Comparing Eight Prognostic Scores in Predicting Mortality of Patients with Acute-On-Chronic Liver Failure Who Were Admitted to an ICU: A Single-Center Experience
Abstract
1. Introduction
2. Methods
2.1. Patient Selection
2.2. Follow-Up Periods
2.3. ACFL Diagnosis and Grading
2.4. Data Collection
2.5. The Primary Endpoint and the Pre-Defined Follow-Up Periods
2.6. Patient Treatment
2.7. Statistical Analysis
3. Results
3.1. Demographics
3.2. Comparisons of the Outcome Prediction Strengths of the Eight Models by AUROC Analysis
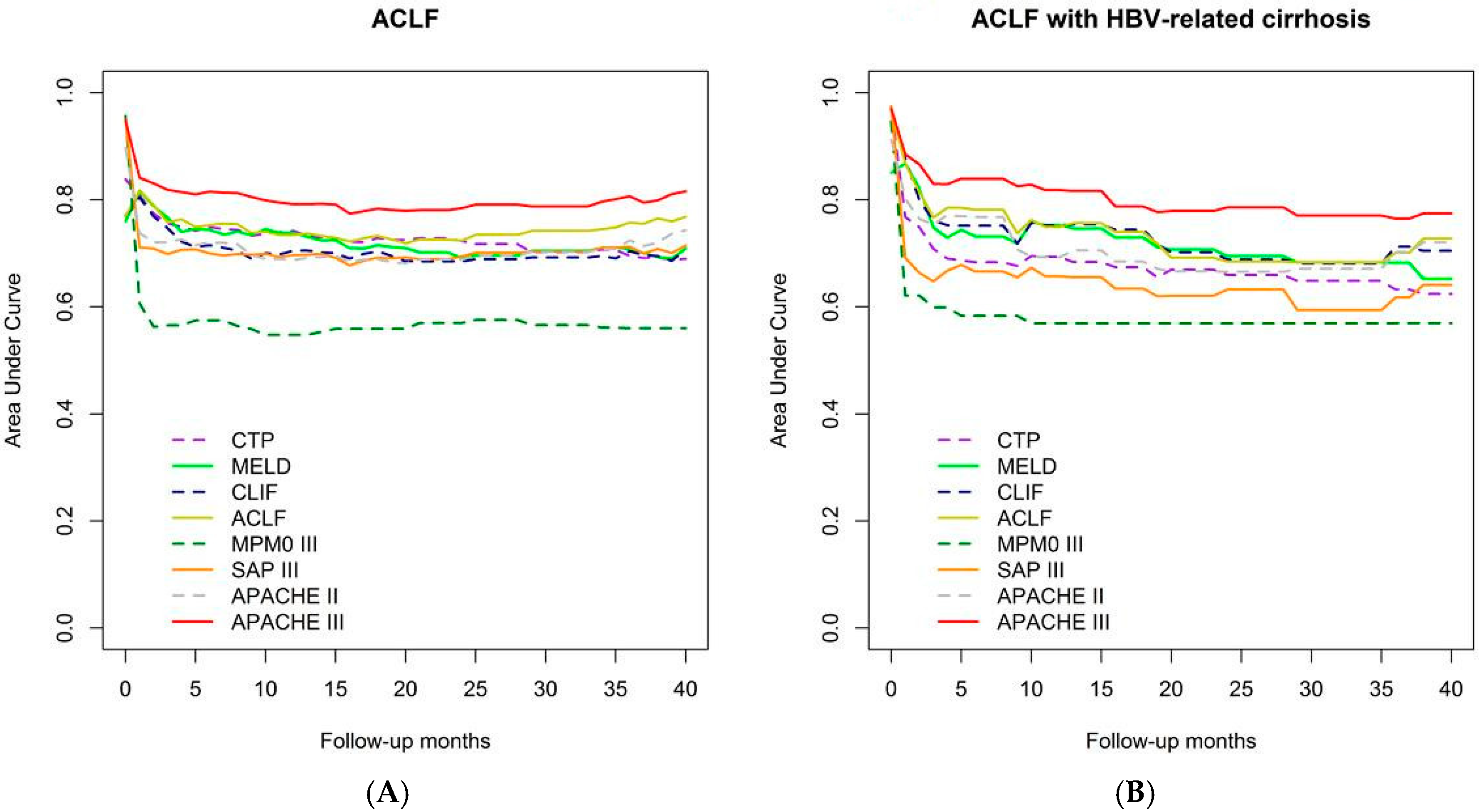
3.2.1. Time-Dependent AUROC Comparison of Overall Mortality Prediction Strength
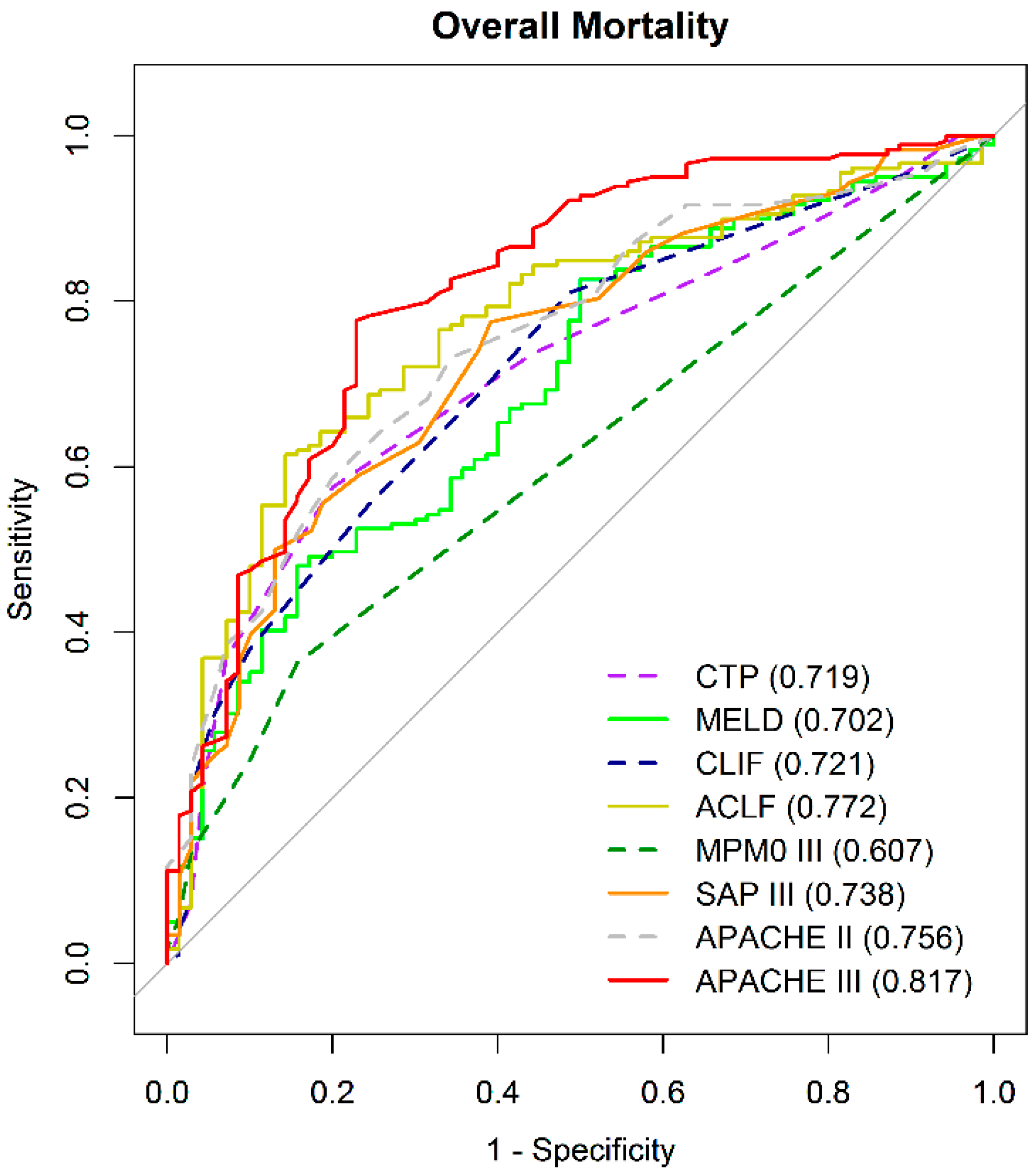
3.2.2. Comparison of the Overall Mortality Prediction Strength by AUROC at Specific Time Point
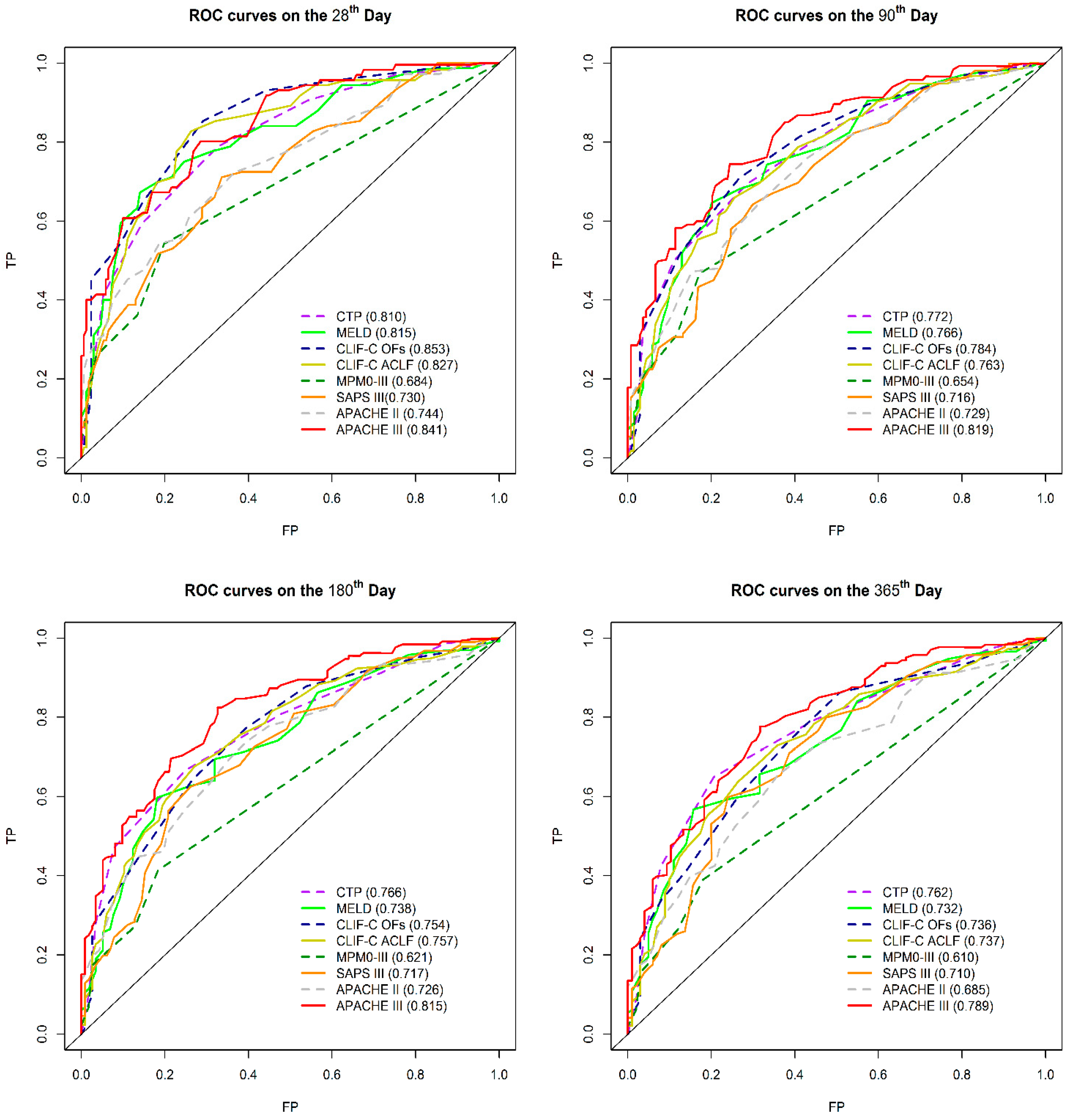
3.2.3. Comparison of 28-Day, 90-Day, 180-Day, and 365-Day Mortality Predictions by AUROC
3.2.4. The Optimal and Futility Cutoff Values for APACHE III and CLIF-C ACLF Scores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| INR | International normalized ratio |
| MAP | Mean arterial pressure |
| CPR | Cardiopulmonary resuscitation |
| SBP | Systolic blood pressure |
| HR | Heart rate |
| ICU | Intensive care unit |
| ACLF | Acute-on-chronic liver failure |
| ICU | Intensive care unit |
| LT | Liver transplantation |
| SOFAs | Sepsis organ failure assessment score |
| CLIF-SOFAs | Chronic liver failure SOFA score |
| CLIF-C OFs | CLIF consortium organ function score |
| CLIF-C ACLFs | CLIF consortium acute on chronic liver failure score |
| APACHE | Acute physiology and chronic health evaluation |
| MPM0-III | Mortality probability model III at zero hours |
| SAPs III | Simplified acute physiology score III |
| CTP | Child–Turcotte–Pugh score |
| MELDs | Model for end-stage liver disease score |
| EASL | European Association for the Study of the Liver |
| APASL | Asia-Pacific Association for the Study of the Liver |
| GCSs | Glasgow coma scale score |
| ROC | Receiver operating characteristic |
| AUROC | Area under the ROC curve |
References
- Jalan, R.; Gines, P.; Olson, J.C.; Mookerjee, R.P.; Moreau, R.; Garcia-Tsao, G. Acute-on-chronic liver failure. J. Hepatol. 2012, 57, 1336–13348. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.; Jalan, R.; Ginès, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. Acute-on-Chronic Liver Failure Is a Distinct Syndrome That Develops in Patients With Acute Decompensation of Cirrhosis. Gastroenterology 2013, 144, 1426–1437.e9. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.C.; Wendon, J.A.; Kramer, D.J.; Arroyo, V.; Jalan, R.; Garcia-Tsao, G.; Kamath, P.S. Intensive care of the patient with cirrhosis. Hepatology 2011, 54, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Party, T.A.A.W.; Kedarisetty, C.K.; Abbas, Z.; Amarapurkar, D.; Bihari, C.; Chan, A.C.; Chawla, Y.K.; Dokmeci, A.K.; Garg, H.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol. Int. 2014, 8, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Ferreira, F.L.; Bota, D.P.; Bross, A.; Melot, C.; Vincent, J.-L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001, 286, 1754–1758. [Google Scholar] [CrossRef]
- Pan, H.-C.; Jenq, C.-C.; Tsai, M.-H.; Fan, P.-C.; Chang, C.-H.; Chang, M.-Y.; Tian, Y.-C.; Hung, C.-C.; Fang, J.-T.; Yang, C.-W.; et al. Scoring systems for 6-month mortality in critically ill cirrhotic patients: A prospective analysis of chronic liver failure—Sequential organ failure assessment score (CLIF-SOFA). Aliment. Pharmacol. Ther. 2014, 40, 1056–1065. [Google Scholar] [CrossRef]
- Jalan, R.; Saliba, F.; Pavesi, M.; Amorós, A.; Moreau, R.; Ginès, P.; Lévesque, É.; Durand, F.; Angeli, P.; Caraceni, P.; et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J. Hepatol. 2014, 61, 1038–1047. [Google Scholar] [CrossRef]
- Nakayama, N.; Uemura, H.; Uchida, Y.; Tomiya, T.; Ido, A.; Inoue, K.; Genda, T.; Takikawa, Y.; Sakaida, I.; Terai, S.; et al. A multicenter pilot survey to clarify the clinical features of patients with acute-on-chronic liver failure in Japan. Hepatol. Res. 2018, 48, 303–312. [Google Scholar] [CrossRef]
- Keegan, M.T.; Gajic, O.; Afessa, B. Severity of illness scoring systems in the intensive care unit. Crit. Care Med. 2011, 39, 163–169. [Google Scholar] [CrossRef]
- Rubenfeld, G.D.; Angus, D.C.; Pinsky, M.R.; Curtis, J.R.; Connors, A.F.; Bernard, G.R. Outcomes research in critical care: Results of the American Thoracic Society Critical Care Assembly Workshop on Outcomes Research. Am. J. Respir. Crit. Care Med. 1999, 160, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Moreno, R.P. Clinical review: Scoring systems in the critically ill. Crit. Care 2010, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.E.; Wagner, D.P.; Draper, E.A.; Wright, L.; Alzola, C.; Knaus, W.A. Evaluation of Acute Physiology and Chronic Health Evaluation III predictions of hospital mortality in an independent database. Crit. Care Med. 1998, 26, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Kuzniewicz, M.W.; Vasilevskis, E.E.; Lane, R.; Dean, M.L.; Trivedi, N.G.; Rennie, D.J.; Clay, T.; Kotler, P.L.; Dudley, R.A. Variation in ICU Risk-Adjusted Mortality. Chest 2008, 133, 1319–1327. [Google Scholar] [CrossRef]
- Higgins, T.; Teres, D.; Copes, W.S.; Nathanson, B.; Stark, M.; Kramer, A. Assessing contemporary intensive care unit outcome: An updated Mortality Probability Admission Model (MPM0-III). Crit. Care Med. 2007, 35, 827–835. [Google Scholar] [CrossRef]
- Metnitz, P.G.H.; Moreno, R.P.; Almeida, E.; Jordan, B.; Bauer, P.; Campos, R.A.; Iapichino, G.; Edbrooke, D.; Capuzzo, M.; Le Gall, J.-R.; et al. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 1: Objectives, methods and cohort description. Intensive Care Med. 2005, 31, 1336–1344. [Google Scholar] [CrossRef]
- Moreno, R.P.; Metnitz, P.G.; Almeida, E.; Jordan, B.; Bauer, P.; Campos, R.A.; Iapichino, C.; Edbrooke, D.; Capuzzo, M.; Le Gall, J.-R.; et al. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005, 31, 1345–1355. [Google Scholar] [CrossRef]
- Olthoff, K.; Brown, R.S.; Delmonico, F.; Freeman, R.B.; McDiarmid, S.V.; Merion, R.M.; Millis, J.M.; Roberts, J.; Shaked, A.; Wiesner, R.H.; et al. Summary report of a national conference: Evolving concepts in liver allocation in the MELD and PELD era. Liver Transplant. 2004, 10, A6–A22. [Google Scholar] [CrossRef]
- Liaw, Y.-F.; Leung, N.; Kao, J.-H.; Piratvisuth, T.; Gane, E.J.; Han, K.-H.; Guan, R.; Lau, G.; Locarnini, S.A. Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2008 update. Hepatol. Int. 2008, 2, 263–283. [Google Scholar] [CrossRef]
- Liaw, Y.-F.; Kao, J.-H.; Piratvisuth, T.; Chan, H.L.Y.; Chien, R.-N.; Liu, C.-J.; Gane, E.; Locarnini, S.; Lim, S.-G.; Han, K.-H.; et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2012 update. Hepatol. Int. 2012, 6, 531–561. [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.Y.; Chen, C.J.; Chen, P.-J.; Chen, H.L.; Chen, P.J.; Chien, R.N.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2015, 10, 1–98. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Choudhury, A. Management of acute-on-chronic liver failure: An algorithmic approach. Hepatol. Int. 2018, 12, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, R.; Solà, E.; Moreau, R.; Ginès, P. Acute-on-chronic liver failure: An update. Gut 2017, 66, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.-C.; Jan, C.-F.; Kuo, H.-S.; Chen, C.-J. Nationwide Hepatitis B Vaccination Program in Taiwan: Effectiveness in the 20 Years After It Was Launched. Epidemiol. Rev. 2006, 28, 126–135. [Google Scholar] [CrossRef]
- Engelmann, C.; Thomsen, K.L.; Zakeri, N.; Sheikh, M.; Agarwal, B.; Jalan, R.; Mookerjee, R. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit. Care 2018, 22, 254. [Google Scholar] [CrossRef]
- Bruno, S.; Saibeni, S.; Bagnardi, V.; Vandelli, C.; De Luca, M.; Felder, M.; Fracanzani, A.L.; Prisco, C.; Vitaliani, G.; Simone, L.; et al. Mortality Risk According to Different Clinical Characteristics of First Episode of Liver Decompensation in Cirrhotic Patients: A Nationwide, Prospective, 3-Year Follow-Up Study in Italy. Am. J. Gastroenterol. 2013, 108, 1112–1122. [Google Scholar] [CrossRef]
- Chen, C.-L.; Wang, K.-L.; Hui, Y.-L.; Shieh, W.-B. Liver transplantation in Taiwan: The Chang Gung experience. Cancer Chemother. Pharmacol. 1992, 31, S162–S165. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II-A Severity of Disease Classification System. Crit. Care Med. 1986, 14, 755. [Google Scholar] [CrossRef]
- Knaus, W.A.; Wagner, D.P.; Draper, E.A.; Zimmerman, J.E.; Bergner, M.; Bastos, P.G.; Sirio, C.A.; Murphy, D.J.; Lotring, T.; Damiano, A.; et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 1991, 100, 1619–1636. [Google Scholar] [CrossRef]
- Zimmerman, J.E.; Kramer, A.; McNair, D.; Malila, F.M. Acute Physiology and Chronic Health Evaluation (APACHE) IV: Hospital mortality assessment for today’s critically ill patients. Crit. Care Med. 2006, 34, 1297–1310. [Google Scholar] [CrossRef] [PubMed]
- Niewiński, G.; Starczewska, M.; Kański, A. Prognostic scoring systems for mortality in intensive care units—The APACHE model. Anaesthesiol. Intensive Ther. 2014, 46, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Keegan, M.T.; Gajic, O.; Afessa, B. Comparison of APACHE III, APACHE IV, SAPS 3, and MPM0III and influence of resuscitation status on model performance. Chest 2012, 142, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Sonika, U.; Jadaun, S.; Ranjan, G.; Rout, G.; Gunjan, D.; Kedia, S.; Nayak, B. Shalimar Alcohol-related acute-on-chronic liver failure—Comparison of various prognostic scores in predicting outcome. Indian J. Gastroenterol. 2018, 37, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, J.-H.; Oh, S.; Jang, Y.; Lee, W.; Lee, H.J.; Yoo, J.-J.; Choi, W.-M.; Cho, Y.Y.; Cho, Y.; et al. CLIF-SOFA scoring system accurately predicts short-term mortality in acutely decompensated patients with alcoholic cirrhosis: A retrospective analysis. Liver Int. 2014, 35, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Chang, Y.; Park, J.Y.; Ahn, H.; Cho, H.; Han, S.J.; Oh, S.; Kim, D.; Jung, Y.J.; Kim, B.G.; et al. Characterization of acute-on-chronic liver failure and prediction of mortality in Asian patients with active alcoholism. J. Gastroenterol. Hepatol. 2016, 31, 427–433. [Google Scholar] [CrossRef]
- Li, N.; Huang, C.; Yu, K.-K.; Lu, Q.; Shi, G.-F.; Zheng, J.-M. Validation of prognostic scores to predict short-term mortality in patients with HBV-related acute-on-chronic liver failure: The CLIF-C OF is superior to MELD, CLIF SOFA, and CLIF-C ACLF. Medicine 2017, 96, e6802. [Google Scholar] [CrossRef]
- Li, H.; Chen, L.-Y.; Zhang, N.-N.; Li, S.-T.; Zeng, B.; Pavesi, M.; Amorós, À.; Mookerjee, R.; Xia, Q.; Xue, F.; et al. Characteristics, Diagnosis and Prognosis of Acute-on-Chronic Liver Failure in Cirrhosis Associated to Hepatitis B. Sci. Rep. 2016, 6, 25487. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.-H.; Shi, Y.; Zhao, H.; Wu, W.; Sheng, J. Acute-on-chronic liver failure in chronic hepatitis B: An update. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 341–350. [Google Scholar] [CrossRef]
- Chiang, C.-J.; Yang, Y.-W.; You, S.-L.; Lai, M.-S.; Chen, C.-J. Thirty-Year Outcomes of the National Hepatitis B Immunization Program in Taiwan. JAMA 2013, 310, 974–976. [Google Scholar] [CrossRef]
- Gustot, T.; Fernandez, J.; Garcia, E.; Morando, F.; Caraceni, P.; Alessandria, C.; Laleman, W.; Trebicka, J.; Elkrief, L.; Hopf, C.; et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 2015, 62, 243–252. [Google Scholar] [CrossRef] [PubMed]
| Patients’ Characteristics | All Patients | Survivors | Non-Survivors | p Value |
|---|---|---|---|---|
| (249 Patients) | (70 Patients) | (179 Patients) | ||
| Age (mean ± SD) | 55 ± 13 | 50 ± 10 | 57 ± 14 | <0.001 |
| Gender = male | 184 (74%) | 51 (73%) | 133 (74%) | 0.81 |
| Etiology | ||||
| HBV | 91 (37%) | 22 (31%) | 69 (38%) | 0.294 |
| HCV | 34 (14%) | 5 (7%) | 29 (16%) | 0.061 |
| ALC | 98 (39%) | 33 (47%) | 65 (36%) | 0.116 |
| HCV + ALC | 10 (4%) | 5 (7%) | 5 (3%) | 0.117 |
| Other † | 16 (6%) | 5 (7%) | 11 (6%) | 0.773 |
| Clinical parameters: | ||||
| Arterial PH | 7.4 (7.3–7.5) | 7.4 (7.4–7.5) | 7.4 (7.3–7.5) | 0.110 |
| PaO2/FiO2 200–300 | 194 | 61 (87%) | 132 (73%) | 0.023 |
| PaO2/FiO2 < 200 | 55 | 9 (13%) | 47 (27%) | 0.023 |
| MAP (mmHg) | 88 (76–102) | 87 (80–104) | 88 (76–101) | 0.581 |
| Temperature (℃) | 36.6 (36.0–37.3) | 36.9 (36.3–37.6) | 36.5 (35.8–37.2) | 0.001 |
| Respiratory rate (/min) | 19 (16–22) | 18 (16–20) | 19 (17–23) | 0.082 |
| Use of vasopressors | 140 | 35 (50%) | 105 (59%) | 0.216 |
| HE I-II | 92 | 17 (24%) | 75 (42%) | 0.01 |
| HE III-IV | 36 | 7 (10%) | 29 (16%) | 0.211 |
| White cell count (×1000/μL) | 9.3 (6.3–12.9) | 8.3 (5.8–10.9) | 9.6 (6.8–15.1) | 0.010 |
| Hematocrit (mg/dL) | 26.7 (22.7–31.2) | 27.5 (22.3–31.5) | 26.7 (22.7–31.2) | 0.907 |
| INR | 1.7 (1.4–2.2) | 1.5 (1.3–1.8) | 1.8 (1.5–2.4) | <0.001 |
| Serum bilirubin (mg/dL) | 3.6 (1.7–9.5) | 2.2 (1.4–4.1) | 4.2 (2.0–12.8) | <0.001 |
| Serum creatinine (mg/dL) | 1.22 (0.82–2.07) | 0.84 (0.66–1.38) | 1.35 (0.95–2.24) | <0.001 |
| Serum sodium (mEq/L) | 138 (134–142) | 138 (136–141) | 138 (133–142) | 0.459 |
| Serum glucose (mg/dL) | 172 (135–229) | 154 (122–198) | 179 (139–241) | 0.013 |
| Albumin (g/dL) | 2.65 (2.29–2.98) | 2.87 (2.54–3.20) | 2.60 (2.20–2.88) | 0.047 |
| Mechanical ventilation use | 85 | 17 (24%) | 68 (38%) | <0.001 |
| ACLF grades of EASL-CLIF consortium: | ||||
| ACLF 1 | 106 (43%) | 43 (61%) | 63 (35%) | 0.35 |
| ACLF 2 | 48 (19%) | 11 (16%) | 37 (21%) | 0.066 |
| ACLF 3 | 95 (38%) | 16 (23%) | 79 (44%) | <0.001 |
| Indications for ICU admission: | ||||
| Gastrointestinal bleeding | 127 (57%) | 43 (61%) | 84 (47%) | 0.040 |
| Hepatic encephalopathy | 35 (12%) | 14 (21%) | 21 (12%) | 0.092 |
| Sepsis | 56 (22%) | 9 (13%) | 47 (26%) | 0.023 |
| Other | 31 (13%) | 4 (5%) | 27 (15%) | 0.053 |
| Score on admission to ICU median (IQR): | ||||
| CTP | 9.0 (8.0–11.0) | 8.0 (7.0–9.0) | 10.0 (8.0–11.0) | <0.001 |
| MELD | 23.0 (18.0–30.0) | 18.5 (16.0–25.0) | 25.0 (19.0–34.0) | <0.001 |
| CLIF-C OF | 10.0 (8.0–12.0) | 8.0 (8.0–10.0) | 11.0 (9.0–13.0) | <0.001 |
| CLIF-C ACLF | 49.2 (41.8–60.5) | 41.8 (37.5–47.9) | 52.6 (46.3–63.4) | <0.001 |
| SAP III | 51.0 (46.0–59.0) | 47.0 (43.0–51.0) | 54.0 (48.0–63.0) | <0.001 |
| MPM0-III | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | 0.001 |
| APACHE II | 16.0 (12.0–22.0) | 13.0 (9.0–16.0) | 18.0 (13.0–23.0) | <0.001 |
| APACHE III | 81.0 (61.0–103.0) | 55.0 (41.0–70.0) | 87.0 (73.0–108.0) | <0.001 |
| AUROC (95%CI) | Pairwise Sig. Mark | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | ||||
| CTP | A | 0.719 | (0.652–0.785) | ||||||||
| MELD | B | 0.702 | (0.631–0.772) | ||||||||
| CLIF-C OF | C | 0.721 | (0.653–0.790) | ||||||||
| CLIF-C ACLF | D | 0.772 | (0.708–0.836) | ||||||||
| MPM0-III | E | 0.607 | (0.552–0.663) | ||||||||
| SAP III | F | 0.739 | (0.671–0.806) | ||||||||
| APACHE II | G | 0.756 | (0.692–0.820) | ||||||||
| APACHE III | H | 0.817 | (0.756–0.878) | ||||||||
| AUROC (95%CI) | Pairwise Sig. Mark | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | ||||
| CTP score | A | 0.810 | (0.736–0.883) | ||||||||
| MELD | B | 0.815 | (0.741–0.887) | ||||||||
| CLIF-C OFs | C | 0.853 | (0.794–0.913) | ||||||||
| CLIF-C ACLF | D | 0.827 | (0.761–0.895) | ||||||||
| MPM0-III | E | 0.684 | (0.602–0.765) | ||||||||
| SAP III | F | 0.730 | (0.653–0.808) | ||||||||
| APACHEII | G | 0.744 | (0.663–0.819) | ||||||||
| APACHEIII | H | 0.841 | (0.784–0.902) | ||||||||
| APACH III | CLIF-C ACLF | ||
|---|---|---|---|
| Optimal cutoff | 79 | 47 | |
| 28th day | Sen | 81.5% | 87.0% |
| Sp | 60.4% | 55.7% | |
| 90th day | Sen | 76.2% | 78.7% |
| Sp | 66.7% | 59.3% | |
| 180th day | Sen | 73.3% | 75.5% |
| Sp | 70.8% | 62.1% | |
| 365th day | Sen | 70.0% | 70.0% |
| Sp | 72.3% | 64.2% | |
| Futility cutoff | 125 | 71 | |
| 28th day | mortality | 80.0% | 80.0% |
| 90th day | mortality | 92.6% | 80.0% |
| 180th day | mortality | 96.3% | 90.0% |
| 365th day | mortality | 96.3% | 90.0% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.-H.; Tseng, H.-J.; Chen, W.-T.; Chen, P.-C.; Ho, Y.-P.; Huang, C.-H.; Lin, C.-Y. Comparing Eight Prognostic Scores in Predicting Mortality of Patients with Acute-On-Chronic Liver Failure Who Were Admitted to an ICU: A Single-Center Experience. J. Clin. Med. 2020, 9, 1540. https://doi.org/10.3390/jcm9051540
Chen B-H, Tseng H-J, Chen W-T, Chen P-C, Ho Y-P, Huang C-H, Lin C-Y. Comparing Eight Prognostic Scores in Predicting Mortality of Patients with Acute-On-Chronic Liver Failure Who Were Admitted to an ICU: A Single-Center Experience. Journal of Clinical Medicine. 2020; 9(5):1540. https://doi.org/10.3390/jcm9051540
Chicago/Turabian StyleChen, Bo-Huan, Hsiao-Jung Tseng, Wei-Ting Chen, Pin-Cheng Chen, Yu-Pin Ho, Chien-Hao Huang, and Chun-Yen Lin. 2020. "Comparing Eight Prognostic Scores in Predicting Mortality of Patients with Acute-On-Chronic Liver Failure Who Were Admitted to an ICU: A Single-Center Experience" Journal of Clinical Medicine 9, no. 5: 1540. https://doi.org/10.3390/jcm9051540
APA StyleChen, B.-H., Tseng, H.-J., Chen, W.-T., Chen, P.-C., Ho, Y.-P., Huang, C.-H., & Lin, C.-Y. (2020). Comparing Eight Prognostic Scores in Predicting Mortality of Patients with Acute-On-Chronic Liver Failure Who Were Admitted to an ICU: A Single-Center Experience. Journal of Clinical Medicine, 9(5), 1540. https://doi.org/10.3390/jcm9051540







