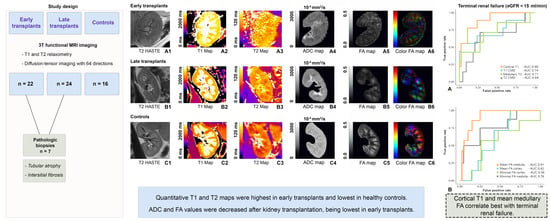
Multiparametric Assessment of Changes in Renal Tissue after Kidney Transplantation with Quantitative MR Relaxometry and Diffusion-Tensor Imaging at 3 T
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design and Population
2.2. Imaging Protocol
2.3. Imaging Analysis
2.4. Statistical Analysis
3. Results
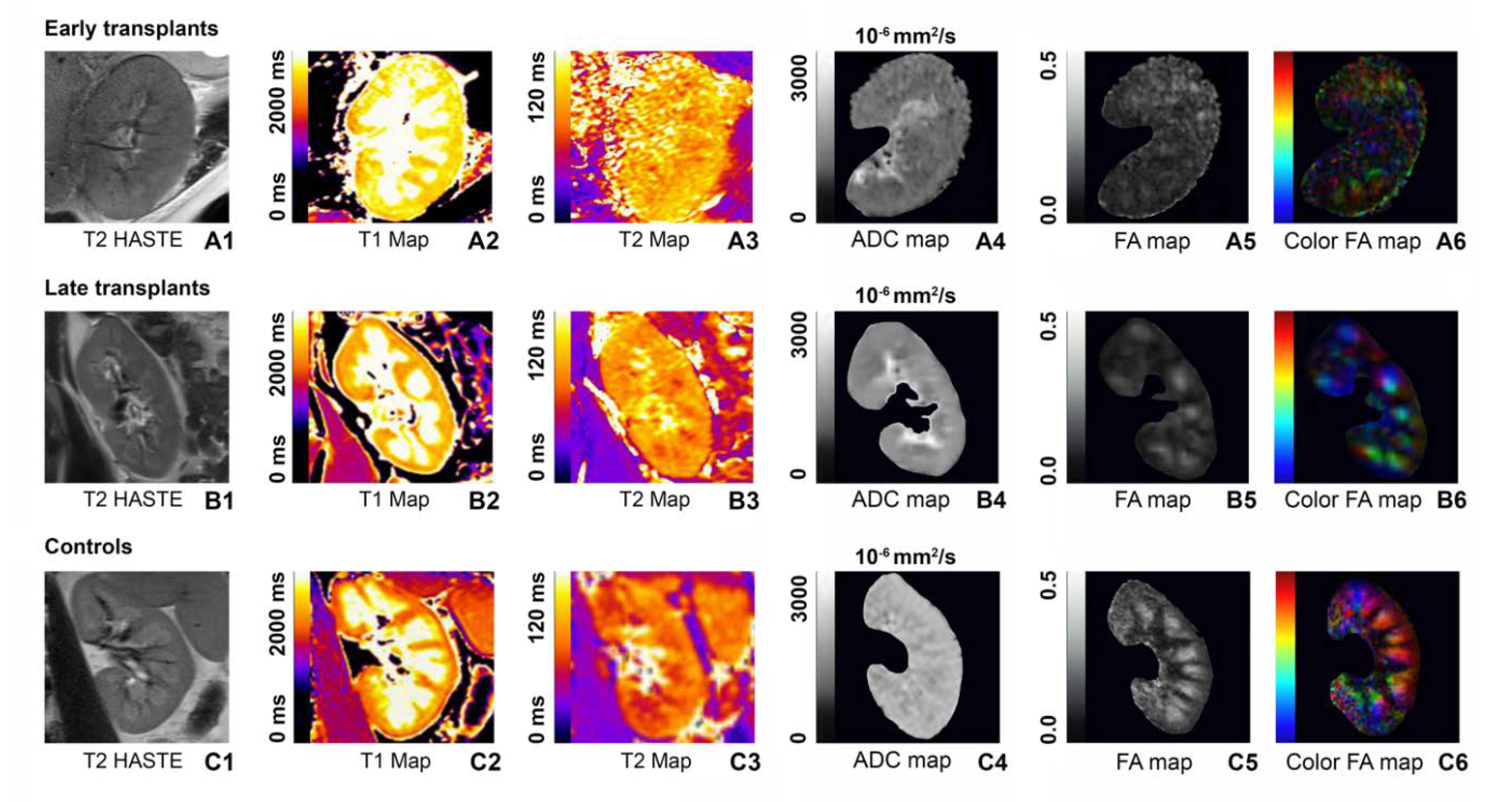
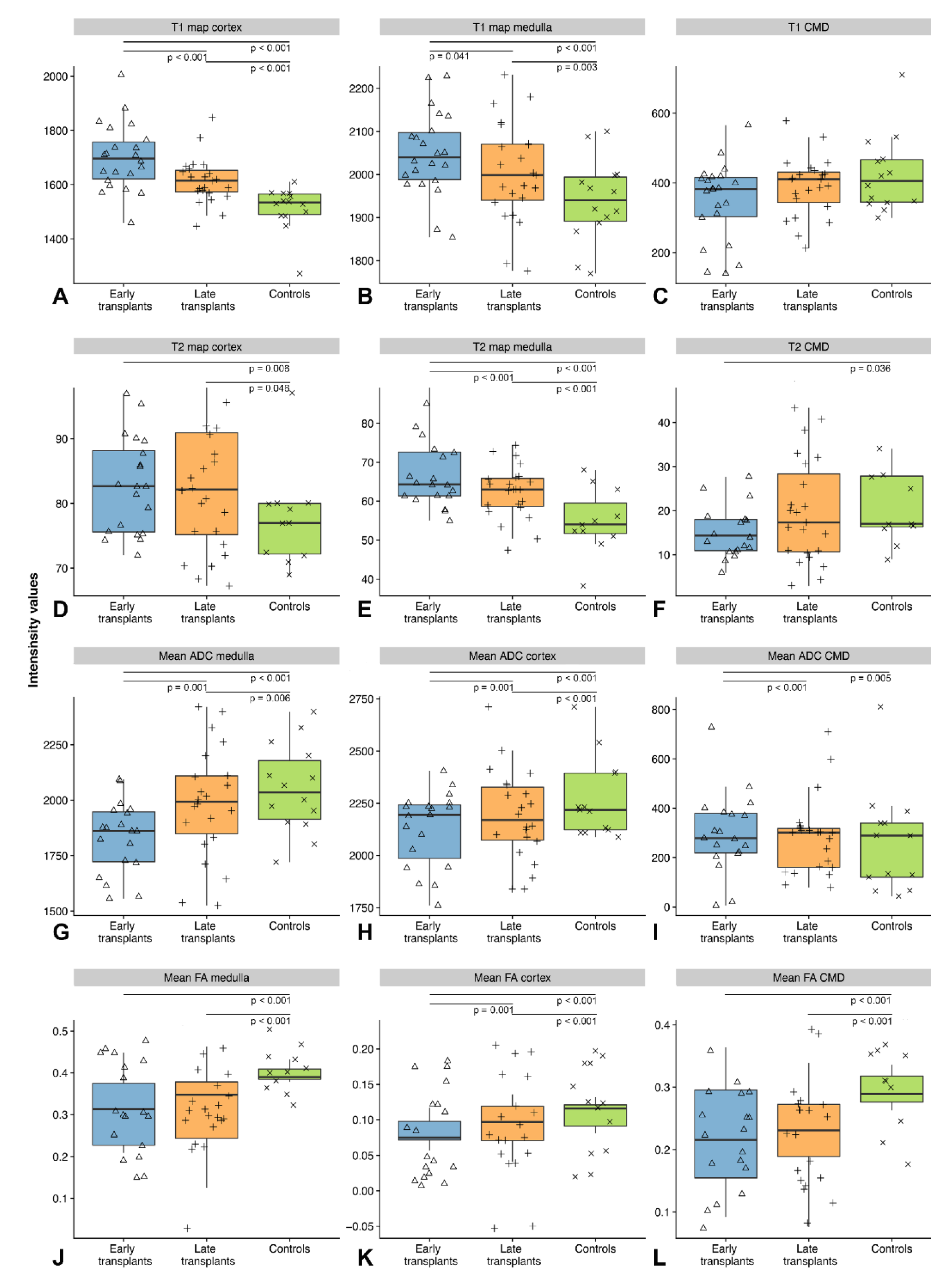
3.1. T1 Relaxometry
3.2. T2 Relaxometry
3.3. Diffusion-Tensor Imaging
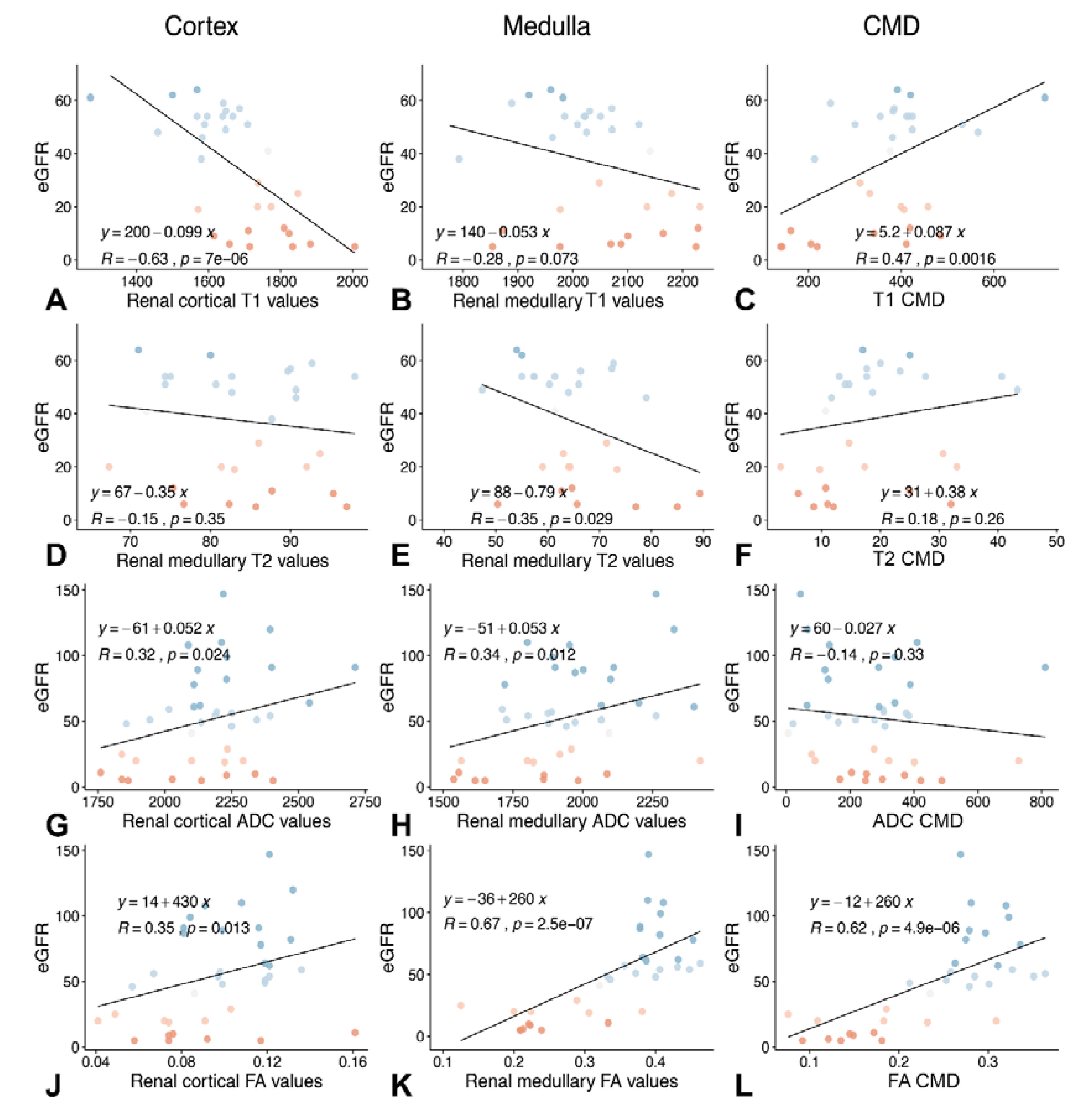
3.4. Association between T1 and T2 Relaxation Times and Cortical and Medullary FA Values and Renal Function
3.5. Diagnostic Performance of MRR and DTI Parameters Compared to Laboratory Markers of Renal Function and Biopsy Results
3.6. Longitudinal Subgroup
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abecassis, M.; Bartlett, S.T.; Collins, A.J.; Davis, C.L.; Delmonico, F.L.; Friedewald, J.J.; Hays, R.; Howard, A.; Jones, E.; Leichtman, A.B.; et al. Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin. J. Am. Soc. Nephrol. 2008, 3, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhang, Z.J. Therapeutic strategies of kidney transplant ischemia reperfusion injury: Insight from mouse models. Biomed. J. Sci. Tech. Res. 2019, 14, 002617. [Google Scholar] [PubMed]
- Basile, D.P. The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int. 2007, 72, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C. Ischaemia-reperfusion injury: A major protagonist in kidney transplantation. Nephrol. Dial. Transplant. 2014, 29, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Faga, T.; Pisani, A.; Perticone, M.; Michael, A. The ischemic/nephrotoxic acute kidney injury and the use of renal biomarkers in clinical practice. Eur. J. Intern. Med. 2017, 39, 1–8. [Google Scholar] [CrossRef]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef]
- Hoste, E.A.; Clermont, G.; Kersten, A.; Venkataraman, R.; Angus, D.C.; De Bacquer, D.; Kellum, J.A. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit. Care 2006, 10, R73. [Google Scholar] [CrossRef]
- Coca, S.G.; Yalavarthy, R.; Concato, J.; Parikh, C.R. Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systematic review. Kidney Int. 2008, 73, 1008–1016. [Google Scholar] [CrossRef]
- Leveridge, M.J.; Finelli, A.; Kachura, J.R.; Evans, A.; Chung, H.; Shiff, D.A.; Fernandes, K.; Jewett, M.A. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur. Urol. 2011, 60, 578–584. [Google Scholar] [CrossRef]
- Berl, T. American society of nephrology renal research report. J. Am. Soc. Nephrol 2005, 16, 1886–1903. [Google Scholar] [CrossRef]
- Mendichovszky, I.; Pullens, P.; Dekkers, I.; Nery, F.; Bane, O.; Pohlmann, A.; de Boer, A.; Ljimani, A.; Odudu, A.; Buchanan, C.; et al. Technical recommendations for clinical translation of renal MRI: A consensus project of the Cooperation in Science and Technology Action PARENCHIMA. Magn. Reson. Mater. Phys. Biol. Med. 2019. [CrossRef] [PubMed]
- Wolf, M.; de Boer, A.; Sharma, K.; Boor, P.; Leiner, T.; Sunder-Plassmann, G.; Moser, E.; Caroli, A.; Jerome, N.P. Magnetic resonance imaging T1- and T2-mapping to assess renal structure and function: A systematic review and statement paper. Nephrol. Dial. Transplant. 2018, 33, ii41–ii50. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.C.; Ralla, B.; Jurmeister, P.; Bressem, K.K.; Fahlenkamp, U.L.; Hamm, B.; Busch, J.; Makowski, M.R. Native T1 mapping as an in vivo biomarker for the identification of higher-grade renal cell carcinoma: Correlation with histopathological findings. Invest. Radiol. 2019, 54, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Hueper, K.; Hensen, B.; Gutberlet, M.; Chen, R.; Hartung, D.; Barrmeyer, A.; Meier, M.; Li, W.; Jang, M.-S.; Mengel, M. Kidney transplantation: Multiparametric functional magnetic resonance imaging for assessment of renal allograft pathophysiology in mice. Investig. Radiol. 2016, 51, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Caroli, A.; Schneider, M.; Friedli, I.; Ljimani, A.; De Seigneux, S.; Boor, P.; Gullapudi, L.; Kazmi, I.; Mendichovszky, I.A.; Notohamiprodjo, M.; et al. Diffusion-weighted magnetic resonance imaging to assess diffuse renal pathology: A systematic review and statement paper. Nephrol. Dial. Transplant. 2018, 33, ii29–ii40. [Google Scholar] [CrossRef] [PubMed]
- Hueper, K.; Khalifa, A.A.; Brasen, J.H.; Vo Chieu, V.D.; Gutberlet, M.; Wintterle, S.; Lehner, F.; Richter, N.; Peperhove, M.; Tewes, S.; et al. Diffusion-Weighted imaging and diffusion tensor imaging detect delayed graft function and correlate with allograft fibrosis in patients early after kidney transplantation. J. Magn. Reson. Imaging 2016, 44, 112–121. [Google Scholar] [CrossRef]
- Friedli, I.; Crowe, L.A.; Berchtold, L.; Moll, S.; Hadaya, K.; de Perrot, T.; Vesin, C.; Martin, P.Y.; de Seigneux, S.; Vallee, J.P. New magnetic resonance imaging index for renal fibrosis assessment: A comparison between diffusion-weighted imaging and T1 mapping with histological validation. Sci. Rep. 2016, 6, 30088. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am. J. Kidney Dis. 2010, 55, 622–627. [Google Scholar] [CrossRef]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Dekkers, I.A.; de Boer, A.; Sharma, K.; Cox, E.F.; Lamb, H.J.; Buckley, D.L.; Bane, O.; Morris, D.M.; Prasad, P.V.; Semple, S.I.K.; et al. Consensus-based technical recommendations for clinical translation of renal T1 and T2 mapping MRI. MAGMA 2020, 33, 163–176. [Google Scholar] [CrossRef]
- Ljimani, A.; Caroli, A.; Laustsen, C.; Francis, S.; Mendichovszky, I.A.; Bane, O.; Nery, F.; Sharma, K.; Pohlmann, A.; Dekkers, I.A.; et al. Consensus-based technical recommendations for clinical translation of renal diffusion-weighted MRI. Magn. Reson. Mater. Phys. Biol. Med. 2020, 33, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Sigmund, E.E.; Rusinek, H.; Chandarana, H.; Storey, P.; Chen, Q.; Lee, V.S. Optimization of b-value sampling for diffusion-weighted imaging of the kidney. Magn. Reson. Med. 2012, 67, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Figini, M.; Scotti, A.; Marcuzzo, S.; Bonanno, S.; Padelli, F.; Moreno-Manzano, V.; Garcia-Verdugo, J.M.; Bernasconi, P.; Mantegazza, R.; Bruzzone, M.G.; et al. Comparison of diffusion MRI acquisition protocols for the in vivo characterization of the mouse spinal cord: Variability analysis and application to an amyotrophic lateral sclerosis model. PLoS ONE 2016, 11, e0161646. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, Y.; Kundel, H. Magnetic resonance imaging following unilateral occlusion of the renal circulation in rabbits. Radiology 1985, 154, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E. Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014, 85, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Peperhove, M.; Vo Chieu, V.D.; Jang, M.S.; Gutberlet, M.; Hartung, D.; Tewes, S.; Warnecke, G.; Fegbeutel, C.; Haverich, A.; Gwinner, W.; et al. Assessment of acute kidney injury with T1 mapping MRI following solid organ transplantation. Eur. Radiol. 2018, 28, 44–50. [Google Scholar] [CrossRef]
- Huang, Y.; Sadowski, E.A.; Artz, N.S.; Seo, S.; Djamali, A.; Grist, T.M.; Fain, S.B. Measurement and comparison of T1 relaxation times in native and transplanted kidney cortex and medulla. J. Magn. Reson. Imaging 2011, 33, 1241–1247. [Google Scholar] [CrossRef]
- Bane, O.; Hectors, S.J.; Gordic, S.; Kennedy, P.; Wagner, M.; Weiss, A.; Khaim, R.; Yi, Z.; Zhang, W.; Delaney, V.; et al. Multiparametric magnetic resonance imaging shows promising results to assess renal transplant dysfunction with fibrosis. Kidney Int. 2020, 97, 414–420. [Google Scholar] [CrossRef]
- Kido, A.; Kataoka, M.; Yamamoto, A.; Nakamoto, Y.; Umeoka, S.; Koyama, T.; Maetani, Y.; Isoda, H.; Tamai, K.; Morisawa, N.; et al. Diffusion tensor MRI of the kidney at 3.0 and 1.5 Tesla. Acta Radiol. 2010, 51, 1059–1063. [Google Scholar] [CrossRef]
- Notohamiprodjo, M.; Dietrich, O.; Horger, W.; Horng, A.; Helck, A.D.; Herrmann, K.A.; Reiser, M.F.; Glaser, C. Diffusion tensor imaging (DTI) of the kidney at 3 tesla-feasibility, protocol evaluation and comparison to 1.5 Tesla. Invest. Radiol. 2010, 45, 245–254. [Google Scholar] [CrossRef]
- Hueper, K.; Peperhove, M.; Rong, S.; Gerstenberg, J.; Mengel, M.; Meier, M.; Gutberlet, M.; Tewes, S.; Barrmeyer, A.; Chen, R.; et al. T1-mapping for assessment of ischemia-induced acute kidney injury and prediction of chronic kidney disease in mice. Eur. Radiol. 2014, 24, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Hueper, K.; Gutberlet, M.; Rodt, T.; Gwinner, W.; Lehner, F.; Wacker, F.; Galanski, M.; Hartung, D. Diffusion tensor imaging and tractography for assessment of renal allograft dysfunction-initial results. Eur. Radiol. 2011, 21, 2427–2433. [Google Scholar] [CrossRef] [PubMed]
- Lanzman, R.S.; Ljimani, A.; Pentang, G.; Zgoura, P.; Zenginli, H.; Kropil, P.; Heusch, P.; Schek, J.; Miese, F.R.; Blondin, D.; et al. Kidney transplant: Functional assessment with diffusion-tensor MR imaging at 3T. Radiology 2013, 266, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Selby, N.M.; Blankestijn, P.J.; Boor, P.; Combe, C.; Eckardt, K.U.; Eikefjord, E.; Garcia-Fernandez, N.; Golay, X.; Gordon, I.; Grenier, N.; et al. Magnetic resonance imaging biomarkers for chronic kidney disease: A position paper from the European Cooperation in Science and Technology Action PARENCHIMA. Nephrol. Dial. Transplant. 2018, 33, ii4–ii14. [Google Scholar] [CrossRef]
- de Boer, A.; Harteveld, A.A.; Stemkens, B.; Blankestijn, P.J.; Bos, C.; Franklin, S.L.; Froeling, M.; Joles, J.A.; Verhaar, M.C.; van den Berg, N.; et al. Multiparametric Renal MRI: An Intrasubject Test-Retest Repeatability Study. J. Magn. Reson. Imaging 2020. [Google Scholar] [CrossRef]

| Sequence | T2 Single-Shot FSE * | Dixon | T1 Mapping | T2 Mapping | DTI |
|---|---|---|---|---|---|
| Scan plane | Oblique coronal | Oblique coronal | Oblique coronal | Oblique coronal | Axial |
| Voxel size (mm) | 0.7 × 0.7 × 4.0 | 1.6 × 1.6 × 1.3 | 0.8 × 0.8 × 4.0 | 1.9 × 1.9 × 4.0 | 2.7 × 2.7 × 2.7 |
| Acquisition time (min) | 1:36 | 00:23 | 3:33 | 1:51 | 8:14 |
| Number of slices | 31 | 31 | 19 | 7 | 50 |
| TR/TE (ms) | 1200/94 | 4.21/1.26; 2.49 | 551.28/1.35 | 766.29/1.44 | 6400/75 |
| Averages | 1 | 1 | 1 | 1 | |
| FoV (mm) | 350 | 400 | 350 | 360 | 350 |
| Flip angle (°) | 153 | 9 | 35 | 12 | 90 |
| Matrix | 512 | 256 | 224 | 192 | 128 |
| Bandwidth (Hz/Px) | 407 | 780 | 1063 | 1184 | 1698 |
| Fat saturation | None/Yes | None | None | None | Strong |
| Number of preparations (duration in ms) | 2 | 5 (0, 30, 34, 38, 42) | |||
| Trigger delay (ms) | 379 | 164 | |||
| Breath-holding procedures | 19 | 7 | 12 | ||
| b-values | 2 (0, 600) | ||||
| Diffusion directions | 64 (2 signals acquired) | ||||
| Echo spacing (ms) | 5.18 | 3.04 | 3.29 | 0.65 |
| Early Transplants | Late Transplants | Controls | |
|---|---|---|---|
| Number (men/women) | 22 (17/5) | 24 (20/4) | 16 (10/6) |
| Age (years) ± SD | 54.90 ± 0.13 | 51.26 ± 12.94 | 59.9 ± 14.4 |
| eGFR (mL/min) ± SD | 31.54 ± 4.95 | 47.48 ± 12.73 | 80.53 ± 21.43 |
| Time since transplantation | 6.8 ± 3.4 days | 5.5 ± 5.1 years | NA |
| T1 in ms ± SD (cortex, medulla) | 1700 ± 53, 2048 ± 72 | 1615 ± 47, 2004 ± 68 | 1514 ± 29, 1939 ± 51 |
| T2 in ms ± SD (cortex, medulla) | 83 ± 6, 67 ± 5 | 82 ± 8, 62 ± 3 | 78 ± 4, 59 ± 2 |
| Mean FA ± SD (cortex, medulla) | 0.085 ± 0.006, 0.310 ± 0.006 | 0.093 ± 0.008, 0.318 ± 0.022 | 0.108 ± 0.005, 0.401 ± 0.006 |
| ADC in 10−6 mm2/s ± SD (cortex, medulla) | 2130 ± 45, 1834 ± 40 | 2189 ± 54, 1988 ± 63 | 2269 ± 48, 2051 ± 49 |
| Patient | T1 Map Cortex (ms) | T1 Map Medulla (ms) | T2 Map Cortex (ms) | T2 Map Medulla (ms) | ||||
|---|---|---|---|---|---|---|---|---|
| TP 1 | TP 2 | TP 1 | TP 2 | TP 1 | TP 2 | TP 1 | TP 2 | |
| 1 | 1710 | 1617 | 1872 | 1884 | 88 | 79 | 63 | 56 |
| 2 | 1582 | 1584 | 1963 | 1924 | 91 | 96 | 79 | 83 |
| 3 | 2005 | 1707 | 2224 | 2030 | 80 | 95 | 72 | 75 |
| 4 | 1736 | 1638 | 2135 | 2057 | 81 | 88 | 64 | 67 |
| 5 | 1882 | 1691 | 2088 | 1975 | 77 | 70 | 66 | 59 |
| 6 | 1675 | 1413 | 1974 | 1839 | 70 | 85 | 63 | 51 |
| Patient | Mean ADC Cortex (10−6 mm2/s) | Mean ADC Medulla (10−6 mm2/s) | Mean FA Cortex | Mean FA Medulla | ||||
| TP 1 | TP 2 | TP 1 | TP 2 | TP 1 | TP 2 | TP 1 | TP 2 | |
| 1 | 1760 | 2222 | 1556 | 2029 | 0.161 | 0.152 | 0.210 | 0.333 |
| 2 | 2250 | 2342 | 1941 | 2207 | 0.057 | 0.106 | 0.279 | 0.336 |
| 3 | 2137 | 2411 | 1650 | 1843 | 0.117 | 0.068 | 0.209 | 0.307 |
| 4 | 2293 | 2329 | 1565 | 1983 | 0.072 | 0.079 | 0.310 | 0.381 |
| 5 | 2028 | 2296 | 1860 | 2100 | 0.092 | 0.136 | 0.213 | 0.226 |
| 6 | 2141 | 2337 | 2327 | 2018 | 0.053 | 0.072 | 0.204 | 0.403 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adams, L.C.; Bressem, K.K.; Scheibl, S.; Nunninger, M.; Gentsch, A.; Fahlenkamp, U.L.; Eckardt, K.-U.; Hamm, B.; Makowski, M.R. Multiparametric Assessment of Changes in Renal Tissue after Kidney Transplantation with Quantitative MR Relaxometry and Diffusion-Tensor Imaging at 3 T. J. Clin. Med. 2020, 9, 1551. https://doi.org/10.3390/jcm9051551
Adams LC, Bressem KK, Scheibl S, Nunninger M, Gentsch A, Fahlenkamp UL, Eckardt K-U, Hamm B, Makowski MR. Multiparametric Assessment of Changes in Renal Tissue after Kidney Transplantation with Quantitative MR Relaxometry and Diffusion-Tensor Imaging at 3 T. Journal of Clinical Medicine. 2020; 9(5):1551. https://doi.org/10.3390/jcm9051551
Chicago/Turabian StyleAdams, Lisa C., Keno K. Bressem, Sonja Scheibl, Max Nunninger, Andre Gentsch, Ute L. Fahlenkamp, Kai-Uwe Eckardt, Bernd Hamm, and Marcus R. Makowski. 2020. "Multiparametric Assessment of Changes in Renal Tissue after Kidney Transplantation with Quantitative MR Relaxometry and Diffusion-Tensor Imaging at 3 T" Journal of Clinical Medicine 9, no. 5: 1551. https://doi.org/10.3390/jcm9051551
APA StyleAdams, L. C., Bressem, K. K., Scheibl, S., Nunninger, M., Gentsch, A., Fahlenkamp, U. L., Eckardt, K.-U., Hamm, B., & Makowski, M. R. (2020). Multiparametric Assessment of Changes in Renal Tissue after Kidney Transplantation with Quantitative MR Relaxometry and Diffusion-Tensor Imaging at 3 T. Journal of Clinical Medicine, 9(5), 1551. https://doi.org/10.3390/jcm9051551