Actual Persistence of Abatacept in Rheumatoid Arthritis: Results of the French-Ric Network
Abstract
1. Introduction
2. Methods
2.1. Patient Selection
- -
- RA according to the 2010 ACR/EULAR criteria [14];
- -
- Age 18 years or older;
- -
- Initiation of ABA therapy by the intravenous or subcutaneous route;
- -
- Attendance at a minimum of 12-months follow-up;
- -
- Written informed consent for participation in the RIC-France Network.
2.2. Data Recorded
- -
- Group 1: patients who initiated ABA therapy from 2007 to 31 July 2010 (ABA indicated after anti-TNF alpha failure);
- -
- Group 2: patients who initiated ABA therapy from 1 August 2010 to 31 March 2014 (ABA indicated after conventional antirheumatic drugs failure including MTX);
- -
- Group 3: patients who initiated ABA therapy from 1 April 2014 to 1 July 2016 (ABA available by the subcutaneous injection) up to 1 July 2016.
2.3. Statistical Analysis
3. Results
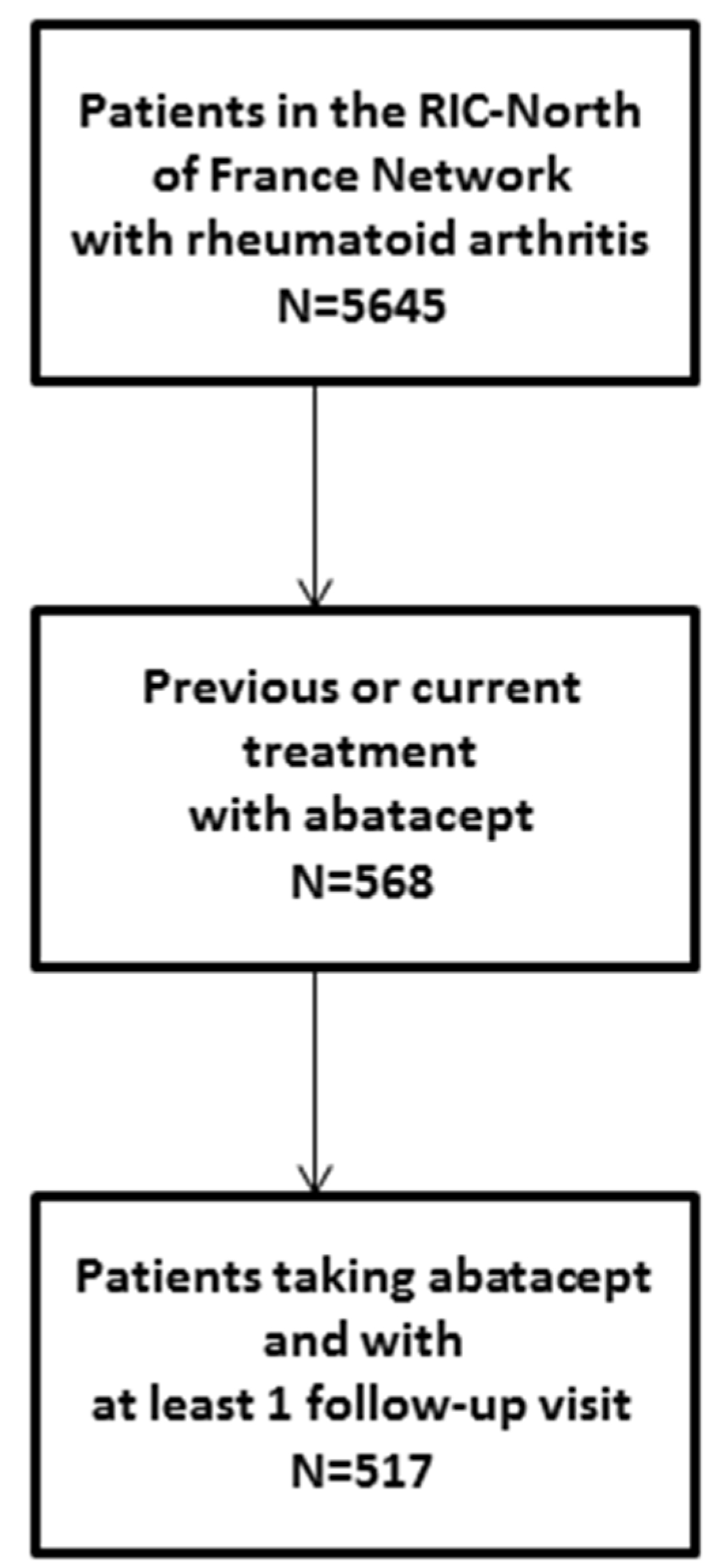
3.1. Patient Selection and Characteristics
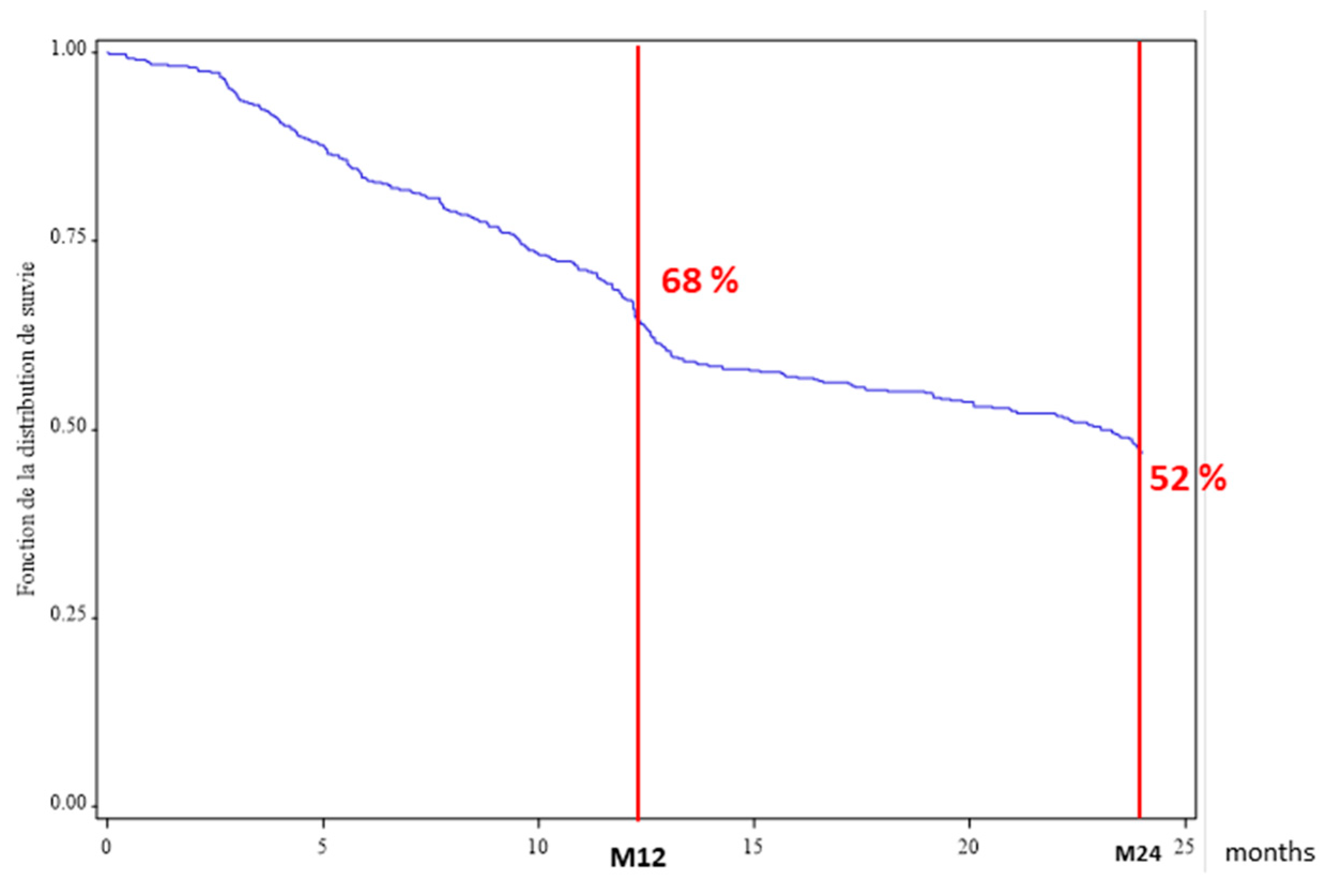
3.2. ABA Persistence in the Overall Population
3.3. Reasons for ABA Discontinuation
3.4. Factors Associated with Drug Persistence at 12 Months
3.5. Efficacy Criteria
3.6. Patient Characteristics According to Date of ABA Initiation
3.7. Relation between Drug Persistence and Other Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tobón, G.J.; Youinou, P.; Saraux, A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J. Autoimmun. 2010, 35, 10–14. [Google Scholar] [CrossRef]
- Newsome, G. American College of Rheumatology Guidelines for the management of rheumatoid arthritis: 2002 update. J. Am. Acad. Nurse Pract. 2002, 14, 432–437. [Google Scholar]
- Scott, D.L.; Wolfe, F.; Huizinga, T.W.J. Rheumatoid arthritis. Lancet Lond. Engl. 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Daien, C.; Hua, C.; Gaujoux-Viala, C.; Cantagrel, A.; Dubremetz, M.; Dougados, M.; Fautrel, B.; Mariette, X.; Nayral, N.; Richez, C.; et al. Update of French society for rheumatology recommendations for managing rheumatoid arthritis. Jt. Bone Spine 2019, 86, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Gottenberg, J.E.; Courvoisier, D.S.; Hernandez, M.V.; Iannone, F.; Lie, E.; Canhão, H.; Pavelka, K.; Hetland, M.L.; Turesson, C.; Mariette, X.; et al. Brief Report: Association of Rheumatoid Factor and Anti-Citrullinated Protein Antibody Positivity With Better Effectiveness of Abatacept: Results From the Pan-European Registry Analysis. Arthritis Rheumatol. 2016, 68, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Cagnotto, G.; Willim, M.; Nilsson, J.-Å.; Compagno, M.; Jacobsson, L.T.H.; Saevarsdottir, S.; Turesson, C. Abatacept in rheumatoid arthritis: Survival on drug, clinical outcomes, and their predictors-data from a large national quality register. Arthritis Res. Ther. 2020, 22, 15. [Google Scholar] [CrossRef]
- Takahashi, N.; Kojima, T.; Kida, D.; Kaneko, A.; Hirano, Y.; Fujibayashi, T.; Yabe, Y.; Takagi, H.; Oguchi, T.; Hanabayashi, M.; et al. Clinical effectiveness and long-term retention of abatacept in elderly rheumatoid arthritis patients: Results from a multicenter registry system. Mod. Rheumatol. 2019, 29, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Choquette, D.; Bessette, L.; Alemao, E.; Haraoui, B.; Postema, R.; Raynauld, J.-P.; Coupal, L. Persistence rates of abatacept and TNF inhibitors used as first or second biologic DMARDs in the treatment of rheumatoid arthritis: 9 years of experience from the Rhumadata® clinical database and registry. Arthritis Res. Ther. 2019, 21, 138. [Google Scholar] [CrossRef]
- Finckh, A.; Neto, D.; Iannone, F.; Loza, E.; Lie, E.; van Riel, P.; Hetland, M.L.; Pavelka, K.; Gottenberg, J.E.; Canhão, H.; et al. The impact of patient heterogeneity and socioeconomic factors on abatacept retention in rheumatoid arthritis across nine European countries. RMD Open 2015, 1, e000040. [Google Scholar] [CrossRef]
- Souto, A.; Maneiro, J.R.; Gómez-Reino, J.J. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: A systematic review and meta-analysis of drug registries and health care databases. Rheumatology 2016, 55, 523–534. [Google Scholar] [CrossRef]
- Mariette, X.; Gottenberg, J.-E.; Ravaud, P.; Combe, B. Registries in rheumatoid arthritis and autoimmune diseases: Data from the French registries. Rheumatology 2011, 50, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.H.; Gottenberg, J.E.; Ravaud, P.; Cantagrel, A.; Combe, B.; Flipo, R.M.; Schaeverbeke, T.; Houvenagel, E.; Gaudin, P.; Loeuille, D.; et al. Predictive risk factors of serious infections in patients with rheumatoid arthritis treated with abatacept in common practice: Results from the Orencia and Rheumatoid Arthritis (ORA) registry. Ann. Rheum. Dis. 2016, 75, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Gottenberg, J.E.; Ravaud, P.; Cantagrel, A.; Combe, B.; Flipo, R.M.; Schaeverbeke, T.; Houvenagel, E.; Gaudin, P.; Loeuille, D.; Rist, S.; et al. Positivity for anti-cyclic citrullinated peptide is associated with a better response to abatacept: Data from the “Orencia and Rheumatoid Arthritis” registry. Ann. Rheum. Dis. 2012, 71, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- van Gestel, A.M.; Haagsma, C.J.; van Riel, P.L. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998, 41, 1845–1850. [Google Scholar] [CrossRef]
- Mack, M.E.; Hsia, E.; Aletaha, D. Comparative Assessment of the Different American College of Rheumatology/European League Against Rheumatism Remission Definitions for Rheumatoid Arthritis for Their Use as Clinical Trial End Points. Arthritis Rheumatol. 2017, 69, 518–528. [Google Scholar] [CrossRef]
- Fleischmann, R.; van der Heijde, D.; Koenig, A.S.; Pedersen, R.; Szumski, A.; Marshall, L.; Bananis, E. How much does Disease Activity Score in 28 joints ESR and CRP calculations underestimate disease activity compared with the Simplified Disease Activity Index? Ann. Rheum. Dis. 2015, 74, 1132–1137. [Google Scholar] [CrossRef]
- Alten, R.; Mariette, X.; Lorenz, H.-M.; Galeazzi, M.; Cantagrel, A.; Nüßlein, H.G.; Chartier, M.; Elbez, Y.; Rauch, C.; Le Bars, M. Real-world predictors of 12-month intravenous abatacept retention in patients with rheumatoid arthritis in the ACTION observational study. RMD Open 2017, 3, e000538. [Google Scholar] [CrossRef]
- Alten, R.; Feist, E.; Lorenz, H.-M.; Nüßlein, H.; Voll, R.E.; Chartier, M.; Elbez, Y.; Rauch, C. Abatacept retention and clinical outcomes in rheumatoid arthritis: Real-world data from the German cohort of the ACTION study and a comparison with other participating countries. Clin. Rheumatol. 2019, 38, 3049–3059. [Google Scholar] [CrossRef]
- Alten, R.; Mariette, X.; Lorenz, H.-M.; Nüßlein, H.; Galeazzi, M.; Navarro, F.; Chartier, M.; Heitzmann, J.; Poncet, C.; Rauch, C.; et al. Predictors of abatacept retention over 2 years in patients with rheumatoid arthritis: Results from the real-world ACTION study. Clin. Rheumatol. 2019, 38, 1413–1424. [Google Scholar] [CrossRef]
- Ebina, K.; Hashimoto, M.; Yamamoto, W.; Hirano, T.; Hara, R.; Katayama, M.; Onishi, A.; Nagai, K.; Son, Y.; Amuro, H.; et al. Drug tolerability and reasons for discontinuation of seven biologics in 4466 treatment courses of rheumatoid arthritis-the ANSWER cohort study. Arthritis Res. Ther. 2019, 21, 91. [Google Scholar] [CrossRef] [PubMed]
- Alten, R.; Mariette, X.; Buch, M.; Caporali, R.; Flipo, R.-M.; Forster, A. ASCORE, A 2-year, observational, prospective multicentre study of subcutaneous abatacept for the treatment of rheumatoid arthritis in routine clinical practice: 1-year interim analysis. Ann. Rheum. Dis. 2019, 78 (Suppl. 2), A1639. [Google Scholar]
- Hetland, M.L.; Christensen, I.J.; Tarp, U.; Dreyer, L.; Hansen, A.; Hansen, I.T.; Kollerup, G.; Linde, L.; Lindegaard, H.M.; Poulsen, U.E.; et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: Results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010, 62, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Belhassen, M.; Tubach, F.; Hudry, C.; Woronoff, M.-C.; Levy-Bachelot, L.; Lamezec, L.; Van Ganse, E.; Fautrel, B. Impact de la non-persistance aux anti-TNF alpha sous-cutanés sur la consommation de soins et les coûts. Rev. DÉpidémiol. Santé Publique 2017, 65, S121. [Google Scholar] [CrossRef]
- Gottenberg, J.-E.; Morel, J.; Perrodeau, E.; Bardin, T.; Combe, B.; Dougados, M.; Flipo, R.-M.; Saraux, A.; Schaeverbeke, T.; Sibilia, J.; et al. Comparative effectiveness of rituximab, abatacept, and tocilizumab in adults with rheumatoid arthritis and inadequate response to TNF inhibitors: Prospective cohort study. BMJ 2019, 364, l67. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef]
- Sokolove, J.; Schiff, M.; Fleischmann, R.; Weinblatt, M.E.; Connolly, S.E.; Johnsen, A.; Zhu, J.; Maldonado, M.A.; Patel, S.; Robinson, W.H. Impact of baseline anti-cyclic citrullinated peptide-2 antibody concentration on efficacy outcomes following treatment with subcutaneous abatacept or adalimumab: 2-year results from the AMPLE trial. Ann. Rheum. Dis. 2016, 75, 709–714. [Google Scholar] [CrossRef]
| Whole Population | Available Data | |
|---|---|---|
| Age | 61.4 ± 13.3 | 517 (100%) |
| Female sex | 381 (73.7%) | 517 (100%) |
| Disease duration (years) | 18.1 ± 10.6 | 503 (97%) |
| Body mass index | 26.9 ± 6 | 467 (90%) |
| RF positive | 365 (77.9%) | 468 (90.5%) |
| ACPA positive | 347 (76.4%) | 454 (88%) |
| Erosion | 327 (70.9%) | 461 (89%) |
| DAS 28 at initiation | 4.7 ± 1.3 | 512 (99%) |
| C-reactive protein | 17.6 ± 25 | 406 (78.5%) |
| Erythrocyte sedimentation rate | 27.7 ± 22.3 | 440 (85%) |
| Methotrexate at initiation | 228 (44.1%) | 517 (100%) |
| -dose in mg/w | 14.2 ± 4.7 | |
| Corticosteroids at initiation | 212 (41%) | 517 (100%) |
| -dose in mg/d | 9.1 ± 5.6 | |
| Mean number of prior conventional DMARDs | 1.5 ± 1.3 | 517 (100%) |
| Mean number of prior bDMARDs | 1.6 ± 1.2 | 517 (100%) |
| 0 | 114 (22%) | |
| 1 | 145 (28%) | |
| 2 | 149 (29%) | |
| ≥3 | 109 (21%) |
| Persistence < 12 Months n = 166 | Persistence ≥ 12 Months n = 351 | Bivariable Analysis OR (95% CI) | Multivariable Analysis OR (95% CI) | |
|---|---|---|---|---|
| Age | 60.8 ± 13.5 | 61.6 ± 13.2 | 1.1 (0.70–1.60) | |
| Female sex | 113 (68%) | 268 (76%) | 1.5 (1–2.30) | 1.6 (0.90–2.70) |
| Disease duration (years) | 18.4 ± 11 | 17.9 ± 10.5 | 1 (0.90–1.10) | |
| Body mass index | 26.8 ± 5.9 | 27 ± 6 | 0.9 (0.60–1.60) | |
| RF positive | 111 (74%) | 254 (80%) | 1.4 (0.90–2.20) | 1.1 (0.60–1.90) |
| ACPA positive + | 105 (73%) | 242 (78%) | 1.3 (0.80–2.10) | |
| Erosion | 98 (67%) | 229 (73%) | 1.3 (0.90–2.10) | 1.5 (0.90–2.40) |
| DAS 28 at initiation | 4.9 ± 1.3 | 4.5 ± 1.4 | 0.3 (0.10–0.80) | 0.3 (0.10–1.10) |
| CRP < 10 mg/L at initiation | 109 (68%) | 197 (43%) | 0.5 (0.40–0.80) | 0.6 (0.30–0.90) |
| ESR | 32.1 ± 25.3 | 25.5 ± 20.3 | 0.98 (0.97–0.99) | 0.99 (0.98–1.10) |
| MTX at initiation | 72 (43%) | 156 (44%) | 1 (0.70–1.50) | |
| Corticosteroids at initiation | 93 (56%) | 212 (60%) | 0.8 (0.50–1.20) | |
| Mean number of prior conventional DMARDs | 1.6 ± 1.4 | 1.4 ± 1.3 | 0.9 (0.70–0.90) | 0.9 (0.70–1.10) |
| Mean number of prior bDMARDs | 1.6 ± 1.2 | 1.6 ± 1.3 | 0.8 (0.50–1.40) |
| Before 1 August 2010 (Anti-TNF Failure) n = 137 | 1 August 2010–31 March 2014 (First-Line Possible) n = 175 | After 1 April 2014 SC Route Possible n = 205 | |
|---|---|---|---|
| Age | 65.4 ± 11.7 | 61.5 ± 12.3 | 58.5 ± 14.4 |
| Disease duration (years) | 23.3 ± 10.5 | 18.9 ± 9.6 | 14 ± 10 |
| Erosion at initiation | 101 (83%) | 101 (57.7%) | 76 (37%) |
| DAS 28 at initiation | 5.3 ± 1.2 | 4.7 ± 1.2 | 4.2 ± 1.4 |
| CRP < 10 mg/L at initiation | 29 (27.3%) | 74 (42.3%) | 72 (35.1%) |
| MTX at initiation | 32 (27.5%) | 76 (43.4%) | 120 (58%) |
| Corticosteroids at initiation | 44 (32%) | 75 (43%) | 93 (45%) |
| Prior conventional DMARDs | |||
| -mean ± SD | 1.5 ± 1.3 | 1.4 ± 1.2 | 1.4 ± 1.3 |
| -median (IQR) | 1 (0–3) | 1 (0–2) | 1 (0–2) |
| Prior bDMARDs | |||
| 0 | 12 (9%) | 39 (22%) | 63 (31%) |
| 1 | 30 (22%) | 58 (33%) | 57 (28%) |
| 2 | 51 (37%) | 43 (25%) | 55 (27%) |
| ≥3 | 44 (32%) | 35 (20%) | 30 (14%) |
| -mean ± SD | 2.1 ± 1.2 | 1.5 ± 1.3 | 1.3 ± 1.2 |
| -median (IQR) | 2 (1–3) | 1 (1–2) | 1 (0–2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmon, J.-H.; Letarouilly, J.-G.; Goëb, V.; Kanagaratnam, L.; Coquerelle, P.; Guyot, M.-H.; Houvenagel, E.; Lecuyer, N.; Marguerie, L.; Morel, G.; et al. Actual Persistence of Abatacept in Rheumatoid Arthritis: Results of the French-Ric Network. J. Clin. Med. 2020, 9, 1528. https://doi.org/10.3390/jcm9051528
Salmon J-H, Letarouilly J-G, Goëb V, Kanagaratnam L, Coquerelle P, Guyot M-H, Houvenagel E, Lecuyer N, Marguerie L, Morel G, et al. Actual Persistence of Abatacept in Rheumatoid Arthritis: Results of the French-Ric Network. Journal of Clinical Medicine. 2020; 9(5):1528. https://doi.org/10.3390/jcm9051528
Chicago/Turabian StyleSalmon, Jean-Hugues, Jean-Guillaume Letarouilly, Vincent Goëb, Lukshe Kanagaratnam, Pascal Coquerelle, Marie-Hélène Guyot, Eric Houvenagel, Nicolas Lecuyer, Laurent Marguerie, Gauthier Morel, and et al. 2020. "Actual Persistence of Abatacept in Rheumatoid Arthritis: Results of the French-Ric Network" Journal of Clinical Medicine 9, no. 5: 1528. https://doi.org/10.3390/jcm9051528
APA StyleSalmon, J.-H., Letarouilly, J.-G., Goëb, V., Kanagaratnam, L., Coquerelle, P., Guyot, M.-H., Houvenagel, E., Lecuyer, N., Marguerie, L., Morel, G., Baudens, G., Gervais, E., & Flipo, R.-M. (2020). Actual Persistence of Abatacept in Rheumatoid Arthritis: Results of the French-Ric Network. Journal of Clinical Medicine, 9(5), 1528. https://doi.org/10.3390/jcm9051528






