Prevalence of Gestational Diabetes in Triplet Pregnancies: A Retrospective Cohort Study and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population and Study Design
2.2. Parameters Analyzed
2.3. Standard GDM Management
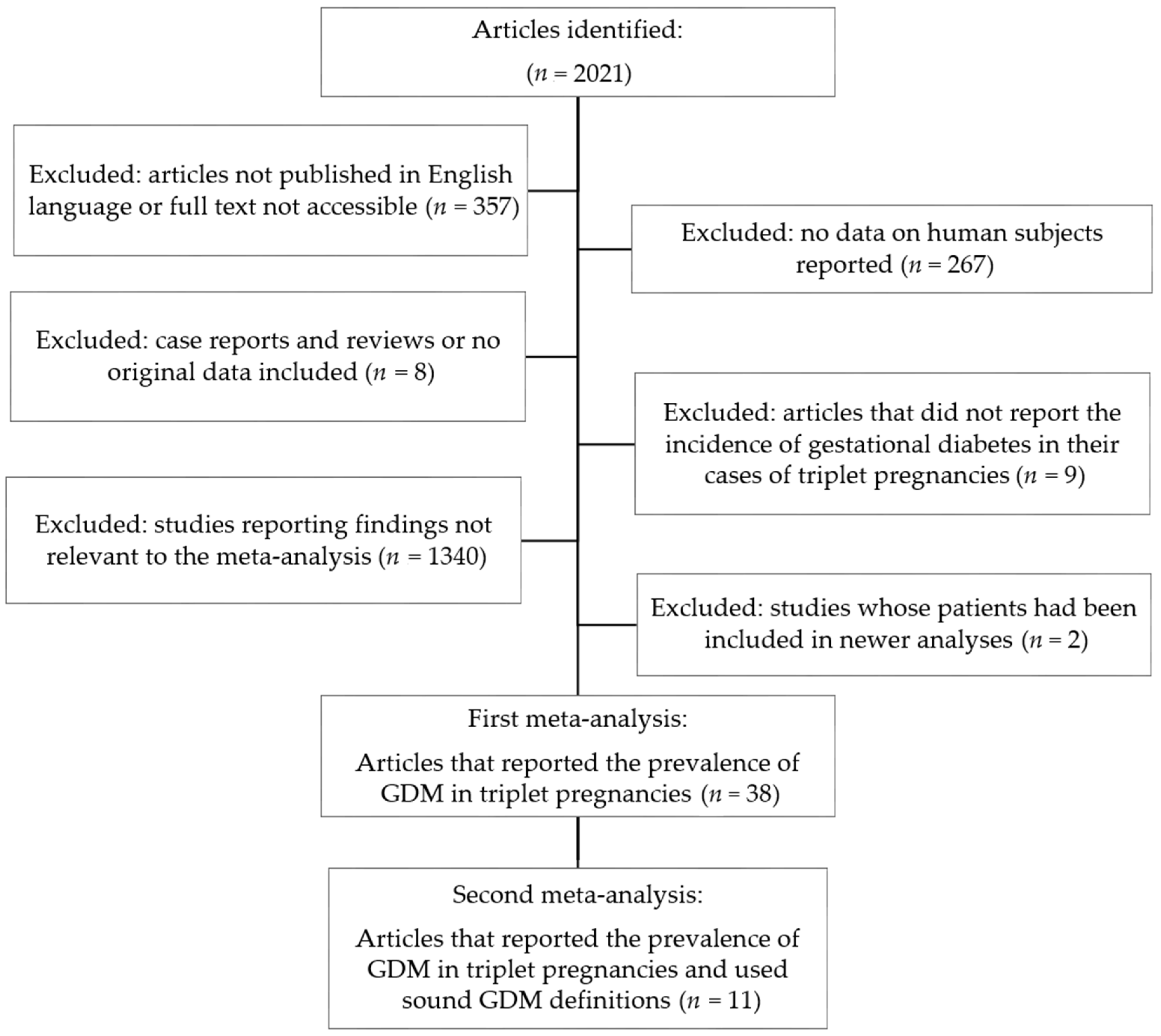
2.4. Meta-Analysis
2.5. Statistical Analyses
3. Results
3.1. Retrospective Cohort Study
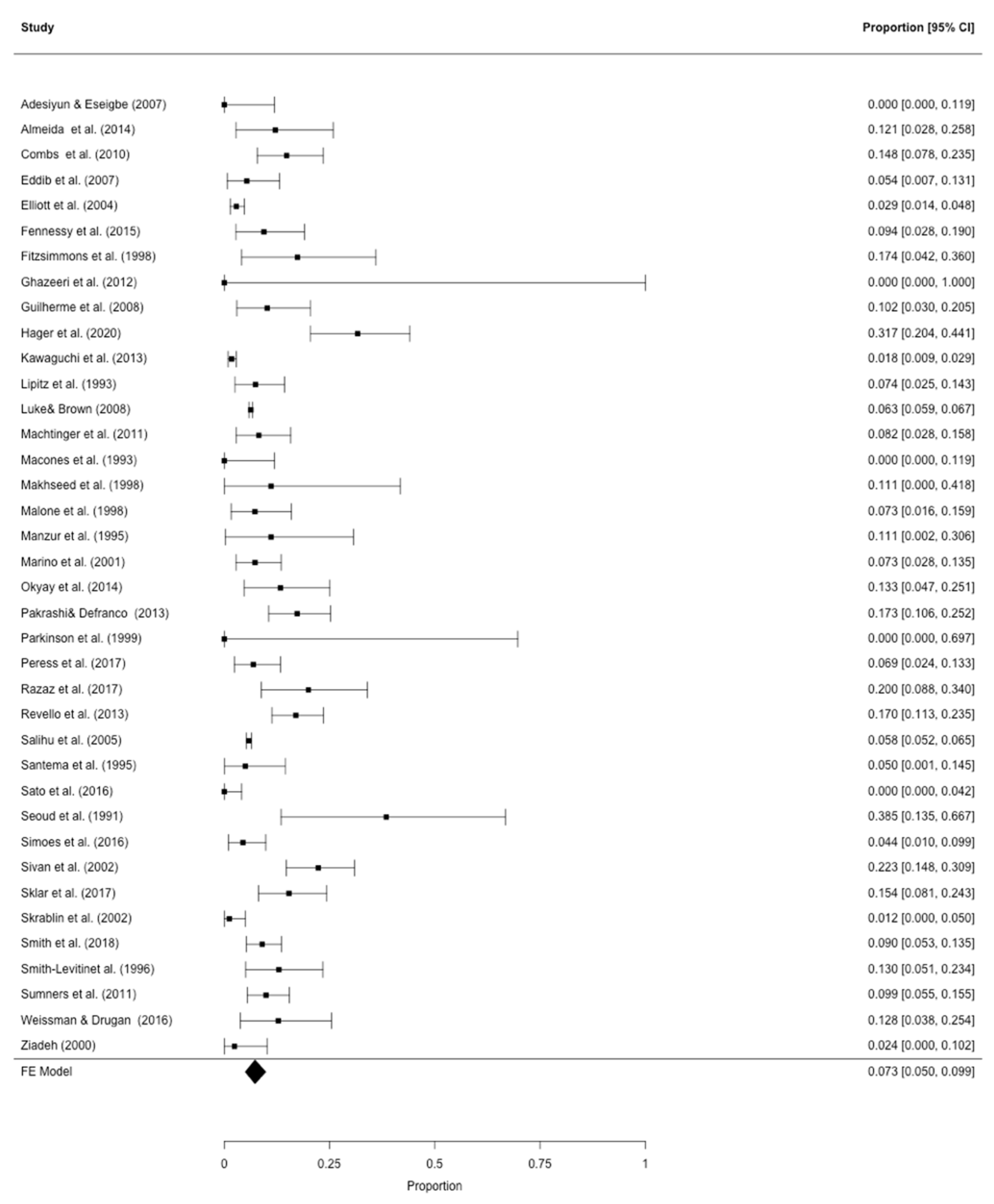
3.2. Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
Availability of Data and Material
References
- Habbema, J.D.; Eijkemans, M.J.; Nargund, G.; Beets, G.; Leridon, H.; Te Velde, E.R. The effect of in vitro fertilization on birth rates in western countries. Hum. Reprod. 2009, 24, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Nyboe Andersen, A.; Goossens, V.; Bhattacharya, S.; Ferraretti, A.P.; Kupka, M.S.; de Mouzon, J.; Nygren, K.G. Assisted reproductive technology and intrauterine inseminations in Europe, 2005: Results generated from European registers by ESHRE: ESHRE. The European IVF Monitoring Programme (EIM), for the European Society of Human Reproduction and Embryology (ESHRE). Hum. Reprod. 2009, 24, 1267–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, R.; Doyle, P.; Maconochie, N. Dramatic reduction in triplet and higher order births in England and Wales. Bjog Int. J. Obstet. Gynaecol. 2004, 111, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.; Kohari, K.S. Higher-order Multiples. Clin. Obstet. Gynecol. 2015, 58, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, P.M.; Tyzbir, E.D.; Roman, N.M.; Amini, S.B.; Sims, E.A. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am. J. Obs. Gynecol. 1991, 165, 1667–1672. [Google Scholar] [CrossRef]
- Roach, V.J.; Lau, T.K.; Wilson, D.; Rogers, M.S. The incidence of gestational diabetes in multiple pregnancy. Aust. N. Z. J. Obstet. Gynaecol. 1998, 38, 56–57. [Google Scholar] [CrossRef]
- Schwartz, D.B.; Daoud, Y.; Zazula, P.; Goyert, G.; Bronsteen, R.; Wright, D.; Copes, J. Gestational diabetes mellitus: Metabolic and blood glucose parameters in singleton versus twin pregnancies. Am. J. Obs. Gynecol. 1999, 181, 912–914. [Google Scholar] [CrossRef]
- Sivan, E.; Maman, E.; Homko, C.J.; Lipitz, S.; Cohen, S.; Schiff, E. Impact of fetal reduction on the incidence of gestational diabetes. Obs. Gynecol. 2002, 99, 91–94. [Google Scholar] [CrossRef]
- Gill, P.; Lende, M.N.; Van Hook, M.D.J.W. Twin Births. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493200/ (accessed on 28 April 2020).
- Chibber, R.; Fouda, M.; Shishtawy, W.; Al-Dossary, M.; Al-Hijji, J.; Amen, A.; Mohammed, A.T. Maternal and neonatal outcome in triplet, quadruplet and quintuplet gestations following ART: A 11-year study. Arch. Gynecol. Obs. 2013, 288, 759–767. [Google Scholar] [CrossRef]
- Moore, E.S.; Elnaggar, A.C.; Wareham, J.A.; Ramsey, C.J.; Sumners, J.E. Neonatal functional lung maturity relative to gestational age at delivery, fetal growth, and pregnancy characteristics in triplet births. J. Matern. Fetal Neonatal. Med. 2012, 25, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Morency, A.M.; Shah, P.S.; Seaward, P.G.; Whittle, W.; Murphy, K.E. Obstetrical and neonatal outcomes of triplet births-spontaneous versus assisted reproductive technology conception. J. Matern. Fetal Neonatal. Med. 2016, 29, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Sameshima, H.; Higo, T.; Ikenoue, T. Longitudinal changes in plasma glucose values of the 75-g glucose tolerance test in triplet pregnancies. Am. J. Perinatol. 2004, 21, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.W.; Demissie, K.; Yang, Q.; Walker, M.C. Maternal morbidity and obstetric complications in triplet pregnancies and quadruplet and higher-order multiple pregnancies. Am. J. Obs. Gynecol. 2004, 191, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Pils, S.; Springer, S.; Wehrmann, V.; Chalubinski, K.; Ott, J. Cervical length dynamics in triplet pregnancies: A retrospective cohort study. Arch. Gynecol. Obs. 2017, 296, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pils, S.; Springer, S.; Seemann, R.; Wehrmann, V.; Worda, C.; Ott, J. Reliability of sonographic fetal weight estimation in triplet pregnancies: A retrospective cohort study. Arch. Gynecol. Obs. 2018, 297, 1441–1447. [Google Scholar] [CrossRef] [Green Version]
- Hadlock, F.P.; Harrist, R.B.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective study. Am. J. Obs. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.; Hod, M.; Kitzmiler, J.L.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Voigt, M.; Olbertz, D.; Hentschel, R.; Kunze, M.; Hagenah, H.P.; Scholz, R.; Wittwer-Backofen, U.; Hesse, V.; Straube, S. Percentile Values for the Anthropometric Dimensions of Triplet Neonates—Analysis of German Perinatal Survey Data of 2007–2011 from all States of Germany. Z. Geburtshilfe Neonatol. 2016, 220, 185. [Google Scholar] [CrossRef]
- Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. Available online: https://apps.who.int/iris/bitstream/handle/10665/85975/WHO_NMH_MND_13.2_eng.pdf;jsessionid=065EDF38C01D18F1C3AB304DFCDC659F?sequence=1 (accessed on 27 April 2020).
- Diabetes Advocacy: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, 152–153. [CrossRef] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 3 May 2020).
- Hager, M.; Wenzl, R.; Riesenhuber, S.; Marschalek, J.; Kuessel, L.; Mayrhofer, D.; Ristl, R.; Kurz, C.; Ott, J. The Prevalence of Incidental Endometriosis in Women Undergoing Laparoscopic Ovarian Drilling for Clomiphene-Resistant Polycystic Ovary Syndrome: A Retrospective Cohort Study and Meta-Analysis. J. Clin. Med. 2019, 8, 1210. [Google Scholar] [CrossRef] [Green Version]
- Luke, B.; Brown, M.B. Maternal morbidity and infant death in twin vs triplet and quadruplet pregnancies. Am. J. Obs. Gynecol. 2008, 198. [Google Scholar] [CrossRef] [PubMed]
- Ghazeeri, G.; Kibbi, A.G.; Abbas, O. Pruritic urticarial papules and plaques of pregnancy: Epidemiological, clinical, and histopathological study of 18 cases from Lebanon. Int. J. Dermatol. 2012, 51, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.; Goudas, L.C.; Steinbok, V.; Craigo, S.D.; Yarnell, R.W. The anesthetic management of triplet cesarean delivery: A retrospective case series of maternal outcomes. Anesth. Analg. 2001, 93, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Adesiyun, A.G.; Eseigbe, E. Triplet gestation: Clinical outcome of 14 cases. Ann. Afr. Med. 2007, 6, 12–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, P.; Domingues, A.P.; Belo, A.; Fonseca, E.; Moura, P. Triplet pregnancies: Perinatal outcome evolution. Rev. Bras Ginecol. Obstet. 2014, 36, 393–397. [Google Scholar] [CrossRef]
- Combs, C.A.; Garite, T.; Maurel, K.; Das, A.; Porto, M. Failure of 17-hydroxyprogesterone to reduce neonatal morbidity or prolong triplet pregnancy: A double-blind, randomized clinical trial. Am. J. Obs. Gynecol 2010, 203. [Google Scholar] [CrossRef]
- Eddib, A.; Penvose-Yi, J.; Shelton, J.A.; Yeh, J. Triplet gestation outcomes in relation to maternal prepregnancy body mass index and weight gain. J. Matern. Fetal Neonatal. Med. 2007, 20, 515–519. [Google Scholar] [CrossRef]
- Elliott, J.P.; Istwan, N.B.; Rhea, D.; Stanziano, G. The occurrence of adverse events in women receiving continuous subcutaneous terbutaline therapy. Am. J. Obs. Gynecol. 2004, 191, 1277–1282. [Google Scholar] [CrossRef]
- Fennessy, K.M.; Doyle, L.W.; Naud, K.; Reidy, K.; Umstad, M.P. Triplet pregnancy: Is the mode of conception related to perinatal outcomes? Twin Res. Hum. Genet. 2015, 18, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Fitzsimmons, B.P.; Bebbington, M.W.; Fluker, M.R. Perinatal and neonatal outcomes in multiple gestations: Assisted reproduction versus spontaneous conception. Am. J. Obs. Gynecol. 1998, 179, 1162–1167. [Google Scholar] [CrossRef]
- Guilherme, R.; Drunat, S.; Delezoide, A.L.; Le Ray, C.; Oury, J.F.; Luton, D. Zygosity and chorionicity in the prognosis of triplet pregnancies: Contribution of microsatellites. Twin Res. Hum. Genet. 2008, 11, 648–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, H.; Ishii, K.; Yamamoto, R.; Hayashi, S.; Mitsuda, N. Perinatal death of triplet pregnancies by chorionicity. Am. J. Obs. Gynecol. 2013, 209, 36.e1–36.e7. [Google Scholar] [CrossRef] [PubMed]
- Lipitz, S.; Seidman, D.S.; Alcalay, M.; Achiron, R.; Mashiach, S.; Reichman, B. The effect of fertility drugs and in vitro methods on the outcome of 106 triplet pregnancies. Fertil. Steril. 1993, 60, 1031–1034. [Google Scholar] [CrossRef]
- Machtinger, R.; Sivan, E.; Maayan-Metzger, A.; Moran, O.; Kuint, J.; Schiff, E. Perinatal, postnatal, and maternal outcome parameters of triplet pregnancies according to the planned mode of delivery: Results of a single tertiary center. J. Matern. Fetal Neonatal. Med. 2011, 24, 91–95. [Google Scholar] [CrossRef]
- Macones, G.A.; Schemmer, G.; Pritts, E.; Weinblatt, V.; Wapner, R.J. Multifetal reduction of triplets to twins improves perinatal outcome. Am. J. Obs. Gynecol. 1993, 169, 982–986. [Google Scholar] [CrossRef]
- Makhseed, M.; Al-Sharhan, M.; Egbase, P.; Al-Essa, M.; Grudzinskas, J.G. Maternal and perinatal outcomes of multiple pregnancy following IVF-ET. Int. J. Gynaecol. Obstet. 1998, 61, 155–163. [Google Scholar] [CrossRef]
- Malone, F.D.; Kaufman, G.E.; Chelmow, D.; Athanassiou, A.; Nores, J.A.; D’Alton, M.E. Maternal morbidity associated with triplet pregnancy. Am. J. Perinatol. 1998, 15, 73–77. [Google Scholar] [CrossRef]
- Manzur, A.; Goldsman, M.P.; Stone, S.C.; Frederick, J.L.; Balmaceda, J.P.; Asch, R.H. Outcome of triplet pregnancies after assisted reproductive techniques: How frequent are the vanishing embryos? Fertil. Steril. 1995, 63, 252–257. [Google Scholar] [CrossRef]
- Okyay, E.; Altunyurt, S.; Soysal, D.; Kaymak, O.; Soysal, S.; Danisman, N.; Gulekli, B. A comparative study of obstetric outcomes in electively or spontaneously reduced triplet pregnancies. Arch. Gynecol. Obs. 2014, 290, 177–184. [Google Scholar] [CrossRef]
- Pakrashi, T.; Defranco, E.A. The relative proportion of preterm births complicated by premature rupture of membranes in multifetal gestations: A population-based study. Am. J. Perinatol. 2013, 30, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkinson, J.; Tran, C.; Tan, T.; Nelson, J.; Batzofin, J.; Serafini, P. Perinatal outcome after in-vitro fertilization-surrogacy. Hum. Reprod. 1999, 14, 671–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peress, D.A.; Peaceman, A.M.; Yee, L.M. Evaluation of Trichorionic versus Dichorionic Triplet Gestations from 2005 to 2016 in a Large, Referral Maternity Center. Am. J. Perinatol. 2017, 34, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Razaz, N.; Avitan, T.; Ting, J.; Pressey, T.; Joseph, K.S. Perinatal outcomes in multifetal pregnancy following fetal reduction. CMAJ 2017, 189, E652–E658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revello, R.; De la Calle, M.; Moreno, E.; Duyos, I.; Salas, P.; Zapardiel, I. Maternal morbidity on 147 triplets: Single institution experience. J. Matern. Fetal Neonatal. Med. 2013, 26, 193–196. [Google Scholar] [CrossRef]
- Salihu, H.M.; Aliyu, M.H.; Akintobi, T.H.; Pierre-Louis, B.J.; Kirby, R.S.; Alexander, G.R. The impact of advanced maternal age (> or = 40 years) on birth outcomes among triplets: A population study. Arch. Gynecol. Obs. 2005, 271, 132–137. [Google Scholar] [CrossRef]
- Santema, J.G.; Bourdrez, P.; Wallenburg, H.C. Maternal and perinatal complications in triplet compared with twin pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 60, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Ishii, K.; Yokouchi, T.; Murakoshi, T.; Kiyoshi, K.; Nakayama, S.; Yonetani, N.; Mitsuda, N. Incidences of Feto-Fetal Transfusion Syndrome and Perinatal Outcomes in Triplet Gestations with Monochorionic Placentation. Fetal Diagn. Ther. 2016, 40, 181–186. [Google Scholar] [CrossRef]
- Seoud, M.A.; Kruithoff, C.; Muasher, S.J. Outcome of triplet and quadruplet pregnancies resulting from in vitro fertilization. Eur. J. Obstet. Gynecol. Reprod. Biol. 1991, 41, 79–84. [Google Scholar] [CrossRef]
- Simoes, T.; Queiros, A.; Goncalves, M.R.; Periquito, I.; Silva, P.; Blickstein, I. Perinatal outcome of dichorionic-triamniotic as compared to trichorionic triplets. J. Perinat Med. 2016, 44, 875–879. [Google Scholar] [CrossRef]
- Sklar, C.; Yaskina, M.; Ross, S.; Naud, K. Accuracy of Prenatal Ultrasound in Detecting Growth Abnormalities in Triplets: A Retrospective Cohort Study. Twin Res. Hum. Genet. 2017, 20, 84–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrablin, S.; Kuvacic, I.; Jukic, P.; Kalafatic, D.; Peter, B. Hospitalization vs. outpatient care in the management of triplet gestations. Int. J. Gynaecol. Obstet. 2002, 77, 223–229. [Google Scholar] [CrossRef]
- Smith, D.D.; Merriam, A.A.; Jung, J.; Gyamfi-Bannerman, C. Effect of Maternal Age and Fetal Number on the Risk of Hypertensive Disorders of Pregnancy. Am. J. Perinatol. 2018, 35, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Smith-Levitin, M.; Kowalik, A.; Birnholz, J.; Skupski, D.W.; Hutson, J.M.; Chervenak, F.A.; Rosenwaks, Z. Selective reduction of multifetal pregnancies to twins improves outcome over nonreduced triplet gestations. Am. J. Obs. Gynecol. 1996, 175, 878–882. [Google Scholar] [CrossRef]
- Sumners, J.E.; Moore, E.S.; Ramsey, C.J.; Eggleston, M.K. Transabdominal cervical cerclage in triplet pregnancies and risk of extreme prematurity and neonatal loss. J. Obstet. Gynaecol. 2011, 31, 111–117. [Google Scholar] [CrossRef]
- Weissman, A.; Drugan, A. Glucose tolerance in singleton, twin and triplet pregnancies. J. Perinat. Med. 2016, 44, 893–897. [Google Scholar] [CrossRef]
- Ziadeh, S.M. The outcome of triplet versus twin pregnancies. Gynecol. Obstet. Investig. 2000, 50, 96–99. [Google Scholar] [CrossRef]
- Barchilon-Tiosano, L.; Sheiner, E.; Wainstock, T.; Sergienko, R.; Okby-Cronin, R. Long-term risk for maternal cardiovascular morbidities in twin pregnancies complicated with gestational diabetes mellitus—A retrospective cohort study. J. Matern. Fetal Neonatal. Med. 2019, 3, 1–5. [Google Scholar] [CrossRef]
- Hiersch, L.; Berger, H.; Okby, R.; Ray, J.G.; Geary, M.; McDonald, S.D.; Murray-Davis, B.; Riddell, C.; Halperin, I.; Hasan, H.; et al. Gestational diabetes mellitus is associated with adverse outcomes in twin pregnancies. Am. J. Obs. Gynecol. 2019, 220, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, A.C.M.; Umstad, M.P.; Cole, S.; Cade, T.J. Does Gestational Diabetes Cause Additional Risk in Twin Pregnancy? Twin Res. Hum. Genet. 2019, 22, 62–69. [Google Scholar] [CrossRef]
- Olarinoye, J.K.; Ohwovoriole, A.E.; Ajayi, G.O. Diagnosis of gestational diabetes mellitus in Nigerian pregnant women--comparison between 75G and 100G oral glucose tolerance tests. West. Afr. J. Med. 2004, 23, 198–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pils, S.; Paternostro, C.; Bekos, C.; Hager, M.; Ristl, R.; Ott, J. Prognostic Laboratory Parameters in Placental Abruption: A Retrospective Case-Control Study. J. Clin. Med. 2019, 8, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Zeev, Y.; Haile, Z.T.; Chertok, I.A. Association between Prenatal Smoking and Gestational Diabetes Mellitus. Obs. Gynecol. 2020, 135, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Blickstein, I. Normal and abnormal growth of multiples. Semin. Neonatol. 2002, 7, 177–185. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]


| Triplet Pregnancies (n = 60) | Singleton Pregnancies (n = 60) | OR (95% CI) | p (LR Test) | ||
|---|---|---|---|---|---|
| Age (years) | 32 (27;35) | 32 (27;35) | 1.000 (0.934;1.071) | 1.000 | |
| BMI (kg/m2) | 23.8 (22.3;26.3) | 23.7 (22.4;26.5) | 0.999 (0.904;1.104) | 0.984 | |
| Parity | 0 | 44 (73.3) | 25 (41.7) | 0.002 | |
| 1 | 12 (20.0) | 30 (50.0) | 0.227 (0.099;0.521) | ||
| ≥2 | 4 (6.7) | 5 (8.3) | 0.455 (0.112;1.850) | ||
| Pregnancy after IVF or ovarian stimulation | 43 (71.7) | 7 (11.7) | 19.151 (7.276;50.407) | <0.001 | |
| Smoking during pregnancy | 8 (13.3) | 11 (18.3) | 0.685 (0.254;1.846) | 0.455 | |
| GDM (abnormal oGTT) | 19 (31.7) | 7 (11.7) | 3.509 (1.347;9.142) | 0.010 | |
| 75 g oGTT: fasting glucose level (mmol/L) | 4.66 (4.38;5.05) | 4.55 (3.94;4.83) | 1.136 (1.044;1.237) | 0.003 | |
| 75 g oGTT: 1-h glucose level (mmol/L) | 8.71 (7.44;9.82) | 6.87 (5.61;8.67) | 1.075 (1.034;1.118) | <0.001 | |
| 75 g oGTT: 2-h glucose level (mmol/L) | 6.67 (5.61;8.05) | 5.49 (4.32;6.67) | 1.049 (1.022;1.077) | <0.001 | |
| Abnormal oGTT (n = 19) | Normal oGTT (n = 41) | OR (95% CI) | p (LR test) | ||
|---|---|---|---|---|---|
| Age (years) | 32 (28;36) | 32 (27;35) | 0.988 (0.900;1.086) | 0.805 | |
| BMI (kg/m2) | 23.5 (22.7;27.3) | 23.7 (22.3;26.1) | 1.033 (0.885;1.206) | 0.677 | |
| Parity | 0 | 15 (78.9) | 29 (70.7) | ||
| 1 | 4 (21.1) | 8 (19.51) | |||
| 2 | 0 | 4 | |||
| Pregnancy after IVF | 13 (68.4) | 25 (61.0) | 1.950 (0.536;7.088) | 0.306 | |
| Pregnancy after ovarian stimulation | 1 (3.1) | 4 (10.0) | 0.563 (0.058;5.440) | 0.615 | |
| Smoking during pregnancy | 5 (23.8) | 3 (7.3) | 4.524 (0.953;21.464) | 0.057 | |
| Pregnancy induced/preexisting hypertension | 1 (5.3) | 4 (9.8) | 0.558 | ||
| Gestational age at delivery (completed weeks) | 33.0 (31.7;34.0) | 33.1 (31.4;34.0) | 1.014 (0.978;1.051) | 0.457 | |
| Median birthweight (g) | 1691 (1517;1893) | 1673 (1299;1933) | 1.001 (0.999;1.002) | 0.43 | |
| Median birth weight (percentile) | 33.8 (14.4;62.2) | 43.1 (24.5;69.2) | 0.990 (0.978;1.002) | 0.098 | |
| 75 g oGTT: fasting glucose level (mmol/L) | 5.16 (4.66;5.66) | 4.55 (4.27;4.72) | 1.136 (1.044;1.237) | 0.003 | |
| 75 g oGTT: 1-h glucose level (mmol/L) | 10.55 (9.66;11.60) | 7.88 (7.10;8.82) | 1.075 (1.034;1.118) | <0.001 | |
| 75 g oGTT: 2-h glucose level (mmol/L) | 8.56 (6.55;10.27) | 6.16 (5.44;7.16) | 1.049 (1.022;1.077) | <0.001 | |
| First Author (Year) | Study Design | Number of Subjects | Country | GDM Definition | Selection of Cases | Mean/Median Prepregnancy BMI (kg/m2) | Mean/Median Maternal Age (Years) | Source of Data Specified | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Fennessy (2015) [33] | Retrospective | 53 | Australia | 75 g, 2-h oGTT | Representative | Not reported | 31.6–32.9 | Yes | Medium |
| Hager (2020) | Retrospective | 60 | Austria | 75 g, 2-h oGTT | Representative | 23.5 | 32.0 | Yes | Lowest |
| Lipitz (1993) [37] | Retrospective | 81 | Israel | 100 g, 3-h oGTT | Representative | Not reported | 31.2 | No | Highest |
| Malone (1998) [41] | Retrospective | 55 | USA | 100 g, 3-h oGTT | Representative | Not reported | 32.0 | Yes | Medium |
| Okyay (2014) [43] | Retrospective | 45 | Turkey | 50 g, 1-h oGTT, followed by a 100 g, 3-h oGTT | Representative | 23.6–26.0 | 28.9–30.1 | No | Medium |
| Revello (2013) [48] | Retrospective | 147 | Spain | 100 g, 3-h oGTT | Representative | Not reported | 34.0 | Yes | Medium |
| Seoud (1991) [52] | Retrospective | 13 | USA | 100 g, 3-h oGTT | IVF only | Not reported | 32.2 | Yes | Highest |
| Simoes (2016) [53] | Retrospective | 90 | Israel | 100 g, 3-h oGTT | Representative | 23.6–23.9 | 32.1–33.1 | Yes | Lowest |
| Sivan (2002) [9] | Retrospective | 103 | Israel | 1-h glucose challenge test, followed by a 100 g, 3-h oGTT | Representative | 23.9 | 29.2 | No | Medium |
| Skrablin (2002) [55] | Retrospective | e85 | Croatia | 75 g, 2-h oGTT | Representative | Not reported | Not reported | No | Highest |
| Weissman (2016) [59] | Retrospective | 39 | Israel | 100 g, 3-h oGTT | Representative | Not reported | 30.9 | Yes | Medium |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hager, M.; Ott, J.; Castillo, D.M.; Springer, S.; Seemann, R.; Pils, S. Prevalence of Gestational Diabetes in Triplet Pregnancies: A Retrospective Cohort Study and Meta-Analysis. J. Clin. Med. 2020, 9, 1523. https://doi.org/10.3390/jcm9051523
Hager M, Ott J, Castillo DM, Springer S, Seemann R, Pils S. Prevalence of Gestational Diabetes in Triplet Pregnancies: A Retrospective Cohort Study and Meta-Analysis. Journal of Clinical Medicine. 2020; 9(5):1523. https://doi.org/10.3390/jcm9051523
Chicago/Turabian StyleHager, Marlene, Johannes Ott, Deirdre Maria Castillo, Stephanie Springer, Rudolf Seemann, and Sophie Pils. 2020. "Prevalence of Gestational Diabetes in Triplet Pregnancies: A Retrospective Cohort Study and Meta-Analysis" Journal of Clinical Medicine 9, no. 5: 1523. https://doi.org/10.3390/jcm9051523
APA StyleHager, M., Ott, J., Castillo, D. M., Springer, S., Seemann, R., & Pils, S. (2020). Prevalence of Gestational Diabetes in Triplet Pregnancies: A Retrospective Cohort Study and Meta-Analysis. Journal of Clinical Medicine, 9(5), 1523. https://doi.org/10.3390/jcm9051523






