Post-Infectious Myocardial Infarction: Does Percutaneous Coronary Intervention Improve Outcomes? A Propensity Score-Matched Analysis †
Abstract
1. Introduction
2. Methods
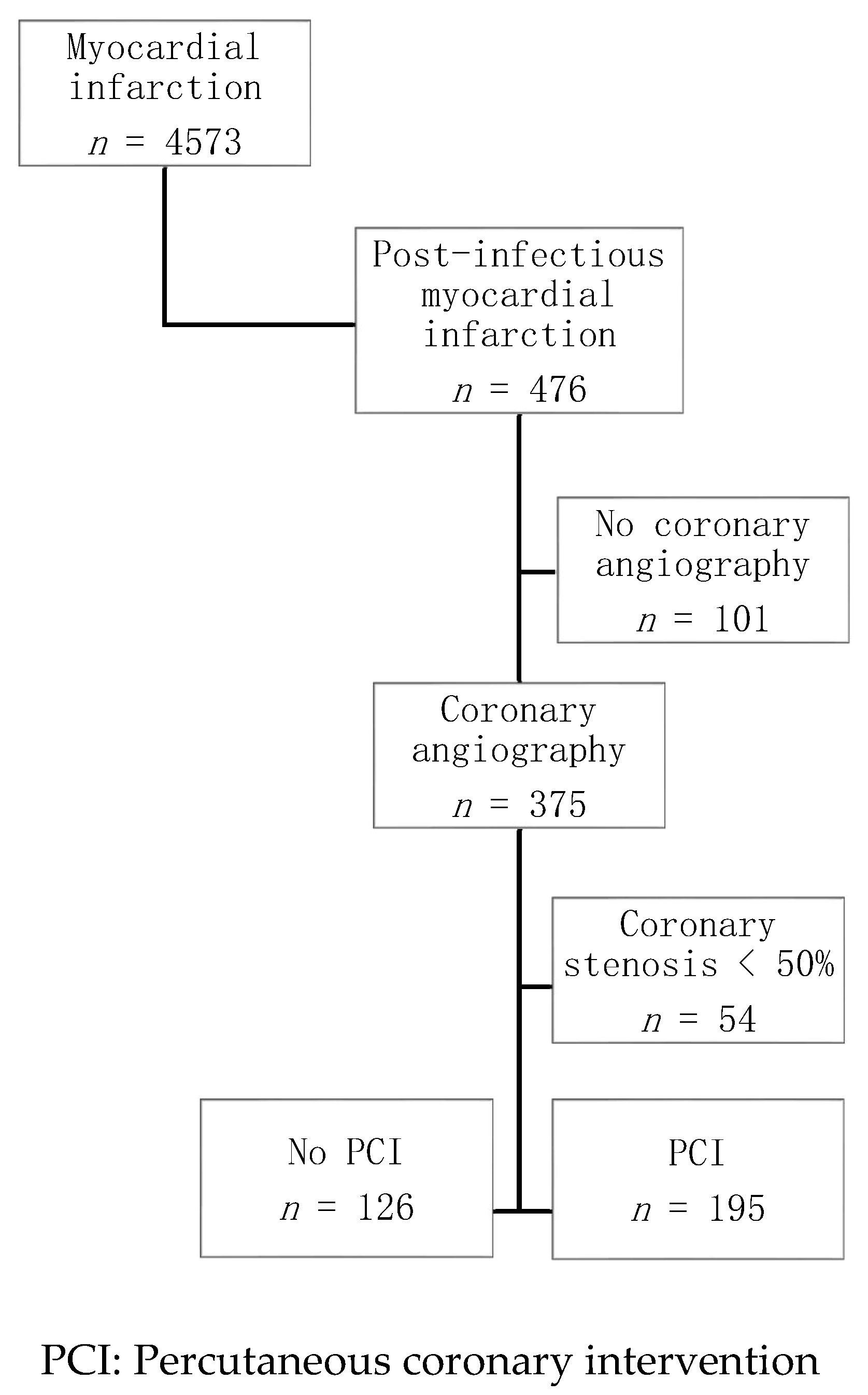
2.1. Study Population
2.2. Definitions
2.3. Data Collection
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
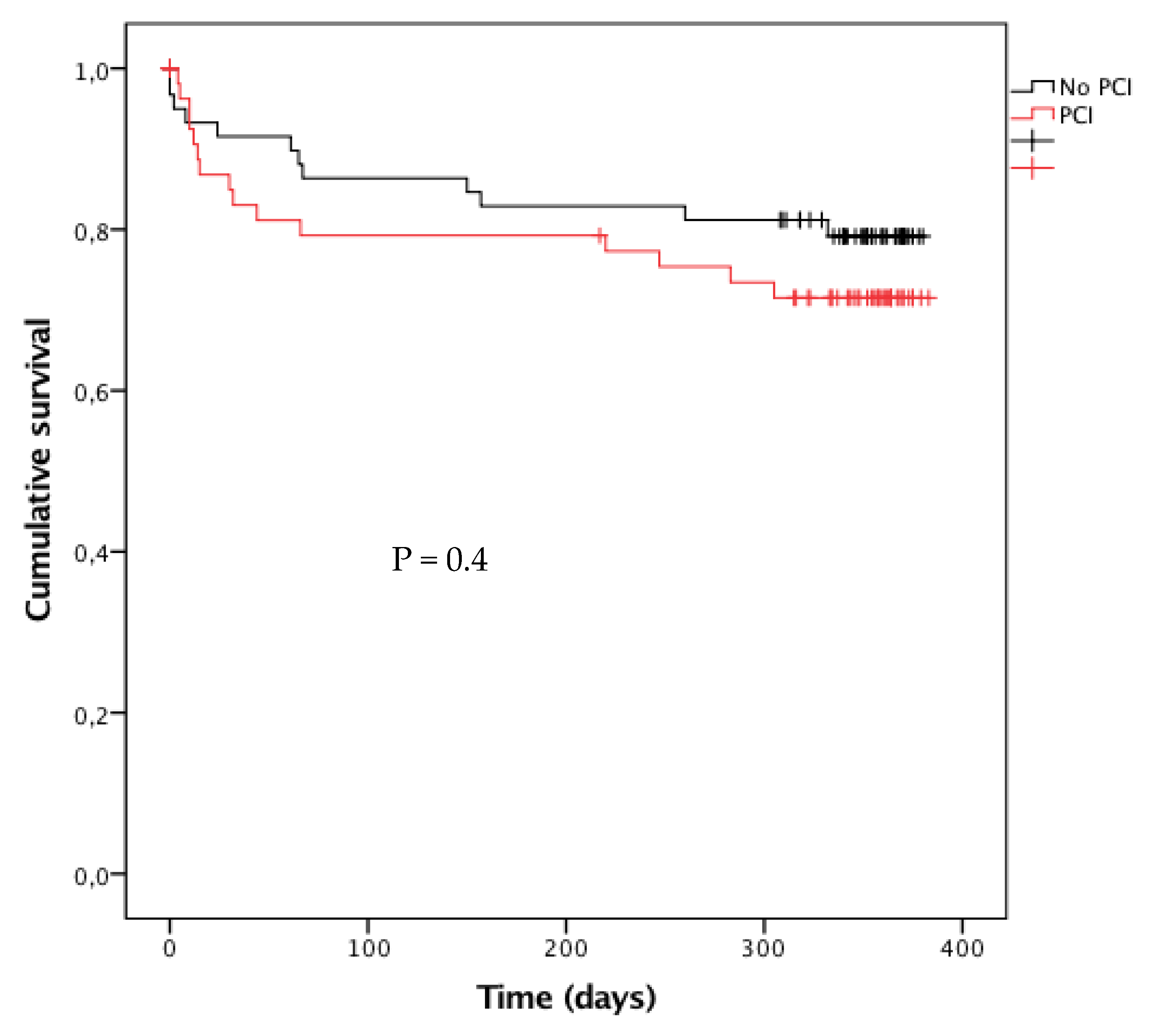
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Musher, D.M.; Abers, M.S.; Corrales-Medina, V.F. Acute Infection and Myocardial Infarction. N. Engl. J. Med. 2019, 380, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Medina, V.F.; Madjid, M.; Musher, D.M. Role of acute infection in triggering acute coronary syndromes. Lancet Infect. Dis. 2010, 10, 83–92. [Google Scholar] [CrossRef]
- Putot, A.; Chague, F.; Manckoundia, P.; Cottin, Y.; Zeller, M. Post-Infectious Myocardial Infarction: New Insights for Improved Screening. J. Clin. Med. 2019, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, N.R.; Gupta, N.; Guo, Y.; Bangalore, S. Comparison of Outcomes of Patients with Sepsis With Versus Without Acute Myocardial Infarction and Comparison of Invasive Versus Noninvasive Management of the Patients With Infarction. Am. J. Cardiol. 2016, 117, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.-S.; Chiang, C.-H.; Hung, W.-T.; Tang, P.-L.; Hung, C.C.; Kuo, S.-H.; Liu, C.-P.; Chen, Y.-S.; Mar, G.-Y.; Huang, W.-C. Comparison of long-term mortality in patients with acute myocardial infarction associated with or without sepsis. Int. J. Infect. Dis. 2019, 79, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Lambrakis, K.; French, J.K.; Scott, I.A.; Briffa, T.; Brieger, D.; Farkouh, M.E.; White, H.; Chuang, A.M.-Y.; Tiver, K.; Quinn, S.; et al. The appropriateness of coronary investigation in myocardial injury and type 2 myocardial infarction (ACT-2): A randomized trial design. Am. Heart J. 2019, 208, 11–20. [Google Scholar] [CrossRef]
- DeFilippis, A.P.; Chapman, A.R.; Mills, N.L.; de Lemos, J.A.; Arbab-Zadeh, A.; Newby, L.K.; Morrow, D.A. Assessment and Treatment of Patients with Type 2 Myocardial Infarction and Acute Nonischemic Myocardial Injury. Circulation 2019, 140, 1661–1678. [Google Scholar] [CrossRef]
- Higuchi, S.; Suzuki, M.; Horiuchi, Y.; Tanaka, H.; Saji, M.; Yoshino, H.; Nagao, K.; Yamamoto, T.; Takayama, M. Higher non-cardiac mortality and lesser impact of early revascularization in patients with type 2 compared to type 1 acute myocardial infarction: Results from the Tokyo CCU Network registry. Heart Vessels 2019, 34, 1140–1147. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group; Thygesen, K.; Alpert, J.S.; et al. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Zeller, M. Impaired fasting glucose and cardiogenic shock in patients with acute myocardial infarction. Eur. Heart J. 2004, 25, 308–312. [Google Scholar] [CrossRef]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G.; et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Putot, A.; Jeanmichel, M.; Chague, F.; Manckoundia, P.; Cottin, Y.; Zeller, M. Type 2 Myocardial Infarction: A Geriatric Population-based Model of Pathogenesis. Aging Dis 2020, 11, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef] [PubMed]
- Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology; Bassand, J.-P.; Hamm, C.W.; Ardissino, D.; Boersma, E.; Budaj, A.; Fernández-Avilés, F.; Fox, K.A.A.; Hasdai, D.; Ohman, E.M.; et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur. Heart J. 2007, 28, 1598–1660. [Google Scholar] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Rich, M.W.; Chyun, D.A.; Skolnick, A.H.; Alexander, K.P.; Forman, D.E.; Kitzman, D.W.; Maurer, M.S.; McClurken, J.B.; Resnick, B.M.; Shen, W.K.; et al. Knowledge Gaps in Cardiovascular Care of the Older Adult Population: A Scientific Statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation 2016, 133, 2103–2122. [Google Scholar] [CrossRef]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; Pieper, K.S.; Eagle, K.A.; Cannon, C.P.; Van De Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef]
- Sianos, G.; Morel, M.-A.; Kappetein, A.P.; Morice, M.-C.; Colombo, A.; Dawkins, K.; van den Brand, M.; Van Dyck, N.; Russell, M.E.; Mohr, F.W.; et al. The SYNTAX Score: An angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005, 1, 219–227. [Google Scholar]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Kwong, J.C.; Schwartz, K.L.; Campitelli, M.A.; Chung, H.; Crowcroft, N.S.; Karnauchow, T.; Katz, K.; Ko, D.T.; McGeer, A.J.; McNally, D.; et al. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. New Engl. J. Med. 2018, 378, 345–353. [Google Scholar] [CrossRef]
- Meier, C.R.; Jick, S.S.; Derby, L.E.; Vasilakis, C.; Jick, H. Acute respiratory-tract infections and risk of first-time acute myocardial infarction. Lancet 1998, 351, 1467–1471. [Google Scholar] [CrossRef]
- D’Souza, M.; Saaby, L.; Poulsen, T.S.; Diederichsen, A.C.P.; Hosbond, S.; Diederichsen, S.Z.; Larsen, T.B.; Schmidt, H.; Gerke, O.; Hallas, J.; et al. Comparison of mortality in patients with acute myocardial infarction accidentally admitted to non-cardiology departments versus that in patients admitted to coronary care units. Am. J. Cardiol. 2014, 114, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.N.; Gade, C.L.; Slotwiner, A.J.; Parikh, M.; Bergman, G.; Wong, S.C.; Minutello, R.M. New York State Angioplasty Registry Comparison of outcomes of percutaneous coronary interventions in patients of three age groups (<60, 60 to 80, and >80 years) (from the New York State Angioplasty Registry). Am. J. Cardiol. 2006, 98, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Modica, A.; Karlsson, F.; Mooe, T. Platelet aggregation and aspirin non-responsiveness increase when an acute coronary syndrome is complicated by an infection. J. Thromb. Haemost. 2007, 5, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Piccaro de Oliveira, P.; Gonzales, V.; Lopes, R.D.; Schmidt, M.M.; Garofallo, S.; Santos, R.P.D.; Carrion, L.; Gottschall, C.; Quadros, A.S. Serious infections among unselected patients with ST-elevation myocardial infarction treated with contemporary primary percutaneous coronary intervention. Am. Heart J. 2016, 181, 52–59. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Sandoval, Y. The Many Faces of Type 2 Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 70, 1569–1572. [Google Scholar] [CrossRef]
- Putot, A.; Derrida, S.B.; Zeller, M.; Avondo, A.; Ray, P.; Manckoundia, P.; Cottin, Y. Short-Term Prognosis of Myocardial Injury, Type 1 and Type 2 Myocardial Infarction in the Emergency Unit. Am. J. Med. 2018, 131, 1209–1219. [Google Scholar] [CrossRef]
- Neumann, J.T.; Sörensen, N.A.; Rübsamen, N.; Ojeda, F.; Renné, T.; Qaderi, V.; Teltrop, E.; Kramer, S.; Quantius, L.; Zeller, T.; et al. Discrimination of patients with type 2 myocardial infarction. Eur. Heart J. 2017, 38, 3514–3520. [Google Scholar] [CrossRef]
- Sandoval, Y.; Jaffe, A.S. Type 2 Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 73, 1846–1860. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
| PCI n = 195 | No PCI n = 126 | p | PCI (Post-Match) n = 62 | No PCI (Post-Match) n = 62 | p | |
|---|---|---|---|---|---|---|
| Risk factors and comorbidities | ||||||
| Age, years | 74 (61–83) | 77 (67–83) | 0.1 | 76 (65–83) | 77 (69–83) | 0.5 |
| Female | 80 (41) | 40 (32) | 0.09 | 34 (50) | 29 (48) | 0.4 |
| Hypertension | 122 (63) | 99 (79) | 0.002 | 46 (74) | 48 (77) | 0.7 |
| Hypercholesterolemia | 96 (49) | 66 (52) | 0.6 | 30 (48) | 30 (48) | 1 |
| Family history of CAD | 50 (26) | 27 (21) | 0.4 | 17 (27) | 12 (19) | 0.3 |
| Smoking | 100 (51) | 75 (59) | 0.1 | 28 (45) | 33 (53) | 0.4 |
| Diabetes | 67 (34) | 59 (47) | 0.03 | 27 (44) | 29 (47) | 0.7 |
| Chronic renal failure | 20 (10) | 24 (19) | 0.02 | 7 (11) | 9 (14) | 0.6 |
| COPD | 33 (17) | 26 (21) | 0.4 | 10 (16) | 9 (14) | 0.8 |
| Neoplasia | 33 (17) | 21 (17) | 1 | 10 (16) | 9 (14) | 0.8 |
| Cardiovascular history | ||||||
| CAD | 57 (29) | 56 (44) | 0.005 | 13 (21) | 20 (32) | 0.2 |
| Stroke | 20 (10) | 17 (13) | 0.4 | 10 (16) | 6 (10) | 0.3 |
| PAD | 26 (13) | 29 (23) | 0.02 | 12 (19) | 8 (13) | 0.3 |
| HF | 13 (7) | 13 (10) | 0.2 | 4 (6) | 2 (3) | 0.4 |
| Atrial fibrillation | 10 (5) | 20 (16) | 0.001 | 4 (6) | 5 (8) | 0.7 |
| Aortic stenosis | 14 (7) | 14 (11) | 0.2 | 4 (6) | 7 (11) | 0.3 |
| Type of MI | ||||||
| Type 1 | 103 (53) | 24 (19) | <0.001 | 22 (35) | 16 (26) | 0.2 |
| Type 2 | 92 (47) | 102 (81) | <0.001 | 40 (63) | 46 (71) | 0.2 |
| Type of infection | ||||||
| Bronchitis | 96 (49) | 45 (36) | 0.02 | 23 (44) | 24 (36) | 0.9 |
| Pneumonia | 37 (19) | 41 (33) | 0.006 | 19 (24) | 16 (26) | 0.5 |
| Urinary tract infection | 28 (14) | 22 (17) | 0.5 | 11 (18) | 11 (18) | 1 |
| Other infections | 34 (17) | 18 (14) | 0.5 | 8 (13) | 8 (13) | 1 |
| Clinical data | ||||||
| HR, beats/min | 80 (67–96) | 85 (73–103) | 0.004 | 81 (70–100) | 81 (72–94) | 1 |
| SBP, mmHg | 135 (114–156) | 135 (118–153) | 0.8 | 130 (117–155) | 136 (119–161) | 0.5 |
| DBP, mmHg | 76 (65–90) | 75 (62–82) | 0.1 | 72 (64–88) | 75 (62–83) | 0.7 |
| GRACE risk score | 168 (139–192) | 177 (153–197) | 0.1 | 172 (141–203) | 171 (151–189) | 0.7 |
| Acute HF * | 92 (47) | 72 (57) | 0.08 | 37 (60) | 36 (58) | 0.9 |
| LVEF < 40% | 66 (35) | 48 (39) | 0.4 | 22 (36) | 18 (30) | 0.5 |
| Cardiac arrest | 14 (7) | 3 (2) | 0.06 | 3 (5) | 1 (2) | 0.6 |
| ECG | ||||||
| STEMI | 100 (51) | 40 (32) | 0.001 | 29 (47) | 23 (37) | 0.3 |
| AF/flutter | 17 (9) | 21 (17) | 0.09 | 8 (13) | 6 (10) | 0.6 |
| LBBB | 21 (11) | 14 (11) | 0.4 | 5 (8) | 8 (13) | 0.4 |
| Biological data | ||||||
| Hemoglobin, g/100 mL | 13.2 (12.2–15.0) | 12.4 (10.6–13.6) | 0.001 | 13.1 (11.6–15.4) | 13.7 (10.5–14.1) | 0.1 |
| Leukocytes, G/L | 13.6 (12.1–14.8) | 11.1 (9.5–15.1) | 0.2 | 13.9 (12.7–19.5) | 11.2(6.7–14.2) | 0.1 |
| CRP, mg/L | 12 (4–78) | 49 (10–105) | <0.001 | 35 (6–122) | 24 (5–89) | 0.7 |
| eGFR, mL/min | 69 (48–89) | 57 (37–84) | 0.01 | 69 (46–88) | 58 (39–78) | 0.1 |
| Troponin I peak, µg/L | 17 (4–72) | 6 (1–20) | <0.001 | 13 (4–53) | 10 (1–23) | 0.1 |
| NT-proBNP, pg/mL | 1950 (368–6302) | 4475 (1673–10915) | <0.001 | 4187 (968–18634) | 4491 (1565–9626) | 0.7 |
| Angiographic data | ||||||
| 1-Vessel disease | 68 (35) | 29 (23) | 0.02 | 20 (32) | 17 (27) | 0.6 |
| 2-Vessel disease | 57 (29) | 31 (25) | 0.4 | 19 (31) | 18 (29) | 0.8 |
| 3-Vessel disease or Left main | 70 (36) | 66 (52) | 0.004 | 23 (37) | 27 (43) | 0.5 |
| SYNTAX score | 11 (6–19) | 19 (11–28) | <0.001 | 14 (8–20) | 15 (10–21) | 0.5 |
| Chronic treatment | ||||||
| Aspirin | 60 (31) | 50 (40) | 0.1 | 19 (31) | 19 (31) | 1 |
| Other antiplatelets | 33 (17) | 35 (28) | 0.02 | 11 (18) | 15 (24) | 0.4 |
| Beta-blockers | 75 (38) | 59 (47) | 0.1 | 21 (34) | 24 (39) | 0.6 |
| CCB | 46 (24) | 39 (31) | 0.1 | 17 (24) | 22 (35) | 0.8 |
| ARB | 40 (20) | 36 (29) | 0.1 | 13 (21) | 21 (34) | 0.1 |
| ACEI | 45 (23) | 46 (37) | 0.009 | 18 (29) | 17 (27) | 0.8 |
| Statins | 72 (37) | 66 (52) | 0.006 | 19 (31) | 28 (45) | 0.1 |
| Diuretics | 79 (40) | 63 (50) | 0.09 | 29 (47) | 32 (52) | 0.6 |
| AVK/DOA | 22 (11) | 23 (18) | 0.08 | 8 (13) | 12 (19) | 0.3 |
| PCI n = 195 | No PCI n = 126 | p | PCI (Post-Match) n = 62 | No PCI (Post-Match) n = 62 | p | |
|---|---|---|---|---|---|---|
| Reperfusion procedures | ||||||
| Thrombolysis | 5 (3) | 1 (1) | 0.4 | 1 (2) | 0 | 1 |
| CABG | 3 (2) | 14 (11) | 0.07 | 2 (3) | 7 (1) | 0.2 |
| First 48-h treatment | ||||||
| Amines | 26 (14) | 10 (8) | 0.1 | 8 (13) | 3 (5) | 0.2 |
| Aspirin | 183 (97) | 115 (94) | 0.2 | 60 (97) | 59 (95) | 1 |
| Other antiplatelets | 179 (95) | 99 (81) | <0.001 | 59 (95) | 51 (82) | 0.04 |
| Beta-blockers | 123 (65) | 80 (65) | 1 | 37 (60) | 43 (69) | 0.3 |
| CCB | 49 (26) | 31 (25) | 0.9 | 16 (26) | 21 (34) | 0.3 |
| ARB | 13 (7) | 11 (9) | 0.5 | 2 (3) | 6 (10) | 0.3 |
| ACEI | 83 (44) | 46 (37) | 0.3 | 30 (48) | 16 (26) | 0.009 |
| Statins | 158 (84) | 111 (90) | 0.1 | 51 (82) | 59 (95) | 0.04 |
| Diuretics | 88 (47) | 76 (62) | 0.008 | 33 (53) | 40 (64) | 0.2 |
| Nitrates | 84 (44) | 41 (33) | 0.05 | 34 (55) | 23 (37) | 0.05 |
| LMWH | 130 (67) | 69 (56) | 0.02 | 41 (66) | 33 (53) | 0.1 |
| UFH | 56 (30) | 50 (41) | 0.05 | 22 (35) | 24 (39) | 0.7 |
| AVK/DOA | 19 (10) | 17 (13) | 0.3 | 5 (8) | 10 (16) | 0.3 |
| Treatment at discharge | ||||||
| Aspirin | 168 (89) | 104 (85) | 0.3 | 53 (85) | 53 (85) | 1 |
| Other antiplatelets | 162 (86) | 45 (37) | <0.001 | 47 (76) | 19 (31) | <0.001 |
| Beta-blockers | 138 (73) | 94 (76) | 0.5 | 41 (66) | 48 (77) | 0.2 |
| CCB | 30 (16) | 22 (18) | 0.6 | 14 (23) | 11 (18) | 0.5 |
| ARB | 13 (7) | 13 (11) | 0.2 | 2 (3) | 7 (11) | 0.2 |
| ACEI | 132 (70) | 61 (50) | <0.001 | 47 (76) | 27 (43) | <0.001 |
| Statins | 157 (83) | 99 (81) | 0.6 | 46 (74) | 50 (81) | 0.4 |
| Diuretics | 67 (35) | 67 (54) | 0.001 | 24 (39) | 37 (60) | 0.02 |
| Nitrates | 20 (11) | 18 (15) | 0.3 | 11 (18) | 5 (8) | 0.1 |
| AVK/DOA | 27 (14) | 38 (30) | <0.001 | 9 (15) | 18 (29) | 0.01 |
| PCI n = 195 | No PCI n = 126 | OR (95% CI) | p | PCI (Post-Match) n = 62 | No PCI (Post-Match) n = 62 | OR (95% CI) | p | |
|---|---|---|---|---|---|---|---|---|
| In-hospital events | ||||||||
| ICU stay >5 days | 69 (35) | 54 (43) | 0.73 (0.46–1.15) | 0.2 | 34 (55) | 32 (52) | 0.88 (0.43–1.78) | 0.7 |
| Severe HF * | 56 (29) | 41 (32) | 0.83 (0.51–1.36) | 0.5 | 21 (34) | 19 (31) | 1.16 (0.55–2.46) | 0.7 |
| VT/VF | 15 (8) | 6 (5) | 1.66 (0.59–5.39) | 0.3 | 6 (10) | 3 (5) | 2.09 (0.42–13.6) | 0.5 |
| Atrial fibrillation | 22 (12) | 23 (19) | 1.74 (0.88–3.47) | 0.2 | 8 (13) | 13 (21) | 0.56 (0.18–1.61) | 0.3 |
| Bleeding # | 14 (7) | 22 (17) | 0.37 (0.17–0.79) | 0.004 | 7 (11) | 11 (18) | 0.59 (0.18–1.82) | 0.3 |
| Re-infarction | 8 (4) | 4 (3) | 1.31 (0.39–4.43) | 0.7 | 4 (6) | 3 (5) | 1.36 (0.29–6.33) | 0.7 |
| All-cause mortality | 17 (9) | 12 (9) | 0.91 (0.42–1.97) | 0.8 | 8 (13) | 5 (8) | 1.69 (0.52–5.48) | 0.4 |
| CV mortality | 15 (8) | 11 (9) | 0.87 (0.39–1.96) | 0.7 | 7 (11) | 5 (8) | 1.45 (0.43–4.85) | 0.5 |
| One-year events | ||||||||
| Recurrent MI | 9 (5) | 5 (5) | 1.12 (0.52–2.20) | 0.8 | 4 (6) | 2 (3) | 2.00 (0.34–11.0) | 0.5 |
| Hospitalization for HF | 7 (4) | 8 (6) | 0.52 (0.18–1.48) | 0.2 | 2 (3) | 6 (10) | 0.29 (0.06–1.51) | 0.2 |
| All-cause mortality | 35 (18) | 30 (24) | 0.71 (0.41–1.22) | 0.2 | 15 (24) | 12 (19) | 1.33 (0.56–3.13) | 0.5 |
| CV mortality | 29 (15) | 16 (13) | 1.17 (0.61–2.27) | 0.6 | 13 (21) | 8 (13) | 1.73 (0.66–4.54) | 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putot, A.; Chagué, F.; Manckoundia, P.; Brunel, P.; Beer, J.-C.; Cottin, Y.; Zeller, M. Post-Infectious Myocardial Infarction: Does Percutaneous Coronary Intervention Improve Outcomes? A Propensity Score-Matched Analysis. J. Clin. Med. 2020, 9, 1608. https://doi.org/10.3390/jcm9061608
Putot A, Chagué F, Manckoundia P, Brunel P, Beer J-C, Cottin Y, Zeller M. Post-Infectious Myocardial Infarction: Does Percutaneous Coronary Intervention Improve Outcomes? A Propensity Score-Matched Analysis. Journal of Clinical Medicine. 2020; 9(6):1608. https://doi.org/10.3390/jcm9061608
Chicago/Turabian StylePutot, Alain, Frédéric Chagué, Patrick Manckoundia, Philippe Brunel, Jean-Claude Beer, Yves Cottin, and Marianne Zeller. 2020. "Post-Infectious Myocardial Infarction: Does Percutaneous Coronary Intervention Improve Outcomes? A Propensity Score-Matched Analysis" Journal of Clinical Medicine 9, no. 6: 1608. https://doi.org/10.3390/jcm9061608
APA StylePutot, A., Chagué, F., Manckoundia, P., Brunel, P., Beer, J.-C., Cottin, Y., & Zeller, M. (2020). Post-Infectious Myocardial Infarction: Does Percutaneous Coronary Intervention Improve Outcomes? A Propensity Score-Matched Analysis. Journal of Clinical Medicine, 9(6), 1608. https://doi.org/10.3390/jcm9061608






