Epidemiological Trend of Sepsis in Patients with Hospital Admissions Related to Hepatitis C in Spain (2000–2015): A Nationwide Study
Abstract
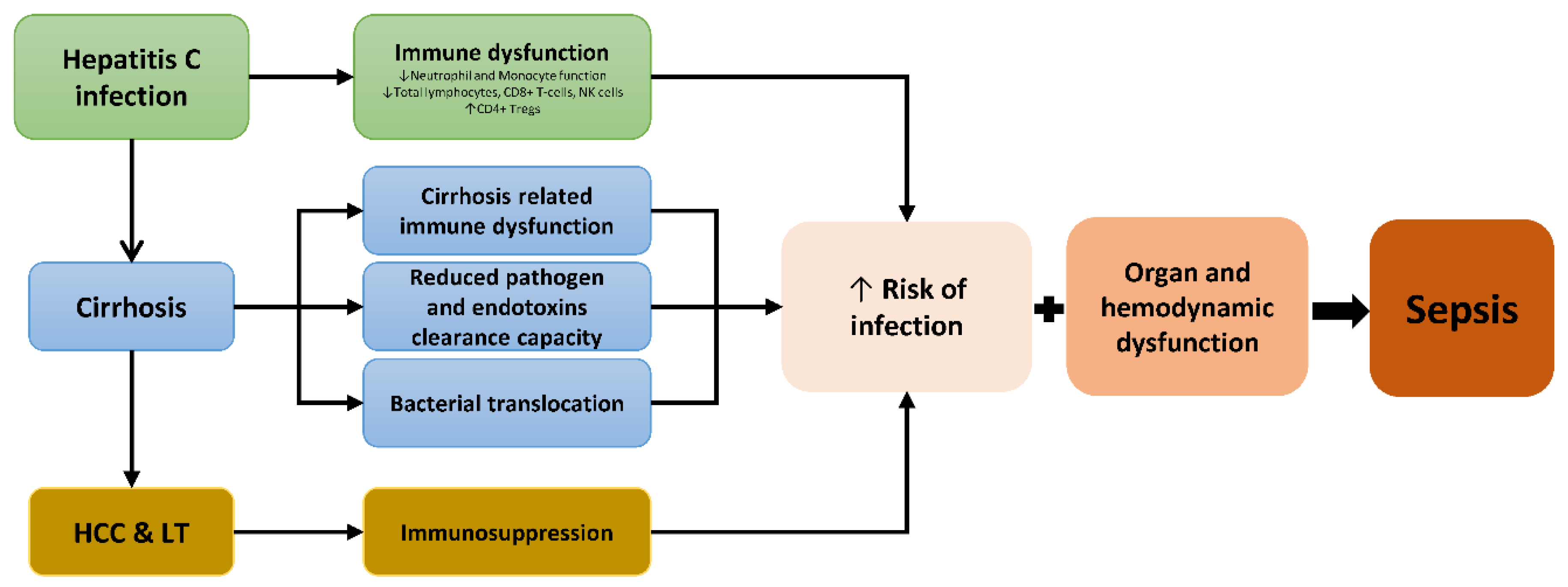
1. Introduction
Objective
2. Methods
2.1. Study Design
2.2. Data Source
2.3. Ethics Statement
2.4. ICD-9-CM Codes and Patients
2.5. Main Study Variables
2.6. Statistical Analyses
3. Results
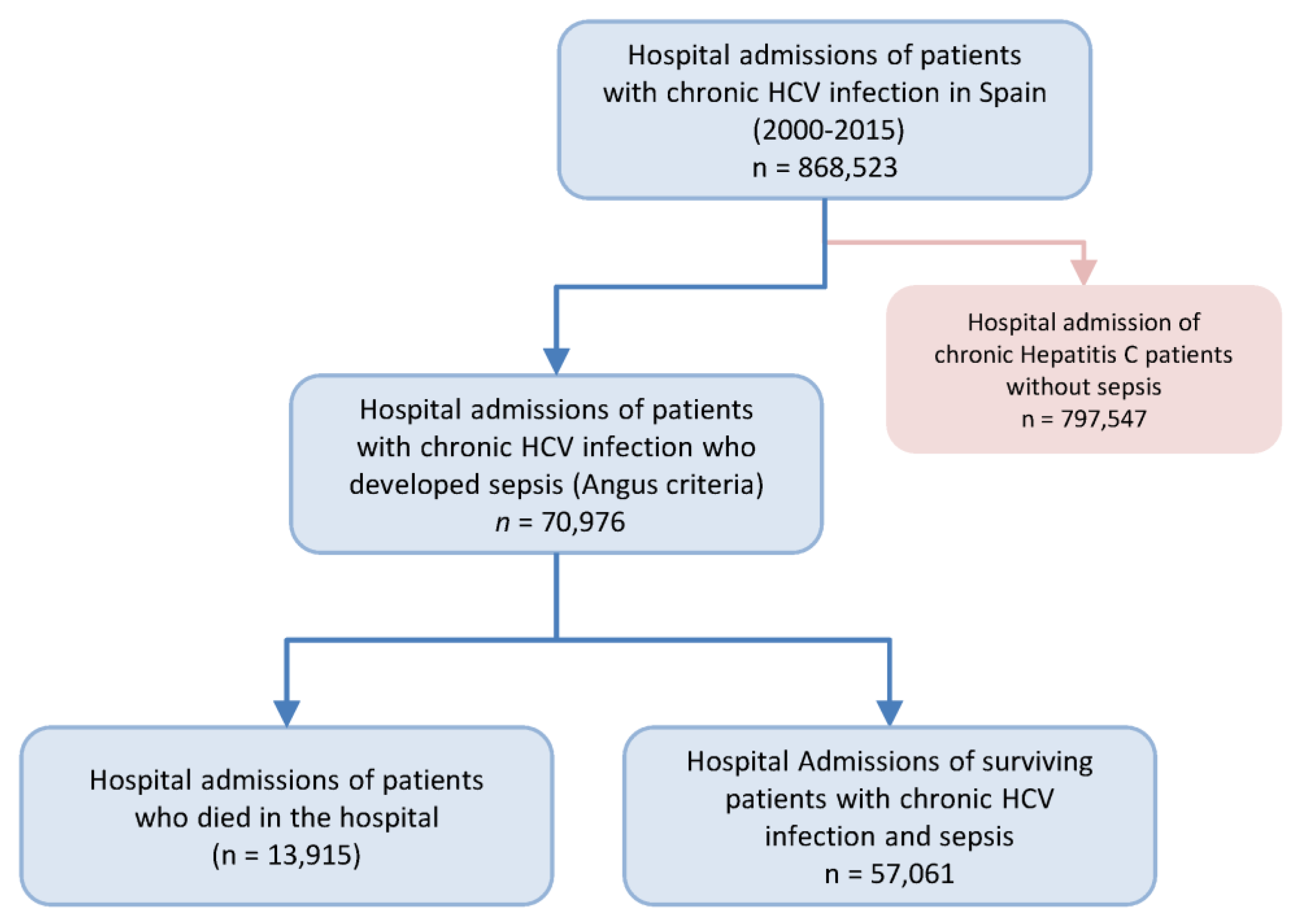
3.1. Study Population
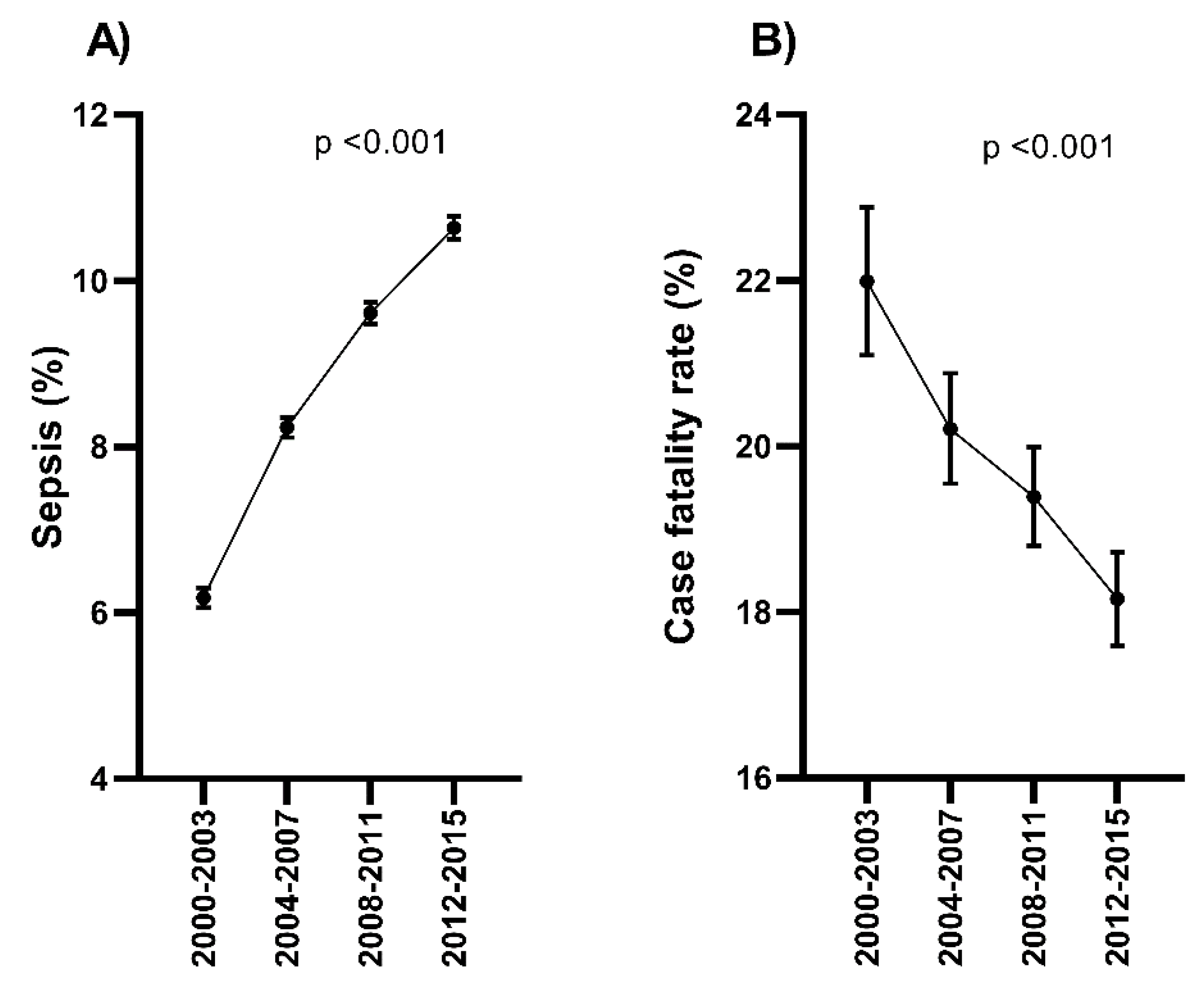
3.2. The Trend of Sepsis-Related Admission and Sepsis-Related Death
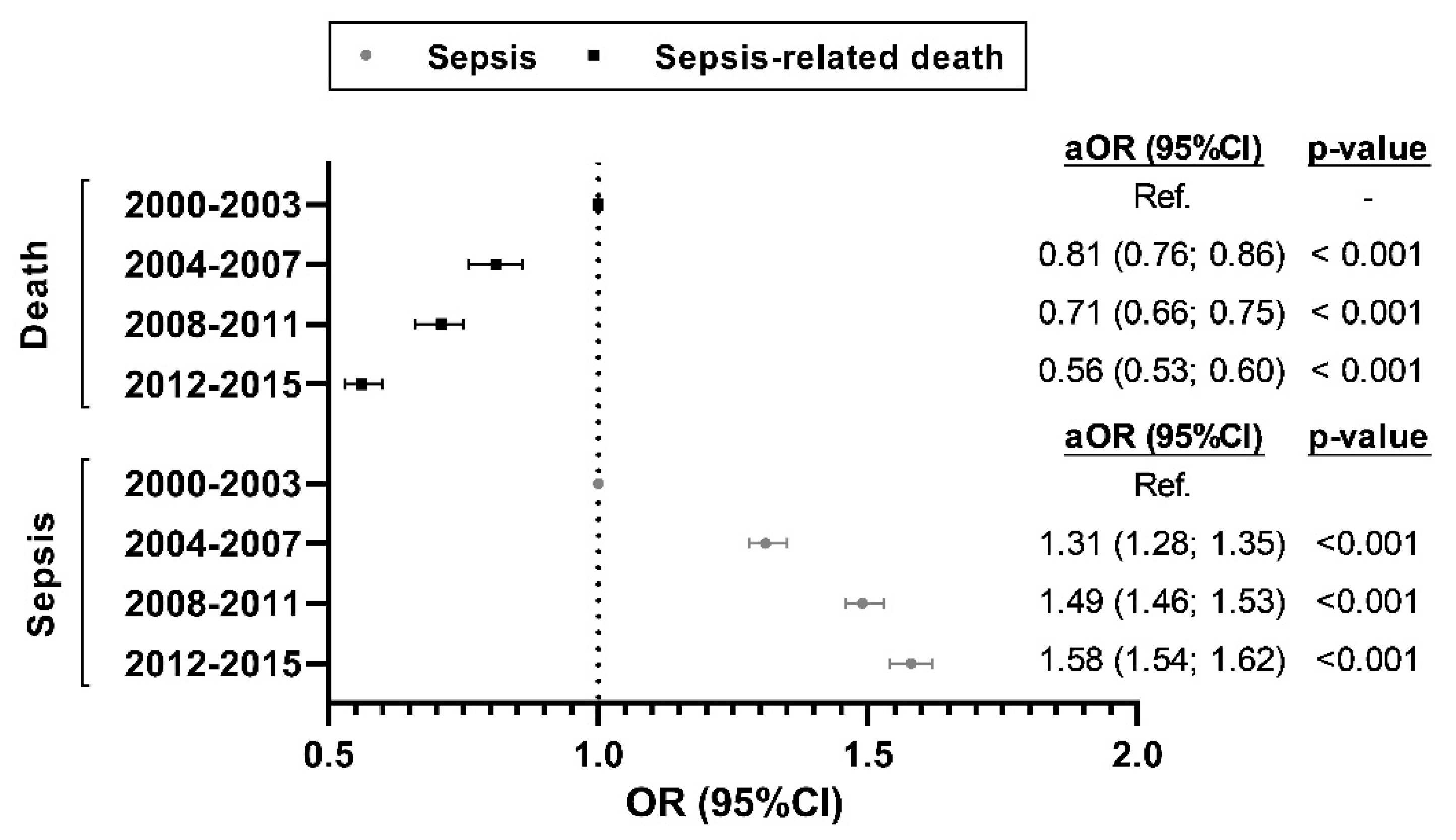
3.3. Temporal Trend of the Risk of Sepsis-Related Admission and Sepsis-Related Death
3.4. Trends in Costs for Hospital Admission with Sepsis
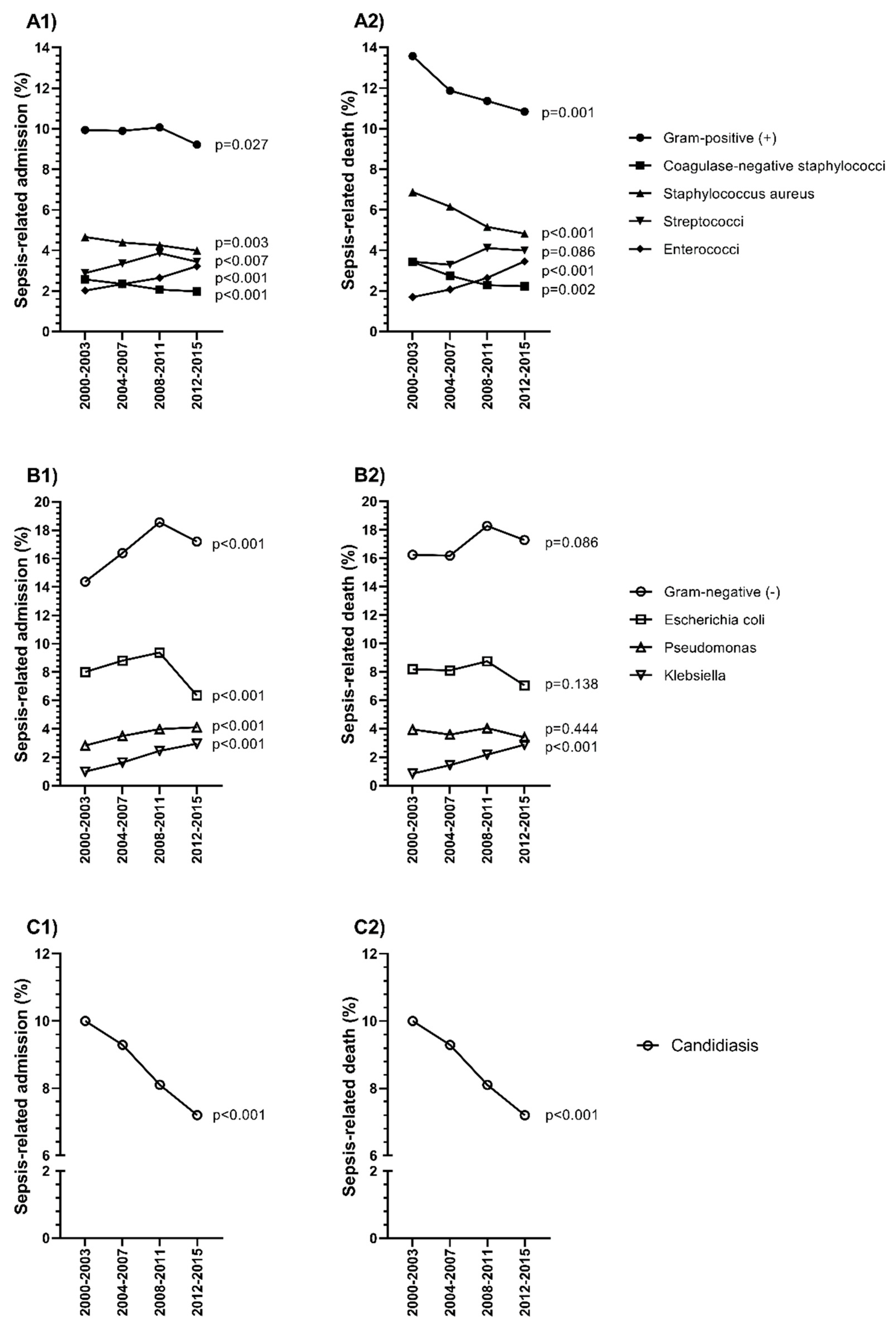
3.5. Epidemiological Trends of Specific Microorganisms
4. Discussion
Limitations and Strengths of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| World Health Organization | (WHO) |
| Hepatitis C virus | (HCV) |
| Hepatocellular carcinoma | (HCC) |
| National Health Services | (NHS) |
| Intensive care unit | (ICU) |
| Cirrhosis-associated immune dysfunction | (CAID) |
| Case-fatality rate | (CFR) |
| Spanish Minimum Basic Data Set | (MBDS) |
| Ministry of Health, Consumption, and Social Welfare | (MHCSW) |
| International Classification of Diseases, 9th ed., Clinical Modification | (ICD-9-CM) |
| Length of hospital stay | (LOHS) |
| Supplementary Table | (ST) |
| Diagnosis-Related Groups | (DRG) |
| Odds ratio | (OR) |
| Adjusted odds ratio | (aOR) |
| Sustained virological response | (SVR) |
| Direct-acting antivirals | (DAAs) |
| Model for End-stage Liver Disease | (MELD) |
| Child-Pugh Turcotte | (CPT) |
| Sequential Organ Failure Assessment | (SOFA) |
| Acute Physiology and Chronic Health Evaluation | (APACHE) |
References
- World Health Organization. Global Hepatitis Report. 2017. Available online: http://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf?sequence=1 (accessed on 10 December 2018).
- Chen, Q.; Ayer, T.; Bethea, E.; Kanwal, F.; Wang, X.; Roberts, M.; Zhuo, Y.; Fagiuoli, S.; Petersen, J.; Chhatwal, J. Changes in hepatitis C burden and treatment trends in Europe during the era of direct-acting antivirals: A modelling study. BMJ Open 2019, 9, e026726. [Google Scholar] [CrossRef]
- Grupo de Trabajo Del Estudio De Prevalencia De La Infección Por Hepatitis C En Población General En España 2017–2018. Resultados Del Segundo Estudio De Seroprevalencia En Espanña (2017–2018). Available online: https://www.mscbs.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/docs/INFORME_INFECCION_VHC_ESPANA2019.pdf (accessed on 31 March 2020).
- Westbrook, R.H.; Dusheiko, G. Natural history of hepatitis C. J. Hepatol. 2014, 61, S58–S68. [Google Scholar] [CrossRef]
- Mate-Cano, I.; Alvaro-Meca, A.; Ryan, P.; Resino, S.; Briz, V. Epidemiological trend of hepatitis C-related liver events in Spain (2000–2015): A nationwide population-based study. Eur. J. Intern. Med. 2020, 75, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Miquel, M.; Cleries, M.; Vergara, M.; Vela, E. Economic burden of cirrhosis in Catalonia: A population-based analysis. BMJ Open 2018, 8, e018012. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Buggisch, P.; Carrion, J.A.; Cooke, G.S.; Zignego, A.L.; Beckerman, R.; Younossi, Z. Direct medical costs associated with the extrahepatic manifestations of hepatitis C infection in Europe. J. Viral Hepat. 2018, 25, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Sicras-Mainar, A.; Navarro-Artieda, R.; Saez-Zafra, M. Comorbidity, concomitant medication, use of resources and healthcare costs associated with chronic hepatitis C virus carriers in Spain. Gastroenterol. Hepatol. 2018, 41, 234–244. [Google Scholar] [CrossRef]
- Marrie, T.J.; Tyrrell, G.J.; Majumdar, S.R.; Eurich, D.T. Concurrent Infection with Hepatitis C Virus and Streptococcus pneumoniae. Emerg. Infect. Dis. 2017, 23, 1118–1123. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Anand, B.; Richardson, P.; Rabeneck, L. Association between hepatitis C infection and other infectious diseases: A case for targeted screening? Am. J. Gastroenterol. 2003, 98, 167–174. [Google Scholar] [CrossRef]
- Wu, P.H.; Lin, Y.T.; Hsieh, K.P.; Chuang, H.Y.; Sheu, C.C. Hepatitis C Virus Infection Is Associated With an Increased Risk of Active Tuberculosis Disease: A Nationwide Population-Based Study. Medicine 2015, 94, e1328. [Google Scholar] [CrossRef]
- Irvine, K.M.; Ratnasekera, I.; Powell, E.E.; Hume, D.A. Causes and Consequences of Innate Immune Dysfunction in Cirrhosis. Front. Immunol. 2019, 10, 293. [Google Scholar] [CrossRef]
- Albillos, A.; Lario, M.; Alvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef]
- Alvaro-Meca, A.; Jimenez-Sousa, M.A.; Boyer, A.; Medrano, J.; Reulen, H.; Kneib, T.; Resino, S. Impact of chronic hepatitis C on mortality in cirrhotic patients admitted to intensive-care unit. BMC Infect. Dis. 2016, 16, 122. [Google Scholar] [CrossRef]
- Terilli, R.R.; Cox, A.L. Immunity and hepatitis C: A review. Curr. HIV/AIDS Rep. 2013, 10, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Wang, J.L.; Dong, Y.H.; Chen, H.C.; Wu, L.C.; Chang, C.H. Incidence of hospitalization for infection among patients with hepatitis B or C virus infection without cirrhosis in Taiwan: A cohort study. PLoS Med. 2019, 16, e1002894. [Google Scholar] [CrossRef] [PubMed]
- Bunchorntavakul, C.; Chamroonkul, N.; Chavalitdhamrong, D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J. Hepatol. 2016, 8, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Spearman, C.W.; Dusheiko, G.M.; Hellard, M.; Sonderup, M. Hepatitis C. Lancet 2019, 394, 1451–1466. [Google Scholar] [CrossRef]
- European Association for Study. EASL Recommendations on Treatment of Hepatitis C 2015. J. Hepatol. 2015, 63, 199–236. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef]
- Vincent, J.L.; Sakr, Y.; Sprung, C.L.; Ranieri, V.M.; Reinhart, K.; Gerlach, H.; Moreno, R.; Carlet, J.; Le Gall, J.R.; Payen, D.; et al. Sepsis in European intensive care units: Results of the SOAP study. Crit. Care Med. 2006, 34, 344–353. [Google Scholar] [CrossRef]
- Mayr, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P. The Surviving Sepsis Campaign: Where have we been and where are we going? Cleve. Clin. J. Med. 2015, 82, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, R.; Artigas, A.; Levy, M.M.; Blanco, J.; Gonzalez-Diaz, G.; Garnacho-Montero, J.; Ibanez, J.; Palencia, E.; Quintana, M.; de la Torre-Prados, M.V.; et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA 2008, 299, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S. Sepsis, severe sepsis and septic shock: Changes in incidence, pathogens and outcomes. Expert Rev. Anti Infect. Ther. 2012, 10, 701–706. [Google Scholar] [CrossRef]
- Gaieski, D.F.; Edwards, J.M.; Kallan, M.J.; Carr, B.G. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit. Care Med. 2013, 41, 1167–1174. [Google Scholar] [CrossRef]
- Vincent, J.L. Increasing awareness of sepsis: World Sepsis Day. Crit. Care 2012, 16, 152. [Google Scholar] [CrossRef]
- Chalupka, A.N.; Talmor, D. The economics of sepsis. Crit. Care Clin. 2012, 28, 57–76. [Google Scholar] [CrossRef]
- Subdirección General de Información Sanitaria e Innovación. Registro de Actividad de Atención Especializada (RAE-CMBD). Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/cmbdhome.htm (accessed on 31 March 2020).
- Dombrovskiy, V.Y.; Martin, A.A.; Sunderram, J.; Paz, H.L. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit. Care Med. 2007, 35, 1244–1250. [Google Scholar] [CrossRef]
- Shen, H.N.; Lu, C.L.; Yang, H.H. Epidemiologic trend of severe sepsis in Taiwan from 1997 through 2006. Chest 2010, 138, 298–304. [Google Scholar] [CrossRef]
- R Development Core Team. R: The R Project for Statistical Computing. The R Foundation for Statistical Computing; R Development Core Team: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 8 March 2020).
- Boix, R.; Cano, R.; Gallego, P.; Vallejo, F.; Fernandez-Cuenca, R.; Noguer, I.; Larrauri, A. Hepatitis C hospitalizations in Spain, 2004–2013: A retrospective epidemiological study. BMC Health Serv. Res. 2017, 17, 461. [Google Scholar] [CrossRef]
- Bouza, C.; Lopez-Cuadrado, T.; Saz-Parkinson, Z.; Amate-Blanco, J.M. Epidemiology and recent trends of severe sepsis in Spain: A nationwide population-based analysis (2006–2011). BMC Infect. Dis. 2014, 14, 3863. [Google Scholar] [CrossRef] [PubMed]
- Alvaro-Meca, A.; Jimenez-Sousa, M.A.; Micheloud, D.; Sanchez-Lopez, A.; Heredia-Rodriguez, M.; Tamayo, E.; Resino, S.; Group of Biomedical Research in Critical Care. Epidemiological trends of sepsis in the twenty-first century (2000–2013): An analysis of incidence, mortality, and associated costs in Spain. Popul Health Metr. 2018, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Blachier, M.; Leleu, H.; Peck-Radosavljevic, M.; Valla, D.C.; Roudot-Thoraval, F. The burden of liver disease in Europe: A review of available epidemiological data. J. Hepatol. 2013, 58, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Razavi, H.; Waked, I.; Sarrazin, C.; Myers, R.P.; Idilman, R.; Calinas, F.; Vogel, W.; Mendes Correa, M.C.; Hezode, C.; Lazaro, P.; et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J. Viral Hepat. 2014, 21 (Suppl. 1), 34–59. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Kempker, J.A.; Martin, G.S. The Changing Epidemiology and Definitions of Sepsis. Clin. Chest Med. 2016, 37, 165–179. [Google Scholar] [CrossRef]
- Stevenson, E.K.; Rubenstein, A.R.; Radin, G.T.; Wiener, R.S.; Walkey, A.J. Two decades of mortality trends among patients with severe sepsis: A comparative meta-analysis*. Crit. Care Med. 2014, 42, 625–631. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Segev, D.L.; Thuluvath, P.J. Nationwide increase in hospitalizations and hepatitis C among inpatients with cirrhosis and sequelae of portal hypertension. Clin. Gastroenterol. Hepatol. 2007, 5, 1092–1099. [Google Scholar] [CrossRef]
- Beste, L.A.; Leipertz, S.L.; Green, P.K.; Dominitz, J.A.; Ross, D.; Ioannou, G.N. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology 2015, 149, 1471–1482.e5. [Google Scholar] [CrossRef]
- Xu, F.; Tong, X.; Leidner, A.J. Hospitalizations and costs associated with hepatitis C and advanced liver disease continue to increase. Health Aff. 2014, 33, 1728–1735. [Google Scholar] [CrossRef]
- Ly, K.N.; Xing, J.; Klevens, R.M.; Jiles, R.B.; Ward, J.W.; Holmberg, S.D. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann. Intern. Med. 2012, 156, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.P.; Liu, M.; Shaheen, A.A. The burden of hepatitis C virus infection is growing: A Canadian population-based study of hospitalizations from 1994 to 2004. Can. J. Gastroenterol. 2008, 22, 381–387. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Angus, D.C. Declining case fatality rates for severe sepsis: Good data bring good news with ambiguous implications. JAMA 2014, 311, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wei, X.; Chen, T.; Huang, C.; Liu, H.; Wang, Y. Characterization of fibrosis changes in chronic hepatitis C patients after virological cure: A systematic review with meta-analysis. J. Gastroenterol. Hepatol. 2017, 32, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Hajarizadeh, B.; Grebely, J.; Dore, G.J.; Matthews, G.V. Management of acute HCV infection in the era of direct-acting antiviral therapy. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 412–424. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Otgonsuren, M.; Henry, L.; Arsalla, Z.; Stepnaova, M.; Mishra, A.; Venkatesan, C.; Hunt, S. Inpatient resource utilization, disease severity, mortality and insurance coverage for patients hospitalized for hepatitis C virus in the United States. J. Viral Hepat. 2015, 22, 137–145. [Google Scholar] [CrossRef]
- Luo, R.; Greenberg, A.; Stone, C.D. Increasing Volume but Decreasing Mortality of Hospitalized Hepatitis C Patients in the United States, 2005 to 2011. J. Clin. Gastroenterol. 2015, 49, 620–627. [Google Scholar] [CrossRef]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef]
- Lagu, T.; Rothberg, M.B.; Shieh, M.S.; Pekow, P.S.; Steingrub, J.S.; Lindenauer, P.K. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit. Care Med. 2012, 40, 754–761. [Google Scholar] [CrossRef]
- Kumar, G.; Kumar, N.; Taneja, A.; Kaleekal, T.; Tarima, S.; McGinley, E.; Jimenez, E.; Mohan, A.; Khan, R.A.; Whittle, J.; et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest 2011, 140, 1223–1231. [Google Scholar] [CrossRef]
- Sutton, J.P.; Friedman, B. Trends in Septicemia Hospitalizations and Readmissions in Selected HCUP States, 2005 and 2010: Statistical Brief #161. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2006. [Google Scholar]
- Quintano Neira, R.A.; Hamacher, S.; Japiassu, A.M. Epidemiology of sepsis in Brazil: Incidence, lethality, costs, and other indicators for Brazilian Unified Health System hospitalizations from 2006 to 2015. PLoS ONE 2018, 13, e0195873. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.; Lee, H.; Ahn, S. Epidemiology of sepsis in Korea: A population-based study of incidence, mortality, cost and risk factors for death in sepsis. Clin. Exp. Emerg. Med. 2019, 6, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Legido-Quigley, H.; Karanikolos, M.; Hernandez-Plaza, S.; de Freitas, C.; Bernardo, L.; Padilla, B.; Sa Machado, R.; Diaz-Ordaz, K.; Stuckler, D.; McKee, M. Effects of the financial crisis and Troika austerity measures on health and health care access in Portugal. Health Policy 2016, 120, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Correia, T.; Dussault, G.; Pontes, C. The impact of the financial crisis on human resources for health policies in three southern-Europe countries. Health Policy 2015, 119, 1600–1605. [Google Scholar] [CrossRef]
- Pereira, P.L.; Casanova, A.P.; Sanz-Barbero, B. A “Before and After” in the Use of Emergency Services in Spain? The Impact of the Economic Crisis. Int. J. Health Serv. 2016, 46, 430–447. [Google Scholar] [CrossRef]
- Nuno Solinis, R.; Arratibel Ugarte, P.; Rojo, A.; Sanchez Gonzalez, Y. Value of Treating All Stages of Chronic Hepatitis C: A Comprehensive Review of Clinical and Economic Evidence. Infect. Dis. Ther. 2016, 5, 491–508. [Google Scholar] [CrossRef]
- Hassan, E.A.; Rehim, A.; Abdel-Malek, M.O.; Ahmed, A.O.; Abbas, N.M. Are there differences in risk factors, microbial aspects, and prognosis of cellulitis between compensated and decompensated hepatitis C virus-related cirrhosis? Clin. Mol. Hepatol. 2019, 25, 317–325. [Google Scholar] [CrossRef]
- Kaka, A.S.; Filice, G.A.; Kuskowski, M.; Musher, D.M. Does active hepatitis C virus infection increase the risk for infection due to Staphylococcus aureus? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1217–1223. [Google Scholar] [CrossRef]
- Bartoletti, M.; Giannella, M.; Lewis, R.E.; Viale, P. Bloodstream infections in patients with liver cirrhosis. Virulence 2016, 7, 309–319. [Google Scholar] [CrossRef]
- Esteban, A.; Frutos-Vivar, F.; Ferguson, N.D.; Penuelas, O.; Lorente, J.A.; Gordo, F.; Honrubia, T.; Algora, A.; Bustos, A.; Garcia, G.; et al. Sepsis incidence and outcome: Contrasting the intensive care unit with the hospital ward. Crit. Care Med. 2007, 35, 1284–1289. [Google Scholar] [CrossRef]
- Andreu Ballester, J.C.; Ballester, F.; Gonzalez Sanchez, A.; Almela Quilis, A.; Colomer Rubio, E.; Penarroja Otero, C. Epidemiology of sepsis in the Valencian Community (Spain), 1995–2004. Infect. Control Hosp. Epidemiol. 2008, 29, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S. Altered Microbiota in Cirrhosis and Its Relationship to the Development of Infection. Clin. Liver Dis. 2019, 14, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Putignani, L.; Paroni Sterbini, F.; Petito, V.; Picca, A.; Del Chierico, F.; Reddel, S.; Calvani, R.; Marzetti, E.; Sanguinetti, M.; et al. Influence of hepatitis C virus eradication with direct-acting antivirals on the gut microbiota in patients with cirrhosis. Aliment. Pharmacol. Ther. 2018, 48, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, X.; Ge, H.; Jiang, Y.; Zhou, H.; Zheng, W. Targeted surveillance of nosocomial infection in intensive care units of 176 hospitals in Jiangsu province, China. J. Hosp. Infect. 2018, 99, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J.; Odden, A.; Rohde, J.; Bonham, C.; Kuhn, L.; Malani, P.; Chen, L.; Flanders, S. Identifying patients with severe sepsis using administrative claims: Patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med. Care 2014, 52, e39–e43. [Google Scholar] [CrossRef]
- Whittaker, S.A.; Mikkelsen, M.E.; Gaieski, D.F.; Koshy, S.; Kean, C.; Fuchs, B.D. Severe sepsis cohorts derived from claims-based strategies appear to be biased toward a more severely ill patient population. Crit. Care Med. 2013, 41, 945–953. [Google Scholar] [CrossRef]
- Mainor, A.J.; Morden, N.E.; Smith, J.; Tomlin, S.; Skinner, J. ICD-10 Coding Will Challenge Researchers: Caution and Collaboration may Reduce Measurement Error and Improve Comparability Over Time. Med. Care 2019, 57, e42–e46. [Google Scholar] [CrossRef]

| Entire Period | 2000–2003 | 2004–2007 | 2008–2011 | 2012–2015 | p-Value (*) | |
|---|---|---|---|---|---|---|
| No. of Hospital Admissions | 70,976 | 10,723 | 17,463 | 20,832 | 21,958 | |
| Gender (Male) | 44,825 (63.2%) | 6781 (63.2%) | 11,227 (64.3%) | 13,102 (62.9%) | 13,715 (62.5%) | 0.006 |
| Age (Years) | 60 (16.7) | 57.4 (17.9) | 58.6 (17.2) | 60.2 (16.5) | 62.3 (15.5) | <0.001 |
| ≥50 Years | 46,544 (65.6%) | 6508 (60.7%) | 10,501 (60.1%) | 13,223 (63.5%) | 16,312 (74.3%) | <0.001 |
| Substances of Abuse | ||||||
| Drugs | 23,819 (33.6%) | 3223 (30.1%) | 5913 (33.8%) | 7384 (35.4%) | 7299 (33.2%) | <0.001 |
| Alcohol | 7881 (11.1%) | 950 (8.8%) | 1787 (10.2%) | 2391 (11.5%) | 2753 (12.5%) | <0.001 |
| Tobacco | 14,571 (20.5%) | 1651 (15.4%) | 3476 (19.9%) | 4636 (22.2%) | 4808 (21.9%) | <0.001 |
| Urgent Admission | 64,398 (90.7%) | 9573 (89.3%) | 15,760 (90.2%) | 18,927 (90.8%) | 20,138 (91.7%) | <0.001 |
| Surgical Condition | 7251 (10.2%) | 1163 (10.8%) | 1853 (10.6%) | 2081 (9.9%) | 2154 (9.8%) | 0.001 |
| Charlson Index | 4.9 (2.9) | 4.8 (2.7) | 4.9 (2.8) | 4.9 (2.9) | 4.9 (2.9) | <0.001 |
| Liver Disease Severity | ||||||
| Non-Complicated Hepatitis C | 39,077 (55.1%) | 5764 (53.7%) | 9690 (55.5%) | 11,625 (55.8%) | 11,998 (54.6%) | 0.418 |
| Compensated Cirrhosis | 23,000 (32.4%) | 3311 (30.9%) | 5498 (31.5%) | 6684 (32.1%) | 7507 (34.2%) | <0.001 |
| End-Stage Liver Disease | 26,207 (36.9%) | 4327 (40.3%) | 6577 (37.6%) | 7569 (36.3%) | 7734 (35.2%) | <0.001 |
| Hepatocellular Carcinoma | 4222 (5.9%) | 471 (4.4%) | 901 (5.1%) | 1295 (6.2%) | 1555 (7.1%) | <0.001 |
| Liver Transplant | 1982 (2.8%) | 217 (2.0%) | 428 (2.4%) | 637 (3.1%) | 700 (3.2%) | <0.001 |
| Number of Acute Organ Dysfunction | ||||||
| Average | 1.3 (0.7) | 1.2 (0.6) | 1.3 (0.6) | 1.3 (0.7) | 1.4 (0.7) | <0.001 |
| 1 | 54,622 (77%) | 8852 (82.5%) | 13,746 (78.7%) | 15,970 (76.6%) | 16,054 (73.1%) | <0.001 |
| 2 | 12,008 (16.9%) | 1451 (13.5%) | 2824 (16.2%) | 3507 (16.8%) | 4226 (19.2%) | <0.001 |
| >2 | 4346 (6.1%) | 420 (3.9%) | 893 (5.1%) | 1355 (6.5%) | 1678 (7.6%) | <0.001 |
| Acute Organ Dysfunction | ||||||
| Cardiovascular | 6721 (9.5%) | 870 (8.1%) | 1547 (8.8%) | 2088 (10%) | 2216 (10.1%) | <0.001 |
| Hematologic | 9487 (13.4%) | 1251 (11.7%) | 2204 (12.6%) | 2649 (12.7%) | 3383 (15.4%) | <0.001 |
| Hepatic | 14,542 (20.5%) | 2931 (27.3%) | 4167 (23.8%) | 4220 (20.2%) | 3224 (14.7%) | <0.001 |
| Neurologic | 3024 (4.3%) | 494 (4.61%) | 711 (4.1%) | 835 (4%) | 984 (4.5%) | 0.898 |
| Renal | 21,264 (30%) | 2752 (25.6%) | 4559 (26.1%) | 6064 (29.1%) | 7889 (35.9%) | <0.001 |
| Respiratory | 35,023 (49.3%) | 4579 (42.7%) | 8568 (49.1%) | 10,755 (51.6%) | 11,121 (50.6%) | <0.001 |
| Metabolic | 3420 (4.8%) | 294 (2.7%) | 628 (3.6%) | 1002 (4.8%) | 1496 (6.8%) | <0.001 |
| Site of Infection | ||||||
| Respiratory | 32,864 (46.3%) | 4999 (46.6%) | 8304 (47.5%) | 9662 (46.4%) | 9899 (45.1%) | <0.001 |
| Digestive | 23,528 (33.1%) | 3984 (37.1%) | 5964 (34.1%) | 6795 (32.6%) | 6785 (30.9%) | <0.001 |
| Genitourinary | 16,576 (23.4%) | 2420 (22.6%) | 3862 (22.1%) | 4811 (23.1%) | 5483 (24.9%) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvaro-Meca, A.; Maté-Cano, I.; Ryan, P.; Briz, V.; Resino, S. Epidemiological Trend of Sepsis in Patients with Hospital Admissions Related to Hepatitis C in Spain (2000–2015): A Nationwide Study. J. Clin. Med. 2020, 9, 1607. https://doi.org/10.3390/jcm9061607
Alvaro-Meca A, Maté-Cano I, Ryan P, Briz V, Resino S. Epidemiological Trend of Sepsis in Patients with Hospital Admissions Related to Hepatitis C in Spain (2000–2015): A Nationwide Study. Journal of Clinical Medicine. 2020; 9(6):1607. https://doi.org/10.3390/jcm9061607
Chicago/Turabian StyleAlvaro-Meca, Alejandro, Irene Maté-Cano, Pablo Ryan, Verónica Briz, and Salvador Resino. 2020. "Epidemiological Trend of Sepsis in Patients with Hospital Admissions Related to Hepatitis C in Spain (2000–2015): A Nationwide Study" Journal of Clinical Medicine 9, no. 6: 1607. https://doi.org/10.3390/jcm9061607
APA StyleAlvaro-Meca, A., Maté-Cano, I., Ryan, P., Briz, V., & Resino, S. (2020). Epidemiological Trend of Sepsis in Patients with Hospital Admissions Related to Hepatitis C in Spain (2000–2015): A Nationwide Study. Journal of Clinical Medicine, 9(6), 1607. https://doi.org/10.3390/jcm9061607





