Increased Cell-Free DNA Plasma Concentration Following Liver Transplantation Is Linked to Portal Hepatitis and Inferior Survival
Abstract
:1. Introduction
2. Experimental Section
2.1. Approval
2.2. Surgical Procedure and Immunosuppression
2.3. Histology Examination
2.4. Blood Collection and Laboratory Analyses
2.5. DNA Extraction
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Haemodialysis In Vivo
2.8. Haemodialysis In Vitro
2.9. Statistical Analysis
3. Results
3.1. Clinical Course and cfDNA
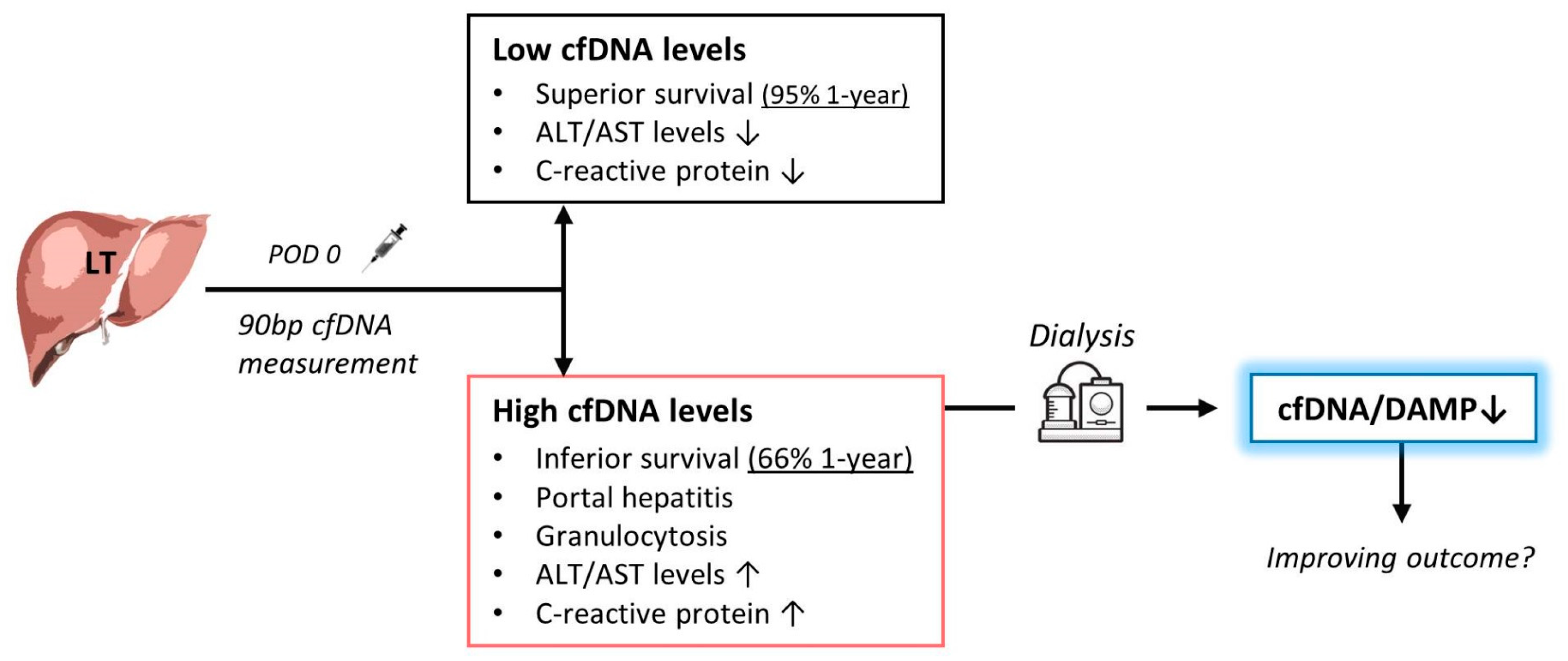
3.2. Association between cfDNA and Survival
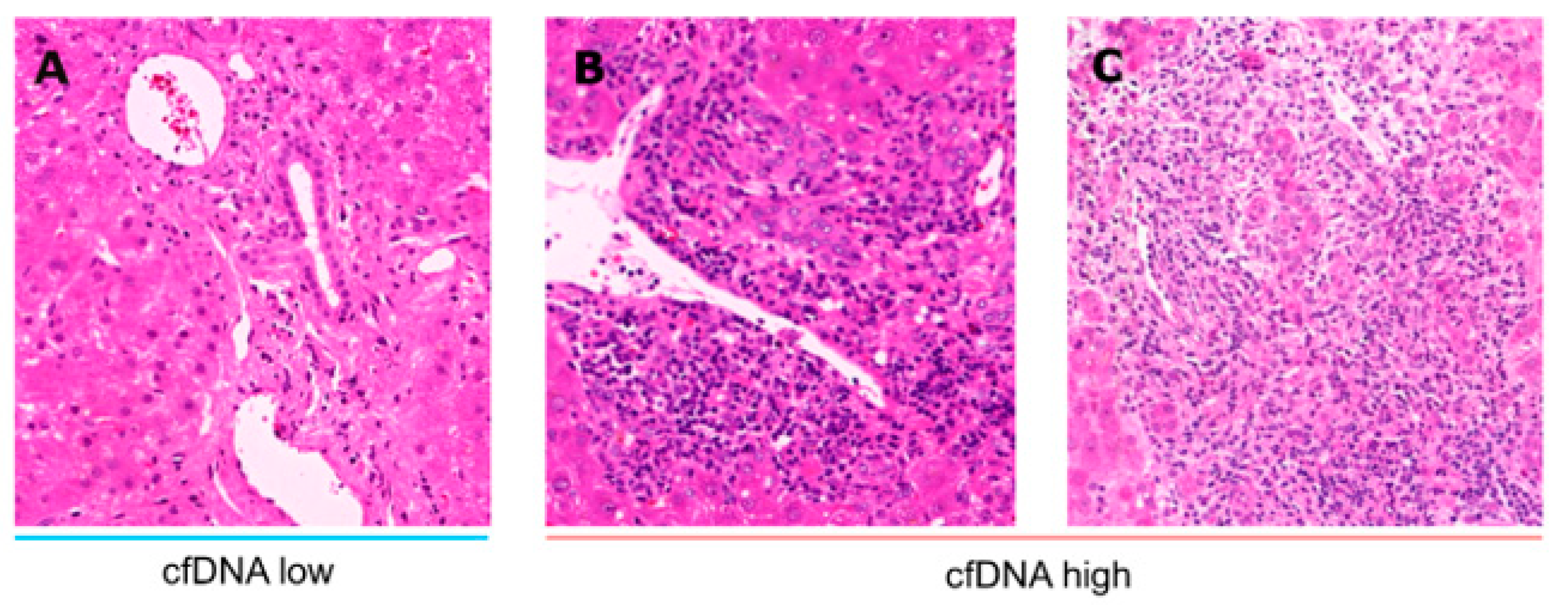
3.3. Inflammation in the Presence of High and Low cfDNA Levels
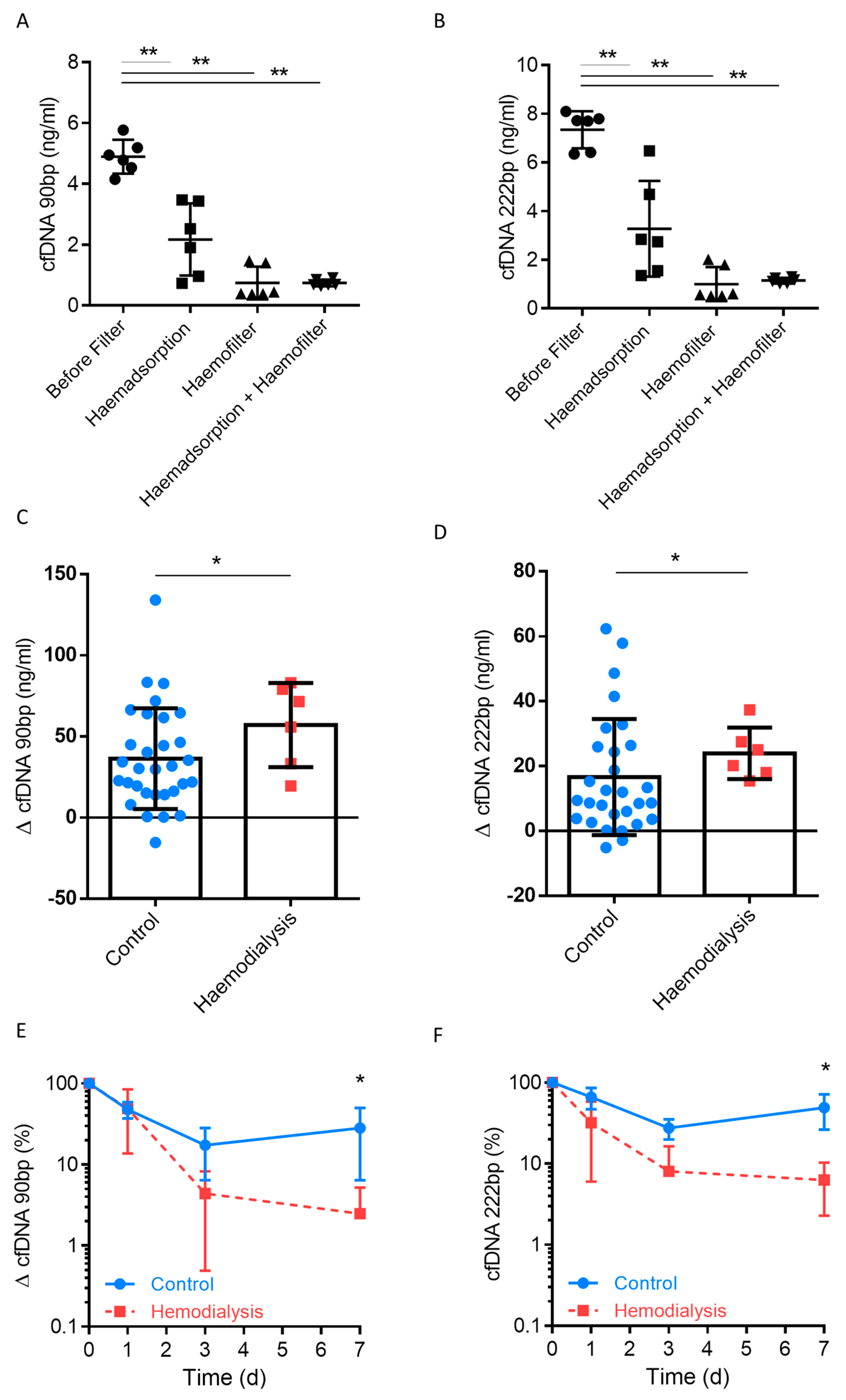
3.4. Clearance of Plasma cfDNA by Haemofiltration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schoening, W.N.; Buescher, N.; Rademacher, S.; Andreou, A.; Kuehn, S.; Neuhaus, R.; Guckelberger, O.; Puhl, G.; Seehofer, D.; Neuhaus, P. Twenty-year longitudinal follow-up after orthotopic liver transplantation: A single-center experience of 313 consecutive cases. Am. J. Transpl. 2013, 13, 2384–2394. [Google Scholar] [CrossRef] [PubMed]
- Åberg, F.; Gissler, M.; Karlsen, T.H.; Ericzon, B.; Foss, A.; Rasmussen, A.; Bennet, W.; Olausson, M.; Line, P.; Nordin, A.; et al. Differences in long-term survival among liver transplant recipients and the general population: A population-based Nordic study. Hepatology 2015, 61, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Benzing, C.; Krezdorn, N.; Förster, J.; Hinz, A.; Krenzien, F.; Atanasov, G.; Schmelzle, M.; Hau, H.-M.; Bartels, M. Health-related quality of life and affective status in liver transplant recipients and patients on the waiting list with low MELD scores. HPB 2016, 18, 449–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacke, F.; Kroy, D.C.; Barreiros, A.P.; Neumann, U.P. Liver transplantation in Germany. Liver Transpl. 2016, 22, 1136–1142. [Google Scholar] [CrossRef]
- Orman, E.S.; Mayorga, M.E.; Wheeler, S.B.; Townsley, R.M.; Toro-Diaz, H.H.; Hayashi, P.H.; Barritt Barritt, A.S., 4th. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transpl. 2015, 21, 1040–1050. [Google Scholar] [CrossRef]
- Mathur, A.K.; Heimbach, J.; Steffick, D.E.; Sonnenday, C.J.; Goodrich, N.P.; Merion, R.M. Donation after cardiac death liver transplantation: Predictors of outcome. Am. J. Transpl. 2010, 10, 2512–2519. [Google Scholar] [CrossRef] [Green Version]
- Barshes, N.R.; Horwitz, I.B.; Franzini, L.; Vierling, J.M.; Goss, J.A. Waitlist mortality decreases with increased use of extended criteria donor liver grafts at adult liver transplant centers. Am. J. Transpl. 2007, 7, 1265–1270. [Google Scholar] [CrossRef]
- Land, W.G.; Agostinis, P.; Gasser, S.; Garg, A.D.; Linkermann, A. Transplantation and Damage-Associated Molecular Patterns (DAMPs). Am. J. Transpl. 2016, 16, 3338–3361. [Google Scholar] [CrossRef]
- Krenzien, F.; Keshi, E.; Splith, K.; Griesel, S.; Kamali, K.; Sauer, I.M.; Feldbrügge, L.; Pratschke, J.; Leder, A.; Schmelzle, M. Diagnostic biomarkers to diagnose acute allograft rejection after liver transplantation: Systematic review and meta-analysis of diagnostic accuracy studies. Front. Immunol. 2019, 10, 758. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Kubes, P.; Mehal, W.Z. Sterile inflammation in the liver. Gastroenterology 2012, 143, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.; Pesiridis, S.; Fitzgerald, K.A. DNA sensing by the cGAS–STING pathway in health and disease. Nat. Rev. Genet. 2019, 20, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Gül, S.; Pascher, A.; Schöning, W.; Al-Abadi, H.; Bahra, M.; Klein, F.; Denecke, T.; Strücker, B.; Puhl, G.; et al. Patient and tumour biology predict survival beyond the Milan criteria in liver transplantation for hepatocellular carcinoma. HPB 2015, 17, 168–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlas, T.; Weise, L.; Kuhn, S.; Krenzien, F.; Mehdorn, M.; Petroff, D.; Linder, N.; Schaudinn, A.; Busse, H.; Keim, V.; et al. Correlation of cell-free DNA plasma concentration with severity of non-alcoholic fatty liver disease. J. Transl. Med. 2017, 15, 106. [Google Scholar] [CrossRef]
- Breitbach, S.; Tug, S.; Helmig, S.; Zahn, D.; Kubiak, T.; Michal, M.; Gori, T.; Ehlert, T.; Beiter, T.; Simon, P. Direct quantification of cell-free, circulating DNA from unpurified plasma. PLoS ONE 2014, 9, e87838. [Google Scholar] [CrossRef] [Green Version]
- Castleberry, A.W.; Worni, M.; Osho, A.A.; Snyder, L.D.; Palmer, S.M.; Pietrobon, R.; Davis, R.D.; Hartwig, M.G. Use of lung allografts from brain-dead donors after cardiopulmonary arrest and resuscitation. Am. J. Respir. Crit. Care Med. 2013, 188, 466–473. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Lázaro, I.J.; Almenar-Bonet, L.; Martínez-Dolz, L.; Buendía-Fuentes, F.; Agüero, J.; Navarro-Manchón, J.; Raso-Raso, R.; Salvador-Sanz, A. Can we accept donors who have suffered a resuscitated cardiac arrest? Transplant. Proc. 2010, 42, 3091–3092. [Google Scholar] [CrossRef]
- Vernon, P.J.; Tang, D. Eat-me: Autophagy, phagocytosis, and reactive oxygen species signaling. Antioxid. Redox Signal. 2013, 18, 677–691. [Google Scholar] [CrossRef] [Green Version]
- Orioles, A.; Morrison, W.E.; Rossano, J.W.; Shore, P.M.; Hasz, R.D.; Martiner, A.C.; Berg, R.A.; Nadkarni, V.M. An under-recognized benefit of cardiopulmonary resuscitation: Organ transplantation. Crit. Care Med. 2013, 41, 2794–2799. [Google Scholar] [CrossRef]
- Dalle Ave, A.L.; Gardiner, D.; Shaw, D.M. Cardio-pulmonary resuscitation of brain-dead organ donors: A literature review and suggestions for practice. Transpl. Int. 2016, 29, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Que, S.; Xu, J.; Peng, T. Alanine aminotransferase-old biomarker and new concept: A review. Int. J. Med. Sci. 2014, 11, 925–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittman, K.; Kubes, P. Damage-associated molecular patterns control neutrophil recruitment. J. Innate Immun. 2013, 5, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zinkova, A.; Brynychova, I.; Svacina, A.; Jirkovska, M.; Korabecna, M. Cell-free DNA from human plasma and serum differs in content of telomeric sequences and its ability to promote immune response. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Atamaniuk, J.; Kopecky, C.; Skoupy, S.; Säemann, M.D.; Weichhart, T. Apoptotic cell-free DNA promotes inflammation in haemodialysis patients. Nephrol. Dial. Transplant. 2012, 27, 902–905. [Google Scholar] [CrossRef] [Green Version]
- Stawski, R.; Walczak, K.; Perdas, E.; Wlodarczyk, A.; Sarniak, A.; Kosielski, P.; Meissner, P.; Budlewski, T.; Padula, G.; Nowak, D. Decreased integrity of exercise-induced plasma cell free nuclear DNA-negative association with the increased oxidants production by circulating phagocytes. Sci. Rep. 2019, 9, 15970. [Google Scholar] [CrossRef] [Green Version]
- von Meijenfeldt, F.A.; Burlage, L.C.; Bos, S.; Adelmeijer, J.; Porte, R.J.; Lisman, T. Elevated plasma levels of cell-free DNA during liver transplantation are associated with activation of coagulation. Liver Transpl. 2018, 24, 1716–1725. [Google Scholar] [CrossRef]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.K.; Coates, E.C.; Smith, D.J.; Karlnoski, R.A.; Hickerson, W.L.; Arnold-Ross, A.L.; Mosier, M.J.; Halerz, M.; Sprague, A.M.; Mullins, R.F.; et al. High-volume hemofiltration in adult burn patients with septic shock and acute kidney injury: A multicenter randomized controlled trial. Crit. Care 2017, 21, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.T.; Lee, H.; Kee, Y.K.; Park, S.; Oh, H.J.; Han, S.H.; Joo, K.W.; Lim, C.-S.; Kim, Y.S.; Kang, S.W. High-dose versus conventional-dose continuous venovenous hemodiafiltration and patient and kidney survival and cytokine removal in sepsis-associated acute kidney injury: A randomized controlled trial. Am. J. Kidney Dis. 2016, 68, 599–608. [Google Scholar] [CrossRef]
- Wu, J.; Ren, J.; Liu, Q.; Hu, Q.; Wu, X.; Wang, G.; Hong, Z.; Ren, H.; Li, J. Effects of changes in the levels of damage-associated molecular patterns following continuous veno-venous hemofiltration therapy on outcomes in acute kidney injury patients with sepsis. Front. Immunol. 2019, 9, 3052. [Google Scholar] [CrossRef] [Green Version]
- Cordaux, R.; Batzer, M.A. The impact of retrotransposons on human genome evolution. Nat. Rev. Genet. 2009, 10, 691–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puszyk, W.M.; Crea, F.; Old, R.W. Unequal representation of different unique genomic DNA sequences in the cell-free plasma DNA of individual donors. Clin. Biochem. 2009, 42, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Buzdin, A.; Ustyugova, S.; Gogvadze, E.; Lebedev, Y.; Hunsmann, G.; Sverdlov, E. Genome-wide targeted search for human specific and polymorphic L1 integrations. Hum. Genet. 2003, 112, 527–533. [Google Scholar] [CrossRef] [PubMed]
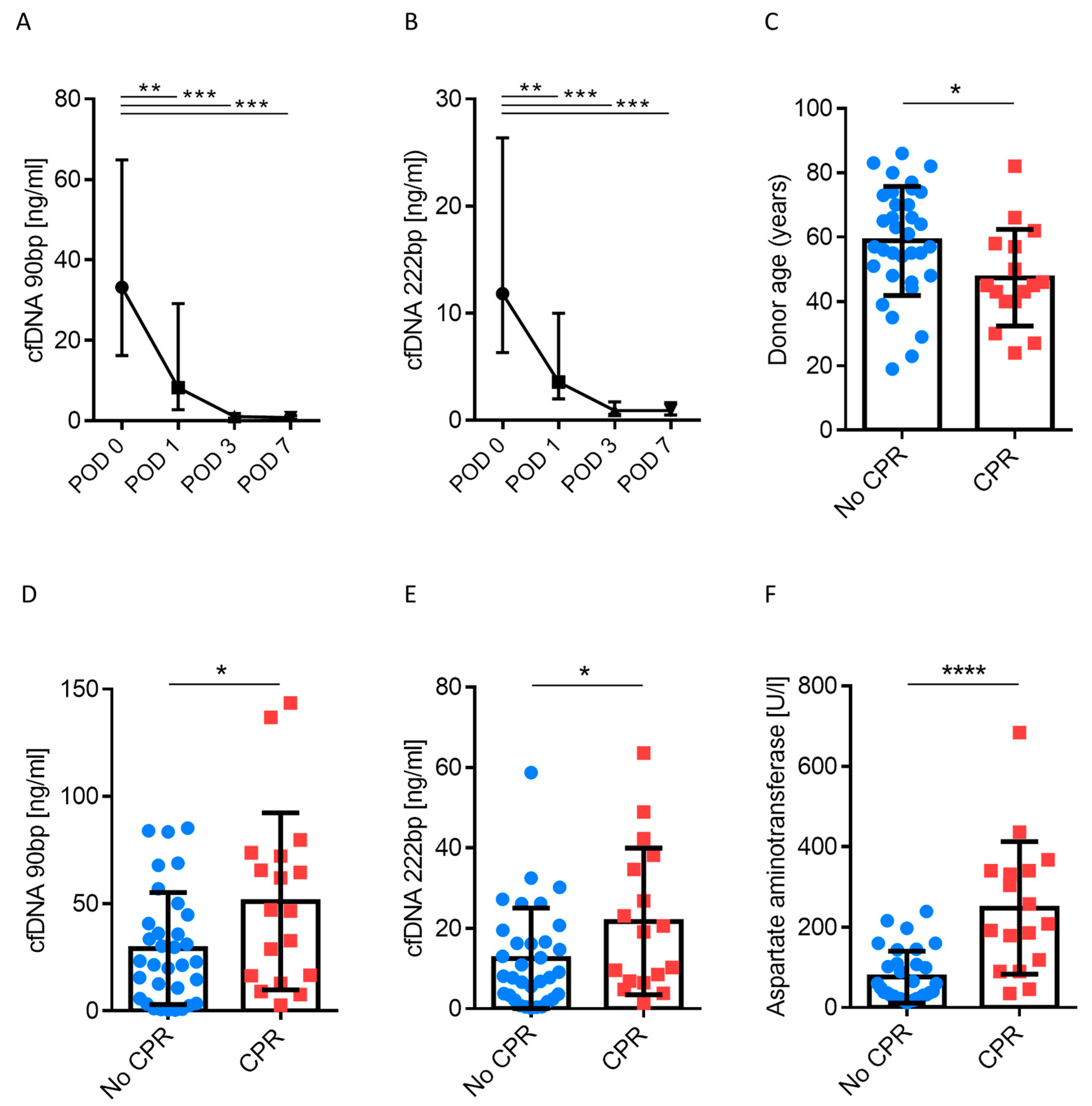
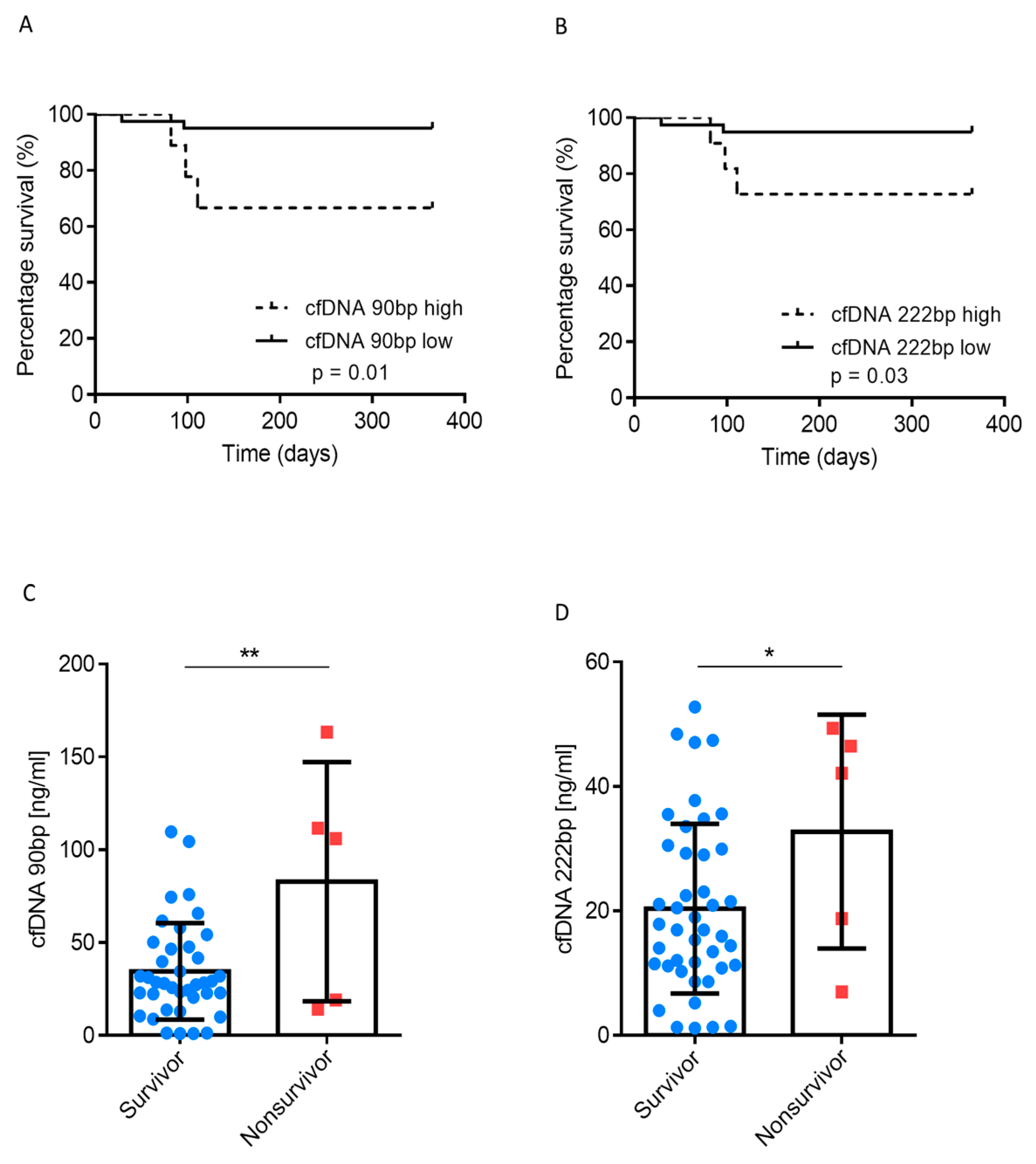
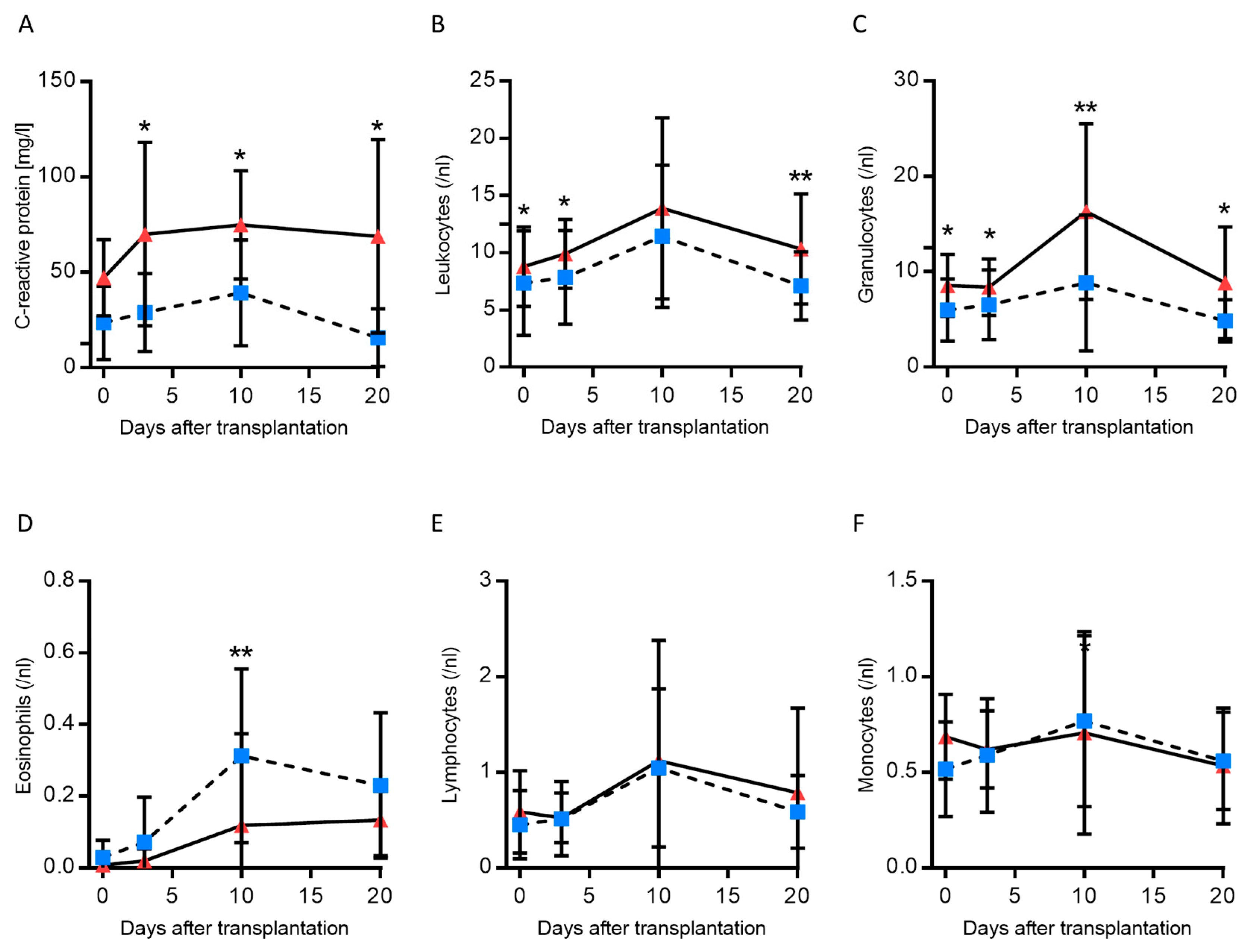
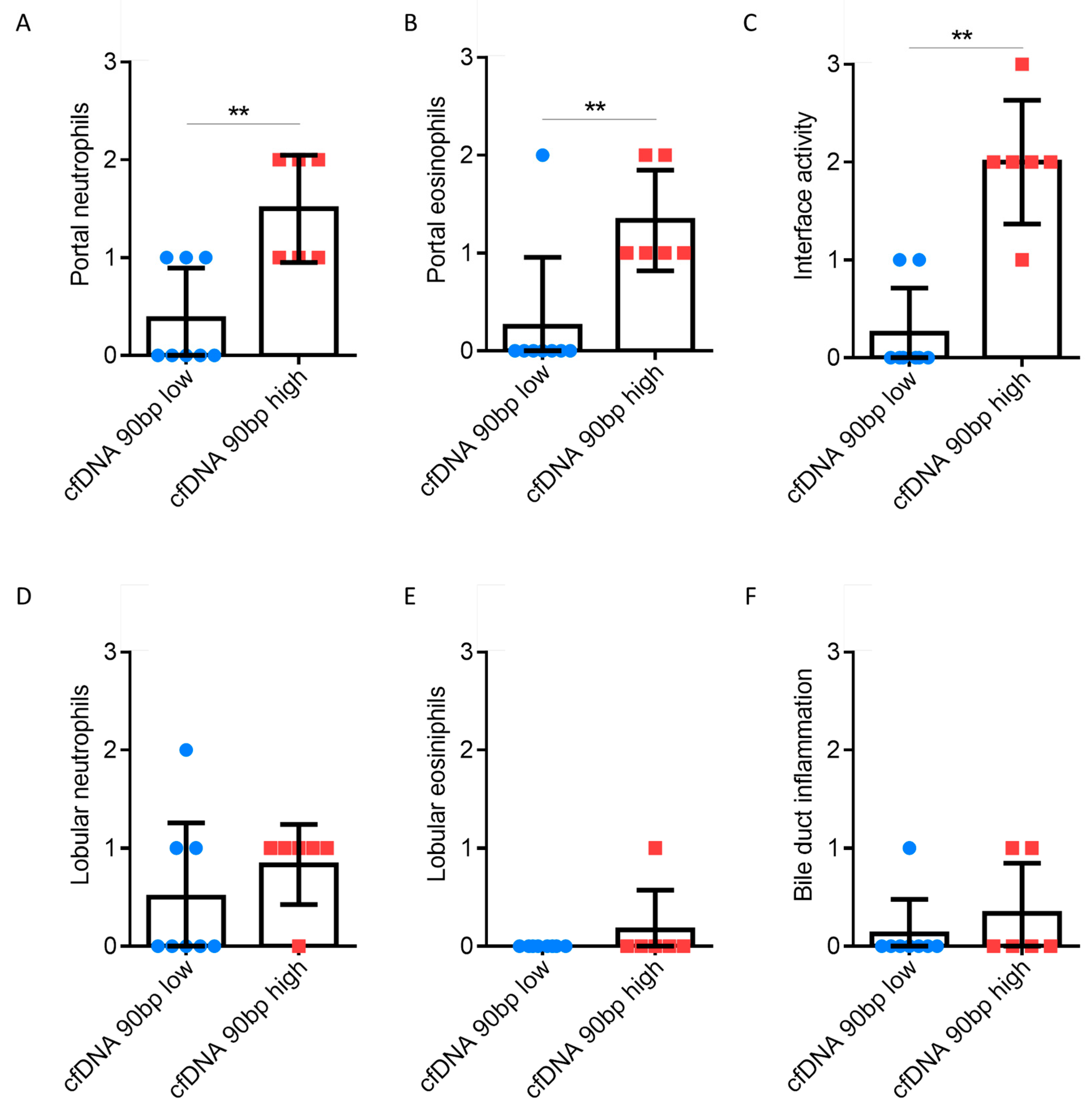
| Clinical Parameters | cfDNA Low (90 bp) n = 41 | cfDNA High (90 bp) n = 9 | p-Value |
|---|---|---|---|
| Age (years) | 57 (22−70) | 61 (28−65) | 0.946 |
| Sex | 0.454 | ||
| Female | 15 | 5 | |
| Male | 26 | 4 | |
| BMI (kg/m2) | 25.0 (17.3−40.0) | 28.6 (19.4−37.9) | 0.393 |
| Primary disease | |||
| Hepatocellular carcinoma | 14 | 2 | 0.573 |
| Cholangiocellular carcinoma | 2 | 1 | 0.844 |
| Alcoholic cirrhosis | 7 | 0 | 0.213 |
| Primary sclerosing cholangitis | 4 | 2 | 0.217 |
| Viral cirrhosis | 4 | 1 | 0.257 |
| Cryptogenic cirrhosis | 3 | 1 | 0.608 |
| Other | 8 | 2 | 0.699 |
| labMELD | 15 (6−40) | 19 (6−40) | 0.713 |
| International normalised ratio | 1.37 (0.97−5.3) | 1.35 (0.99−2.14) | 0.945 |
| Bilirubin total (mg/dL) | 2.1 (0.3−30.3) | 4.7 (0.5−20.2) | 0.838 |
| Creatinine (mg/dL) | 0.80 (0.47−4.67) | 0.82 (0.48−4.0) | 0.925 |
| Sodium (mmol/L) | 138 (128−144) | 138 (129−142) | 0.300 |
| Clinical Parameters | Univariate HR (95% CI) | p-Value | Multivariate HR (95% CI) | p-Value |
|---|---|---|---|---|
| BMI kg/m2 | 0.92 (0.76−1.11) | 0.402 | ||
| ICU (>10 days) | 0.29 (0.05−1.76) | 0.180 | ||
| Acute rejection (BANF ≥ 1) | 62.86 (0.47−83803) | 0.259 | ||
| AST recipient POD 0 | 1.00 (1.00−1.00) | 0.807 | ||
| ALT recipient POD 0 | 1.00 (0.99−1.01) | 0.780 | ||
| AST donor | 1.00 (0.99−1.01) | 0.754 | ||
| ALT donor | 0.99 (0.98−1.01) | 0.428 | ||
| Bilirubin donor | 0.57 (0.11−2.88) | 0.500 | ||
| Creatinine donor | 0.98 (0.96−1.01) | 0.392 | ||
| Lipase donor | 1.00 (0.99−1.01) | 0.663 | ||
| Resuscitation of donor | 1.37 (0.23−8.19) | 0.731 | ||
| labMELD | 1.07(1.00−1.16) | 0.043 | 1.056 (0.92−1.21) | 0.422 |
| cfDNAhigh/low 90 bp | 8.34(1.39−50.03) | 0.020 | 11.96 (1.11−128.96) | 0.041 |
| EROD | 11.78 (1.32−105.82) | 0.027 | 6.134 (0.18−203.99) | 0.310 |
| WIT (>45 min) | 5.45 (0.91−32.71) | 0.064 | 3.184 (0.30−33.95) | 0.337 |
| CIT (>6 h) | 7.63 (0.85−68.35) | 0.069 | 14.66 (0.95−226.59) | 0.055 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krenzien, F.; Katou, S.; Papa, A.; Sinn, B.; Benzing, C.; Feldbrügge, L.; Kamali, C.; Brunnbauer, P.; Splith, K.; Lorenz, R.R.; et al. Increased Cell-Free DNA Plasma Concentration Following Liver Transplantation Is Linked to Portal Hepatitis and Inferior Survival. J. Clin. Med. 2020, 9, 1543. https://doi.org/10.3390/jcm9051543
Krenzien F, Katou S, Papa A, Sinn B, Benzing C, Feldbrügge L, Kamali C, Brunnbauer P, Splith K, Lorenz RR, et al. Increased Cell-Free DNA Plasma Concentration Following Liver Transplantation Is Linked to Portal Hepatitis and Inferior Survival. Journal of Clinical Medicine. 2020; 9(5):1543. https://doi.org/10.3390/jcm9051543
Chicago/Turabian StyleKrenzien, Felix, Shadi Katou, Alba Papa, Bruno Sinn, Christian Benzing, Linda Feldbrügge, Can Kamali, Philipp Brunnbauer, Katrin Splith, Ralf Roland Lorenz, and et al. 2020. "Increased Cell-Free DNA Plasma Concentration Following Liver Transplantation Is Linked to Portal Hepatitis and Inferior Survival" Journal of Clinical Medicine 9, no. 5: 1543. https://doi.org/10.3390/jcm9051543
APA StyleKrenzien, F., Katou, S., Papa, A., Sinn, B., Benzing, C., Feldbrügge, L., Kamali, C., Brunnbauer, P., Splith, K., Lorenz, R. R., Ritschl, P., Wiering, L., Öllinger, R., Schöning, W., Pratschke, J., & Schmelzle, M. (2020). Increased Cell-Free DNA Plasma Concentration Following Liver Transplantation Is Linked to Portal Hepatitis and Inferior Survival. Journal of Clinical Medicine, 9(5), 1543. https://doi.org/10.3390/jcm9051543






