Associations of Sedentary Behaviour, Physical Activity, Cardiorespiratory Fitness and Body Composition with Risk of Sleep-Related Breathing Disorders in Children with Overweight/Obesity: A Cross-Sectional Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Procedures and Measurements
2.2.1. Sedentary Behaviour and Physical Activity
2.2.2. Cardiorespiratory Fitness
2.2.3. Body Composition Parameters
2.2.4. Sleep-Related Breathing Disorders
2.2.5. Covariates
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Sateia, M.J. International classification of sleep disorders: Third edition: Highlights and Modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Tsara, V.; Amfilochiou, A.; Papagrigorakis, J.; Georgopoulos, D.; Liolios, E.; Kadiths, A.; Koudoumnakis, E.; Aulonitou, E.; Emporiadou, M.; Tsakanikos, M.; et al. Guidelines for Diagnosing and Treating Sleep related Breathing Disorders in Adults and Children (Part 3: Obstructive Sleep Apnea in Children, Diagnosis and Treatment). Hippokratia 2010, 14, 57–62. [Google Scholar]
- Ma, Y.; Peng, L.; Kou, C.; Hua, S.; Yuan, H. Associations of Overweight, Obesity and Related Factors with Sleep-Related Breathing Disorders and Snoring in Adolescents: A Cross-Sectional Survey. Int. J. Environ. Res. Public Health 2017, 14, 194. [Google Scholar] [CrossRef]
- Scalzitti, N.J.; Sarber, K.M. Diagnosis and perioperative management in pediatric sleep-Disordered breathing. Pediatr. Anesth. 2018, 28, 940–946. [Google Scholar] [CrossRef]
- Ikävalko, T.; Tuomilehto, H.; Pahkala, R.; Tompuri, T.; Laitinen, T.; Myllykangas, R.; Vierola, A.; Lindi, V.; Närhi, M.; Lakka, T.A. Craniofacial morphology but not excess body fat is associated with risk of having sleep-disordered breathing-The PANIC Study (a questionnaire-based inquiry in 6-8-year-olds). Eur. J. Pediatr. 2012, 171, 1747–1752. [Google Scholar] [CrossRef]
- Dehlink, E.; Tan, H. Update on paediatric obstructive sleep apnoea. J. Thorac. Dis. 2016, 8, 224–235. [Google Scholar]
- Urquhart, D.S.; Hill, E.A.; Morley, A. Sleep-Disordered breathing in children. Paediatr. Child Health (Oxford) 2017, 27, 328–336. [Google Scholar] [CrossRef]
- Marcus, C.L.; Brooks, L.J.; Ward, S.D.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Lehmann, C.; Schechter, M.S.; Sheldon, S.; et al. Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome. Am. Acad. Pediatr. 2012, 130, e714–e755. [Google Scholar]
- Lumeng, J.C.; Chervin, R.D. Epidemiology of Pediatric Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008, 5, 242–252. [Google Scholar] [CrossRef] [Green Version]
- Kaditis, A.G.; Alvarez, M.L.A.; Boudewyns, A.; Alexopoulos, E.I.; Ersu, R.; Joosten, K.; Larramona, H.; Miano, S.; Narang, I.; Trang, H.; et al. Obstructive sleep disordered breathing in 2-To 18-Year-Old children: Diagnosis and management. Eur. Respir. J. 2016, 47, 69–94. [Google Scholar] [CrossRef]
- Ehsan, Z.; Ishman, S.L.; Kimball, T.R.; Zhang, N.; Zou, Y.; Amin, R.S. Longitudinal Cardiovascular Outcomes of Sleep Disordered Breathing in Children: A Meta-Analysis and Systematic Review. Sleep 2017, 40. [Google Scholar] [CrossRef]
- Hou, H.; Zhao, Y.; Yu, W.; Dong, H.; Xue, X.; Ding, J.; Xing, W.; Wang, W. Association of obstructive sleep apnea with hypertension: A systematic review and meta-Analysis. J. Glob. Health 2018, 8. [Google Scholar] [CrossRef]
- Xu, S.; Wan, Y.; Xu, M.; Ming, J.; Xing, Y.; An, F.; Ji, Q. The association between obstructive sleep apnea and metabolic syndrome: A systematic review and meta-Analysis. BMC Pulm. Med. 2015, 15, 105. [Google Scholar] [CrossRef] [Green Version]
- Galland, B.; Spruyt, K.; Dawes, P.; McDowall, P.S.; Elder, D.; Schaughency, E. Sleep disordered breathing and academic performance: A meta-analysis. Pediatrics 2015, 136, e934–e946. [Google Scholar] [CrossRef] [Green Version]
- Beebe, D.W.; Ris, M.D.; Kramer, M.E.; Long, E.; Amin, R. The association between sleep disordered breathing, academic grades, and cognitive and behavioral functioning among overweight subjects during middle to late childhood. Sleep 2010, 33, 1447–1456. [Google Scholar] [CrossRef] [Green Version]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-Analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Reilly, J.J.; Kelly, J. Long-Term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int. J. Obes. 2011, 35, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Walter, L.M.; Tamanyan, K.; Limawan, A.P.; Biggs, S.N.; Weichard, A.J.; Davey, M.J.; Nixon, G.M.; Horne, R.S.C. Overweight and obese children with sleep disordered breathing have elevated arterial stiffness. Sleep Med. 2018, 48, 187–193. [Google Scholar] [CrossRef]
- Wing, Y.K.; Hui, S.H.; Pak, W.M.; Ho, C.K.; Cheung, A.; Li, A.M.; Fok, T.F. A controlled study of sleep related disordered breathing in obese children. Arch. Dis. Child. 2003, 88, 1043–1047. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, R.; Hakim, F.; Gozal, D. Sleep, sleep-Disordered breathing and lipid homeostasis: Translational evidence from murine models and children. Clin. Lipidol. 2012, 7, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Verhulst, S.L.; Van Gaal, L.; De Backer, W.; Desager, K. The prevalence, anatomical correlates and treatment of sleep-disordered breathing in obese children and adolescents. Sleep Med. Rev. 2008, 12, 339–346. [Google Scholar] [CrossRef]
- Verhulst, S.L.; Schrauwen, N.; Haentjens, D.; Suys, B.; Rooman, R.P.; Van Goal, L.; De Backer, W.A.; Desager, K.N. Sleep-Disordered breathing in overweight and obese children and adolescents: Prevalence, characteristics and the role of fat distribution. Arch. Dis. Child. 2007, 92, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Bixler, E.O.; Vgontzas, A.N.; Lin, H.-M.; Liao, D.; Calhoun, S.; Vela-Bueno, A.; Fedok, F.; Vlasic, V.; Graff, G. Sleep Disordered Breathing in Children in a General Population Sample: Prevalence and Risk Factors. Sleep 2009, 32, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Tsaoussoglou, M.; Bixler, E.O.; Calhoun, S.; Chrousos, G.P.; Sauder, K.; Vgontzas, A.N. Sleep-Disordered Breathing in Obese Children Is Associated with Prevalent Excessive Daytime Sleepiness, Inflammation, and Metabolic Abnormalities. J. Clin. Endocrinol. Metab. 2010, 95, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, L.; Tesse, R.; Miniello, V.L.; Colella, I.; Delvecchio, M.; Logrillo, V.P.; Francavilla, R.; Armenio, L. Sleep-Disordered breathing in obese children: The Southern Italy experience. Chest 2010, 137, 1085–1090. [Google Scholar] [CrossRef]
- Andersen, I.G.; Holm, J.C.; Homøe, P. Obstructive sleep apnea in obese children and adolescents, treatment methods and outcome of treatment-A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2016, 87, 190–197. [Google Scholar] [CrossRef]
- Andersen, I.G.; Holm, J.C.; Homøe, P. Obstructive sleep apnea in children and adolescents with and without obesity. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 871–878. [Google Scholar] [CrossRef]
- Mathew, J.L.; Narang, I. Sleeping too Close Together: Obesity and Obstructive Sleep Apnea in Childhood and Adolescence. Paediatr. Respir. Rev. 2014, 15, 211–218. [Google Scholar] [CrossRef]
- Kline, C.E.; Krafty, R.T.; Mulukutla, S.; Hall, M.H. Associations of sedentary time and moderate-Vigorous physical activity with sleep-Disordered breathing and polysomnographic sleep in community-Dwelling adults. Sleep Breath 2017, 21, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Shin, J.C.; Li, D.; An, R. Sedentary Behavior and Sleep Problems: A Systematic Review and Meta-Analysis. Int. J. Behav. Med. 2017, 24, 481–492. [Google Scholar] [CrossRef]
- Mendelson, M.; Bailly, S.; Marillier, M.; Flore, P.; Borel, J.C.; Vivodtzev, I.; Doutreleau, S.; Verges, S.; Tamisier, R.; Pépin, J.L. Obstructive sleep apnea syndrome, objectively measured physical activity and exercise training interventions: A systematic review and meta-analysis. Front. Neurol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Beitler, J.R.; Awad, K.M.; Bakker, J.P.; Edwards, B.A.; DeYoung, P.; Djonlagic, I.; Forman, D.E.; Quan, S.F.; Malhotra, A. Obstructive sleep apnea is associated with impaired exercise capacity: A cross-Sectional study. J. Clin. Sleep Med. 2014, 10, 1199–1204. [Google Scholar] [CrossRef]
- Vanhecke, T.E.; Franklin, B.A.; Ajluni, S.C.; Sangal, R.B.; McCullough, P.A. Cardiorespiratory fitness and sleep-related breathing disorders. Expert Rev. Cardiovasc. Ther. 2008, 6, 745–758. [Google Scholar] [CrossRef]
- Cadenas-Sánchez, C.; Mora-González, J.; Migueles, J.H.; Martín-Matillas, M.; Gómez-Vida, J.; Escolano-Margarit, M.V.; Maldonado, J.; Enriquez, G.M.; Pastor-Villaescusa, B.; de Teresa, C.; et al. An exercise-Based randomized controlled trial on brain, cognition, physical health and mental health in overweight/obese children (ActiveBrains project): Rationale, design and methods. Contemp. Clin. Trials 2016, 47, 315–324. [Google Scholar] [CrossRef]
- Keadle, S.K.; Arem, H.; Moore, S.C.; Sampson, J.N.; Matthews, C.E. Impact of changes in television viewing time and physical activity on longevity: A prospective cohort study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, M.S.; LeBlanc, A.G.; Kho, M.E.; Saunders, T.J.; Larouche, R.; Colley, R.C.; Goldfield, G.; Gorber, S.C. Systematic review of sedentary behaviour and health indicators in school-Aged children and youth. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 98. [Google Scholar] [CrossRef] [Green Version]
- Saint-Maurice, P.F.; Welk, G.J. Web-Based assessments of physical activity in youth: Considerations for design and scale calibration. J. Med. Internet Res. 2014, 16. [Google Scholar] [CrossRef] [Green Version]
- Saint-Maurice, P.F.; Welk, G.J. Validity and calibration of the youth activity profile. PLoS ONE 2015, 10, e0143949. [Google Scholar] [CrossRef] [Green Version]
- Fairclough, S.J.; Christian, D.L.; Saint-Maurice, P.F.; Hibbing, P.R.; Noonan, R.J.; Welk, G.J.; Dixon, P.M.; Boddy, L.M. Calibration and validation of the youth activity profile as a physical activity and sedentary behaviour surveillance tool for english youth. Int. J. Environ. Res. Public Health 2019, 16, 3711. [Google Scholar] [CrossRef] [Green Version]
- van Hees, V.T.; Renström, F.; Wright, A.; Gradmark, A.; Catt, M.; Chen, K.Y.; Löf, M.; Bluck, L.; Pomeroy, J.; Wareham, N.J.; et al. Estimation of daily energy expenditure in pregnant and Non-Pregnant women using a Wrist-Worn Tri-Axial accelerometer. PLoS ONE 2011, 6, e22922. [Google Scholar] [CrossRef] [Green Version]
- van Hees, V.T.; Sabia, S.; Anderson, K.N.; Denton, S.J.; Oliver, J.; Catt, M.; Abell, J.G.; Kivimäki, M.; Trenell, M.I.; Singh-Manoux, A. A novel, open access method to assess sleep duration using a wrist-Worn accelerometer. PLoS ONE 2015, 10, e0142533. [Google Scholar] [CrossRef] [Green Version]
- van Hees, V.T.; Gorzelniak, L.; Dean León, E.C.; Eder, M.; Pias, M.; Taherian, S.; Ekelund, U.; Renström, F.; Franks, P.W.; Horsch, A.; et al. Separating Movement and Gravity Components in an Acceleration Signal and Implications for the Assessment of Human Daily Physical Activity. PLoS ONE 2013, 8, e61691. [Google Scholar] [CrossRef] [Green Version]
- van Hees, V.T.; Fang, Z.; Langford, J.; Assah, F.; Mohammad, A.; da Silva, I.C.M.; Trenell, M.I.; White, T.; Wareham, N.J.; Brage, S. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: An evaluation on four continents. J. Appl. Physiol. 2014, 117, 738–744. [Google Scholar] [CrossRef] [Green Version]
- Hildebrand, M.; Van Hees, V.T.; Hansen, B.H.; Ekelund, U. Age group comparability of raw accelerometer output from wrist-And hip-Worn monitors. Med. Sci. Sports Exerc. 2014, 46, 1816–1824. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Castro-Piñero, J.; España-Romero, V.; Artero, E.G.; Ortega, F.B.; Cuenca, M.A.M.; Enez-Pavón, D.J.; Chillón, P.; Girela-Rejón, M.J.; Mora, J.; et al. Field-Based fitness assessment in young people: The ALPHA health-Related fitness test battery for children and adolescents. Br. J. Sports Med. 2011, 45, 518–524. [Google Scholar] [CrossRef]
- Artero, E.G.; España-Romero, V.; Castro-Piero, J.; Ortega, F.B.; Suni, J.; Castillo-Garzon, M.J.; Ruiz, J.R. Reliability of field-Based fitness tests in youth. Int. J. Sports Med. 2011, 32, 159–169. [Google Scholar] [CrossRef]
- Castro-Piñero, J.; Artero, E.G.; España-Romero, V.; Ortega, F.B.; Sjostrom, M.; Suni, J.; Ruiz, J.R. Criterion-Related validity of field-Based fitness tests in youth: A systematic review. Br. J. Sport. Med. 2010, 44, 934–943. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Castro-Piñero, J.; Artero, E.G.; Ortega, F.B.; Sjöström, M.; Suni, J.; Castillo, M.J. Predictive validity of health-related fitness in youth: A systematic review. Br. J. Sports Med. 2009, 43, 909–923. [Google Scholar] [CrossRef]
- Léger, L.A.; Mercier, D.; Gadoury, C.; Lambert, J. The multistage 20 metre shuttle run test for aerobic fitness. J. Sports Sci. 1988, 6, 93–101. [Google Scholar] [CrossRef]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-Offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Bervoets, L.; Massa, G. Defining morbid obesity in children based on BMI 40 at age 18 using the extended international (IOTF) cut-offs. Pediatr. Obes. 2014, 9, e94–e98. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Marco, L.; Moreno, L.A.; Ortega, F.B.; Len, F.; Sioen, I.; Kafatos, A.; Martinez-Gomez, D.; Widhalm, K.; Castillo, M.J.; Vicente-Rodrguez, G. Levels of physical activity that predict optimal bone mass in adolescents: The HELENA study. Am. J. Prev. Med. 2011, 40, 599–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracia-Marco, L.; Ortega, F.B.; Jiménez-Pavón, D.; Rodríguez, G.; Castillo, M.J.; Vicente-Rodríguez, G.; Moreno, L.A. Adiposity and bone health in Spanish adolescents. the HELENA study. Osteoporos. Int. 2012, 23, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.T.; Torres, A.M.; Soto, B.B. Spanish Version of the Pediatric Sleep Questionnaire (PSQ). A useful instrument in investigation of sleep disturbances in childhood. Reliability analysis. An. Pediatr. 2007, 66, 121–128. [Google Scholar]
- Chervin, R.D.; Weatherly, R.A.; Garetz, S.L.; Ruzicka, D.L.; Giordani, B.J.; Hodges, E.K.; Dillon, J.E.; Guire, K.E. Pediatric sleep questionnaire. Prediction of sleep apnea and outcomes. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Rosiek, A.; Maciejewska, N.F.; Leksowski, K.; Rosiek-Kryszewska, A.; Leksowski, Ł. Effect of television on obesity and excess of weight and consequences of health. Int. J. Environ. Res. Public Health 2015, 12, 9408–9426. [Google Scholar] [CrossRef] [Green Version]
- Carson, V.; Hunter, S.; Kuzik, N.; Gray, C.E.; Poitras, V.J.; Chaput, J.; Saunders, T.J.; Katzmarzyk, P.T.; Okely, A.D.; Gorber, S.C.; et al. Systematic review of sedentary behaviour and health indicators in school-Aged children and youth: An update. Appl. Physiol. Nutr. Metab. 2016, 41, S240–S265. [Google Scholar] [CrossRef]
- Paavonen, E.J.; Pennonen, M.; Roine, M.; Valkonen, S.; Lahikainen, A.R. TV exposure associated with sleep disturbances in 5- to 6-year-old children. J. Sleep Res. 2006, 15, 154–161. [Google Scholar] [CrossRef]
- Davis, C.L.; Tkacz, J.; Gregoski, M.; Boyle, C.A.; Lovrekovic, G. Aerobic Exercise and Snoring in Overweight Children: A Randomized Controlled Trial. Obesity (Silver Spring) 2006, 14, 1985–1991. [Google Scholar] [CrossRef]
- Ortega, F.; Ruiz, J.; Castillo, M.; Sjöström, M. Physical fitness in childhood and adolescence: A powerful marker of health. Int. J. Obes. 2008, 32, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Stojek, M.M.K.; Montoya, A.K.; Drescher, C.F.; Newberry, A.; Sultan, Z.; Williams, C.F.; Pollock, N.K.; Davis, C.L. Fitness, Sleep-Disordered Breathing, Symptoms of Depression, and Cognition in Inactive Overweight Children: Mediation Models. Public Health Rep. 2017, 132, 65S–73S. [Google Scholar] [CrossRef]
- Twig, G.; Afek, A.; Derazne, E.; Tzur, D.; Cukierman-Yaffe, T.; Gerstein, H.C.; Tirosh, A. Diabetes risk among overweight and obese metabolically healthy young adults. Diabetes Care 2014, 37, 2989–2995. [Google Scholar] [CrossRef] [Green Version]
- Ikävalko, T.; Närhi, M.; Eloranta, A.M.; Lintu, N.; Myllykangas, R.; Vierola, A.; Tuomilehto, H.; Lakka, T.; Pahkala, R. Predictors of sleep disordered breathing in children: The PANIC study. Eur. J. Orthod. 2018, 40, 268–272. [Google Scholar] [CrossRef]
- Carotenuto, M.; Bruni, O.; Santoro, N.; del Giudice, E.M.; Perrone, L.; Pascotto, A. Waist circumference predicts the occurrence of sleep-disordered breathing in obese children and adolescents: A questionnaire-Based study. Sleep Med. 2006, 7, 357–361. [Google Scholar] [CrossRef]
- Bixler, E.O.; Fernandez-Mendoza, J.; Liao, D.; Calhoun, S.; Rodriguez-Colon, S.M.; Gaines, J.; He, F.; Vgontzas, A.N. Natural history of sleep disordered breathing in prepubertal children transitioning to adolescence. Eur. Respir. J. 2016, 47, 1402–1409. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, R.; Lesser, D.J.; Oliveira, F.G.S.A.; Tran, W.H.; Keens, T.G.; Khoo, M.C.K.; Ward, S.L.D. Body fat composition: A predictive factor for sleep related breathing disorder in obese children. J. Clin. Sleep Med. 2015, 11, 1039–1045. [Google Scholar] [CrossRef] [Green Version]
- Piovezan, R.D.; Hirotsu, C.; Moizinho, R.; de Sá Souza, H.; D’Almeida, V.; Tufik, S.; Poyares, D. Associations between sleep conditions and body composition states: Results of the EPISONO study. J. Cachexia Sarcopenia Muscle 2019, 10, 962–973. [Google Scholar] [CrossRef]
- Lovin, S.; Bercea, R.; Cojocaru, C.; Rusu, G.; Mihãescu, T. Body composition in obstructive sleep apneahypopnea syndrome: Bio-impedance reflects the severity of sleep apnea. Multidiscip. Respir. Med. 2010, 5, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Kuźnar-Kamińska, B.; Grabicki, M.; Trafas, T.; Szulińska, M.; Cofta, S.; Piorunek, T.; Brajer-Luftmann, B.; Nowicka, A.; Bromińska, B.; Batura-Gabryel, H. Body composition, anthropometric indices and hydration status of obstructive sleep apnea patients: Can cachexia coexist with obesity? Adv. Exp. Med. Biol. 2017, 1020, 43–51. [Google Scholar]
- Ortega, F.B.; Sui, X.; Lavie, C.J.; Blair, S.N. Body Mass Index, the Most Widely Used but also Widely Criticized Index: Would a Gold-Standard Measure of Total Body Fat be a Better Predictor of Cardiovascular Disease Mortality? Mayo Clin. Proc. 2016, 91, 443–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| All | Boys | Girls | |
|---|---|---|---|
| n (%) | 109 (100) | 64 (59) | 45 (41) |
| Age (years) | 10.0 ± 1.1 | 10.2 ± 1.1 | 9.9 ± 1.1 |
| Sedentary behaviour | |||
| Television viewing time, n (%) (n = 107) | |||
| None | 1 (1) | 0 (0) | 1 (2) |
| <1 h/day | 41 (38) | 24 (38) | 17 (39) |
| 1–2 h/day | 42 (39) | 22 (34) | 20 (47) |
| <2–3 h/day | 13 (12) | 8 (12) | 5 (12) |
| >3 h/day | 10 (9) | 10 (16) | 0 (0) |
| Sedentary time (min/day) (n = 103) | 520.1 ± 54.7 | 521.3 ± 52.0 | 518.4 ± 58.9 |
| Physical activity | |||
| Moderate-to-vigorous physical activity (min/day) (n = 103) | 51.4 ± 20.1 | 59.1 ± 21.0 | 40.2 ± 11.7 |
| Cardiorespiratory fitness | |||
| 20 m shuttle run test (laps) | 16.0 ± 7.7 | 17.2 ± 8.1 | 14.4 ± 6.9 |
| 20 m shuttle run test (VO2max, mL/kg/min) a | 40.7 ± 2.7 | 40.8 ± 2.7 | 40.6 ± 2.7 |
| Body composition parameters | |||
| Weight (kg) | 56.2 ± 11.2 | 57.1 ± 11.2 | 54.9 ± 11.3 |
| Height (cm) | 144.2 ± 8.4 | 145.0 ± 8.0 | 143.1 ± 9.0 |
| Body mass index (kg/m2) | 26.8 ± 3.6 | 27.0 ± 3.7 | 26.6 ± 3.5 |
| Fat mass index (kg/m2) | 11.8 ± 2.9 | 11.5 ± 2.9 | 12.1 ± 2.8 |
| Lean mass index (kg/m2) | 14.0 ± 1.4 | 14.3 ±1.3 | 13.5 ± 1.4 |
| Waist circumference (cm) | 90.2 ± 9.9 | 91.3 ± 9.4 | 88.7 ± 10.5 |
| Sleep-related breathing disorders | |||
| SRBD scale (0 to 1) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Presence of SRBD, n (%) | 17(16) | 10 (16) | 7 (16) |
| SRBD Scale (0 to 1) | ||
|---|---|---|
| r | p | |
| Sedentary behaviour | ||
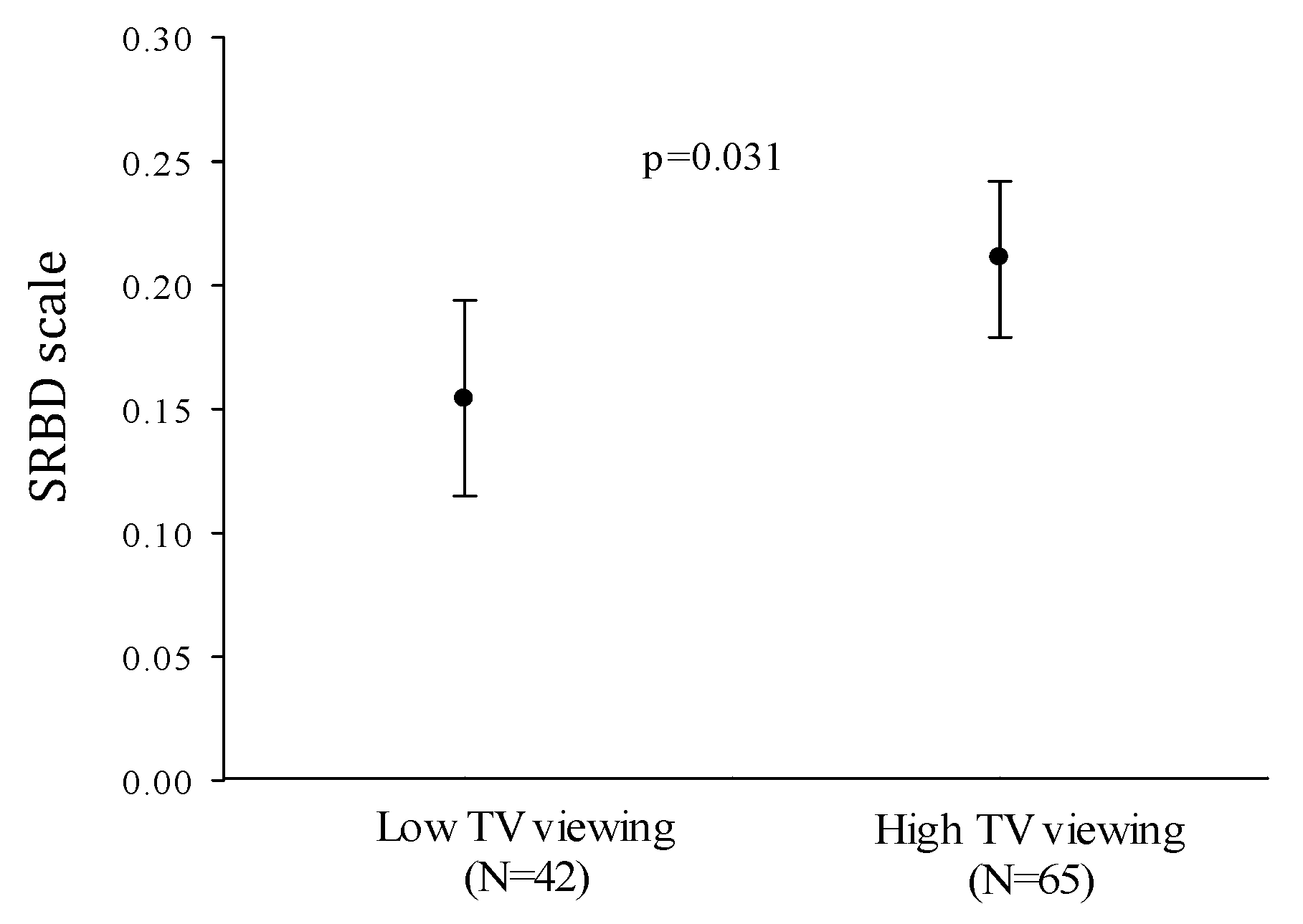
| Television viewing time (h/day) | 0.222 | 0.021 |
| Sedentary time (min/day) | 0.129 | 0.193 |
| Physical activity | ||
| Moderate-to-vigorous physical activity (min/day) | 0.054 | 0.585 |
| Cardiorespiratory fitness (VO2max mL/kg/min) a | −0.210 | 0.030 |
| Body composition parameters | ||
| Body mass index (kg/m2) | 0.209 | 0.029 |
| Fat mass index (kg/m2) | 0.153 | 0.114 |
| Lean mass index (kg/m2) | 0.223 | 0.020 |
| Waist circumference (cm) | 0.191 | 0.047 |
| β | p-Value | Change R2 | R2 | |
|---|---|---|---|---|
| Model 1 | 0.063 | 0.063 | ||
| Body mass index | 0.251 | 0.011 | ||
| Model 2 | 0.048 | 0.111 | ||
| Body mass index | 0.249 | 0.010 | ||
| Television viewing time | 0.220 | 0.022 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Lopez, L.V.; Cadenas-Sanchez, C.; Migueles, J.H.; Adelantado-Renau, M.; Plaza-Florido, A.; Solis-Urra, P.; Molina-Garcia, P.; Ortega, F.B. Associations of Sedentary Behaviour, Physical Activity, Cardiorespiratory Fitness and Body Composition with Risk of Sleep-Related Breathing Disorders in Children with Overweight/Obesity: A Cross-Sectional Study. J. Clin. Med. 2020, 9, 1544. https://doi.org/10.3390/jcm9051544
Torres-Lopez LV, Cadenas-Sanchez C, Migueles JH, Adelantado-Renau M, Plaza-Florido A, Solis-Urra P, Molina-Garcia P, Ortega FB. Associations of Sedentary Behaviour, Physical Activity, Cardiorespiratory Fitness and Body Composition with Risk of Sleep-Related Breathing Disorders in Children with Overweight/Obesity: A Cross-Sectional Study. Journal of Clinical Medicine. 2020; 9(5):1544. https://doi.org/10.3390/jcm9051544
Chicago/Turabian StyleTorres-Lopez, Lucia V., Cristina Cadenas-Sanchez, Jairo H. Migueles, Mireia Adelantado-Renau, Abel Plaza-Florido, Patricio Solis-Urra, Pablo Molina-Garcia, and Francisco B. Ortega. 2020. "Associations of Sedentary Behaviour, Physical Activity, Cardiorespiratory Fitness and Body Composition with Risk of Sleep-Related Breathing Disorders in Children with Overweight/Obesity: A Cross-Sectional Study" Journal of Clinical Medicine 9, no. 5: 1544. https://doi.org/10.3390/jcm9051544






