Bad, p21, p73) |
| Aprepitant-ATO decrease the expression of c-myc and regulates NF-κB activity |
| Diminished antitumor effect when the NF-κB pathway is overactivated |
| AML xenograft model: fosaprepitant increases the median survival 4–7 days |
| Induces AML cell death by apoptosis |
| A high dose of aprepitant does not affect lymphocytes proliferation |
| Exerts a higher damage in AML cells than in non-cancer cells |
| Blocks the formation of colonies of AML cells (size, circularity, perimeter) |
| Necrotic mechanisms are observed in AML cells upgrading aprepitant concentration |
| Increases mitochondrial reactive oxygen species |
| Mitochondrial oxidative stress is due to Ca++ flux from endoplasmic reticulum to mitochondria |
| Exerts an antinociceptive effect in myeloid leukemia-induced bone pain |
| Increases the level of apoptotic markers (annexin-V/propidium iodide) |
| Increases G0/G1 phase and decreases cells in S phase |
| No effect on human normal hematopoietic cells and no hemolytic toxicity on red blood cells |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, M.; Coveñas, R. The Neurokinin-1 Receptor Antagonist Aprepitant, a New Drug for the Treatment of Hematological Malignancies: Focus on Acute Myeloid Leukemia. J. Clin. Med. 2020, 9, 1659. https://doi.org/10.3390/jcm9061659
Muñoz M, Coveñas R. The Neurokinin-1 Receptor Antagonist Aprepitant, a New Drug for the Treatment of Hematological Malignancies: Focus on Acute Myeloid Leukemia. Journal of Clinical Medicine. 2020; 9(6):1659. https://doi.org/10.3390/jcm9061659
Chicago/Turabian StyleMuñoz, Miguel, and Rafael Coveñas. 2020. "The Neurokinin-1 Receptor Antagonist Aprepitant, a New Drug for the Treatment of Hematological Malignancies: Focus on Acute Myeloid Leukemia" Journal of Clinical Medicine 9, no. 6: 1659. https://doi.org/10.3390/jcm9061659
APA StyleMuñoz, M., & Coveñas, R. (2020). The Neurokinin-1 Receptor Antagonist Aprepitant, a New Drug for the Treatment of Hematological Malignancies: Focus on Acute Myeloid Leukemia. Journal of Clinical Medicine, 9(6), 1659. https://doi.org/10.3390/jcm9061659






