Managing Bladder Cancer Care during the COVID-19 Pandemic Using a Team-Based Approach
Abstract
1. Introduction
2. Balancing the Need for Bladder Cancer Treatments and Risk of Exposure to COVID-19
2.1. Patients with Bladder Cancer Undergoing Treatments Are at a Higher Risk for COVID-19 Infections and Worse Outcomes Compared to the General Population without Cancer
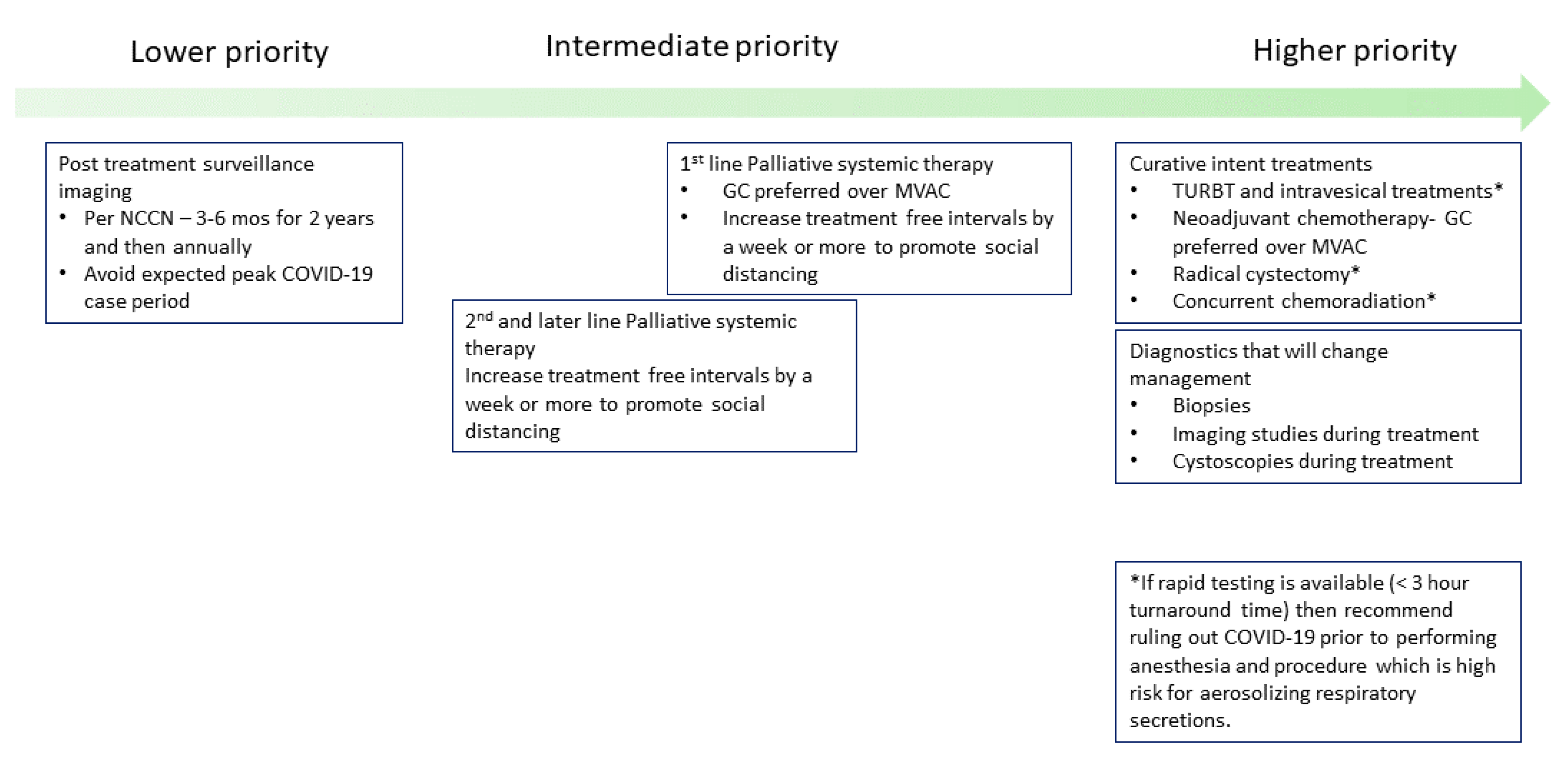
2.2. Prioritizing Treatments Appropriately and Applying Social Distancing
2.3. Applying COVID-19 Risk Mitigation Measures for Bladder Cancer Treatment
2.3.1. Delay Post-Treatment Surveillance Visits until There Is a Decrease in COVID-19 Cases
2.3.2. Continue Curative Intent Treatments for Localized Bladder Cancer with COVID-19 Precautions
2.3.3. Increasing Off-Treatment Period between Cycles for Palliative Systemic Therapy in Metastatic Urothelial Carcinoma Patients
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| COVID-19 | coronavirus disease 2019 |
| FDA | Federal Drug administration |
| GC | gemcitabine/cisplatin |
| ddMVAC | dose-dense methotrexate, vinblastine, doxorubicin, cisplatin |
| NCCN | National Comprehensive Cancer Network |
References
- Novel Coronavirus (2019-nCoV) Situation Reports. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 4 April 2020).
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 46, 854–887. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020, 21, e181. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, R.; Zhao, J.; Li, W.; Shen, H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020, 21, e180. [Google Scholar] [CrossRef]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, N.M.; Mahmud, S.; Aprikian, A.G. Delay in the surgical treatment of bladder cancer and survival: Systematic review of the literature. Eur. Urol. 2006, 50, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Audenet, F.; Sfakianos, J.P.; Waingankar, N.; Ruel, N.H.; Galsky, M.D.; Yuh, B.E.; Gin, G.E. A delay ≥8 weeks to neoadjuvant chemotherapy before radical cystectomy increases the risk of upstaging. Urol. Oncol. 2019, 37, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Gawande, A. Keeping the Coronavirus from Infecting Health-Care Workers. Available online: https://www.newyorker.com/news/news-desk/keeping-the-coronavirus-from-infecting-health-care-workers (accessed on 12 April 2020).
- Dash, A.; Pettus, J.A.; Herr, H.W.; Bochner, B.H.; Dalbagni, G.; Donat, S.M.; Russo, P.; Boyle, M.G.; Milowsky, M.I.; Bajorin, D.F. A Role for Neoadjuvant Gemcitabine Plus Cisplatin in Muscle-Invasive Urothelial Carcinoma of the Bladder: A Retrospective Experience. Cancer 2008, 113, 2471–2477. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Jacobus, S.; Bellmunt, J.; Qu, A.; Appleman, L.J.; Tretter, C.; Bubley, G.J.; Stack, E.C.; Signoretti, S.; Walsh, M.; et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: Pathologic, radiologic, and biomarker correlates. J. Clin. Oncol. 2014, 32, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Liu, S.; Joseph, T.; Lyou, Y. Managing Bladder Cancer Care during the COVID-19 Pandemic Using a Team-Based Approach. J. Clin. Med. 2020, 9, 1574. https://doi.org/10.3390/jcm9051574
Wang T, Liu S, Joseph T, Lyou Y. Managing Bladder Cancer Care during the COVID-19 Pandemic Using a Team-Based Approach. Journal of Clinical Medicine. 2020; 9(5):1574. https://doi.org/10.3390/jcm9051574
Chicago/Turabian StyleWang, Tina, Sariah Liu, Thomas Joseph, and Yung Lyou. 2020. "Managing Bladder Cancer Care during the COVID-19 Pandemic Using a Team-Based Approach" Journal of Clinical Medicine 9, no. 5: 1574. https://doi.org/10.3390/jcm9051574
APA StyleWang, T., Liu, S., Joseph, T., & Lyou, Y. (2020). Managing Bladder Cancer Care during the COVID-19 Pandemic Using a Team-Based Approach. Journal of Clinical Medicine, 9(5), 1574. https://doi.org/10.3390/jcm9051574




