The Role of the CYP11B2 Promoter Polymorphism in the Diagnosis of Primary Aldosteronism
Abstract
1. Introduction
2. Material and Methods
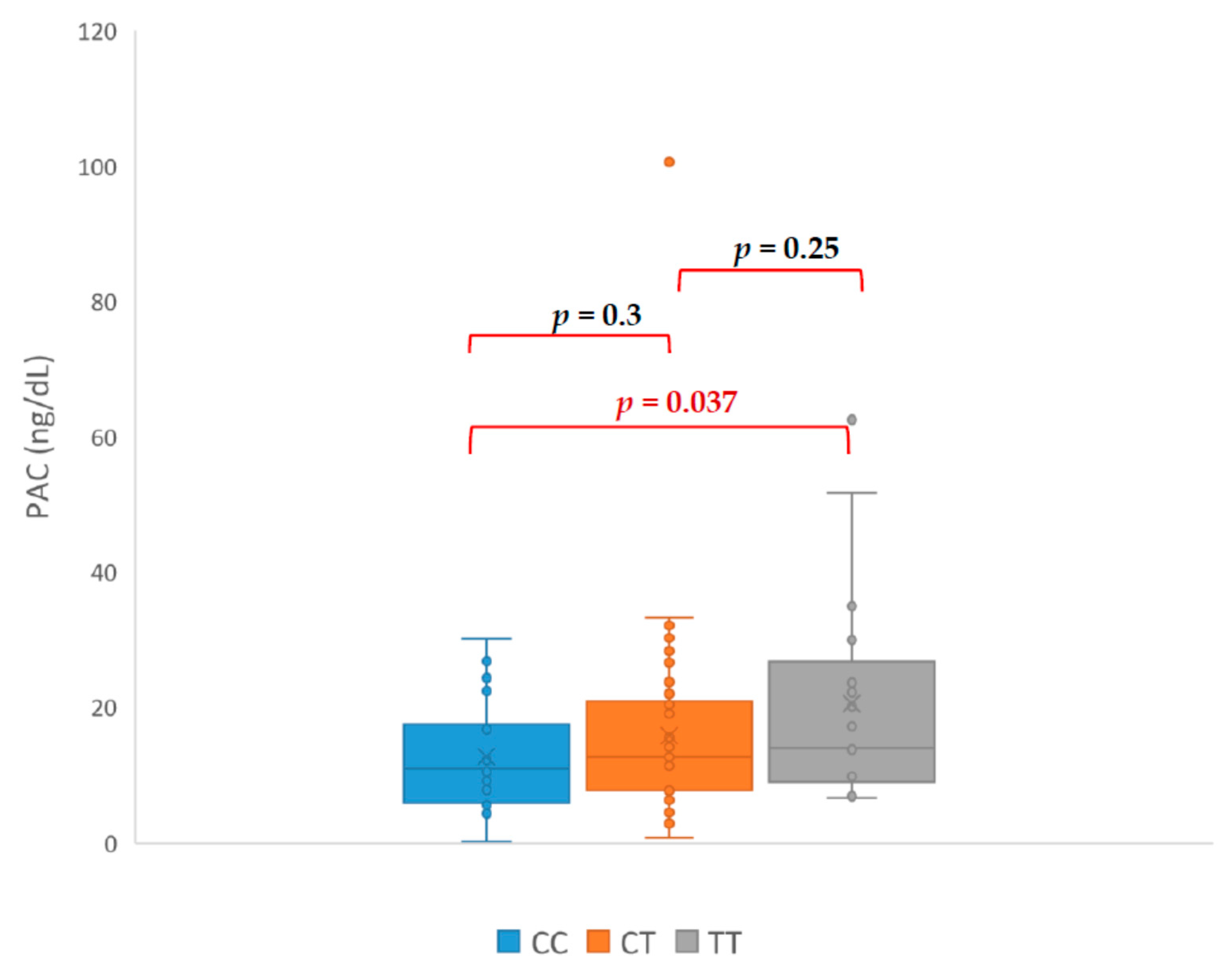
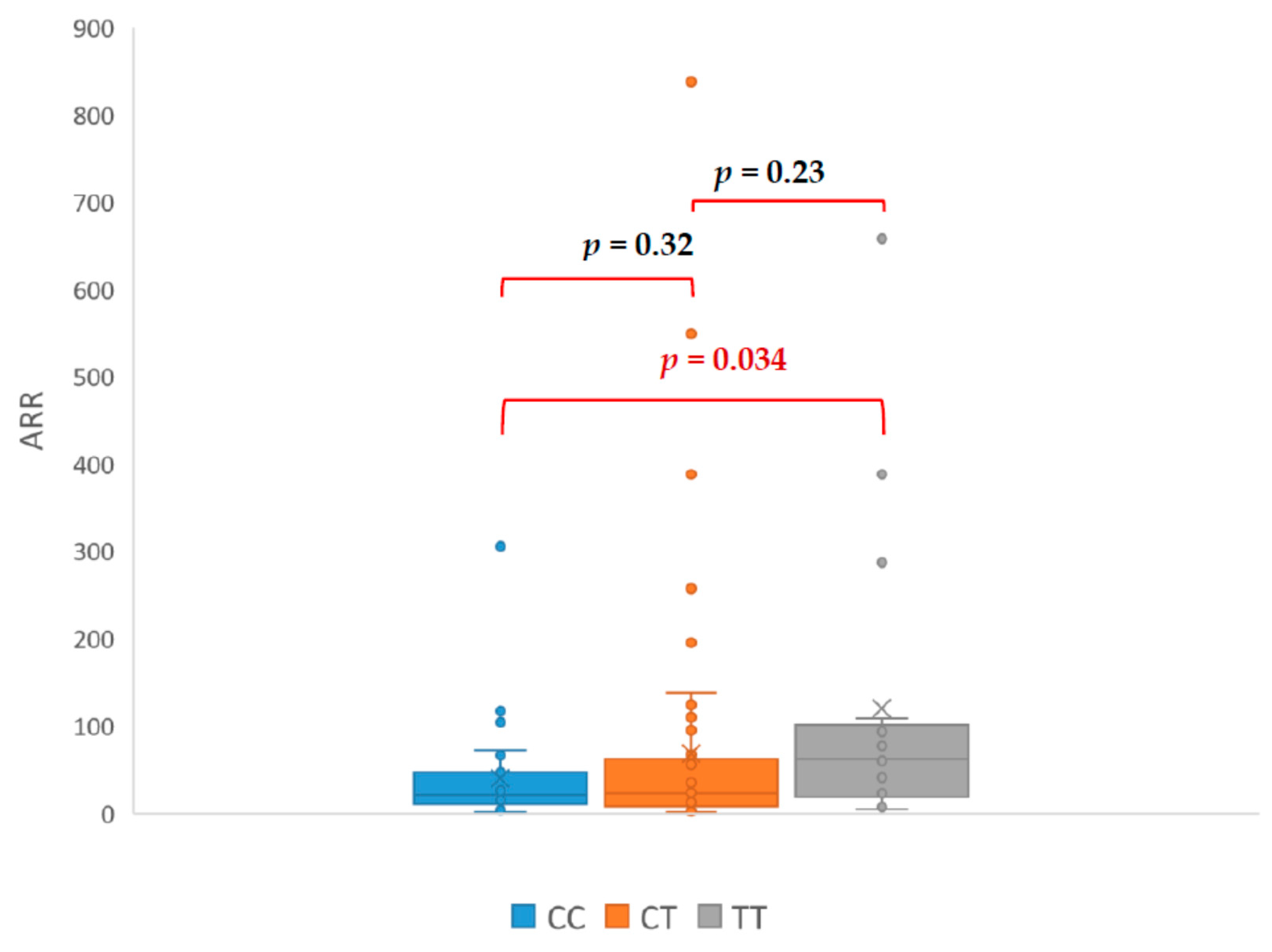
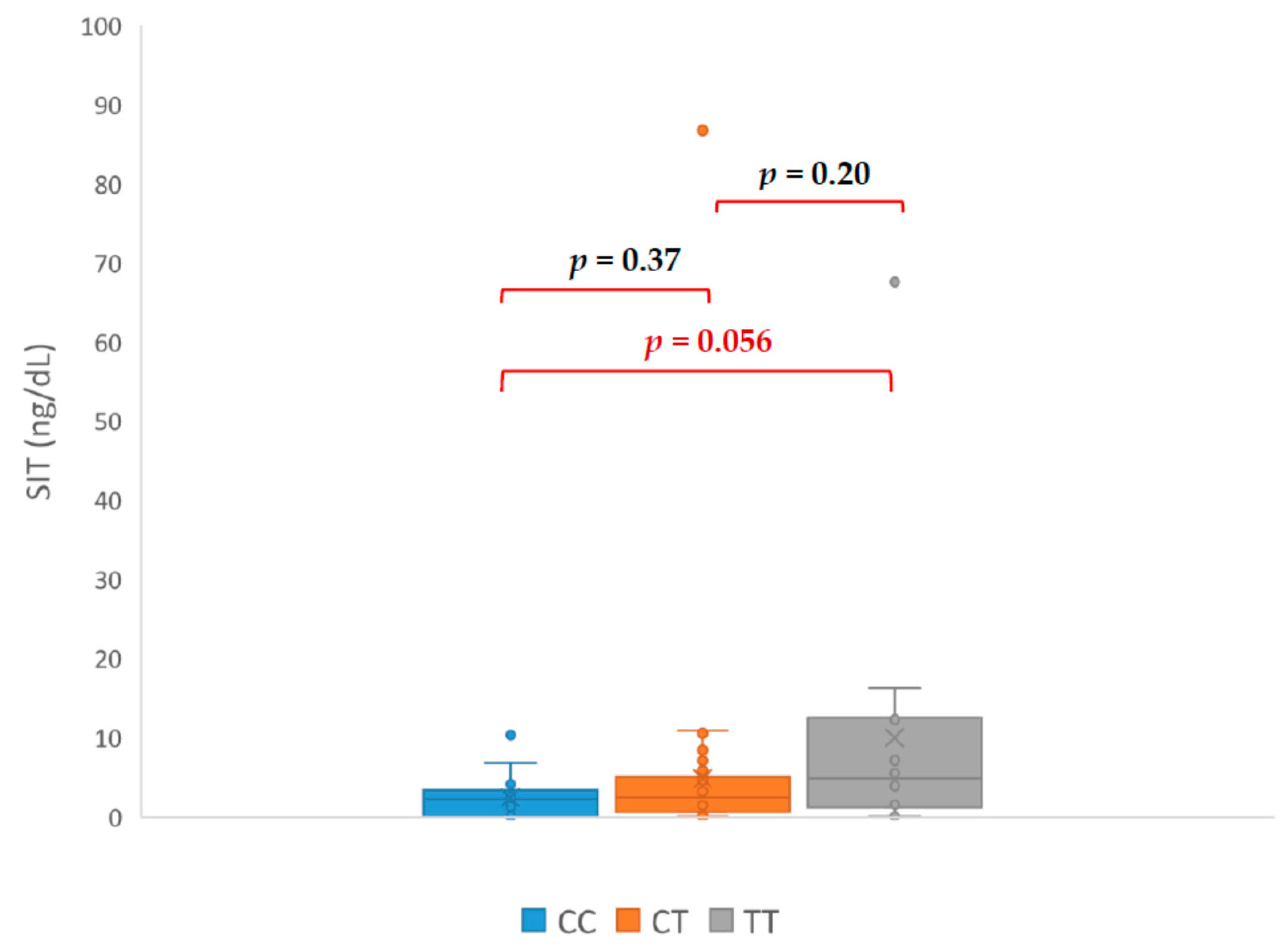
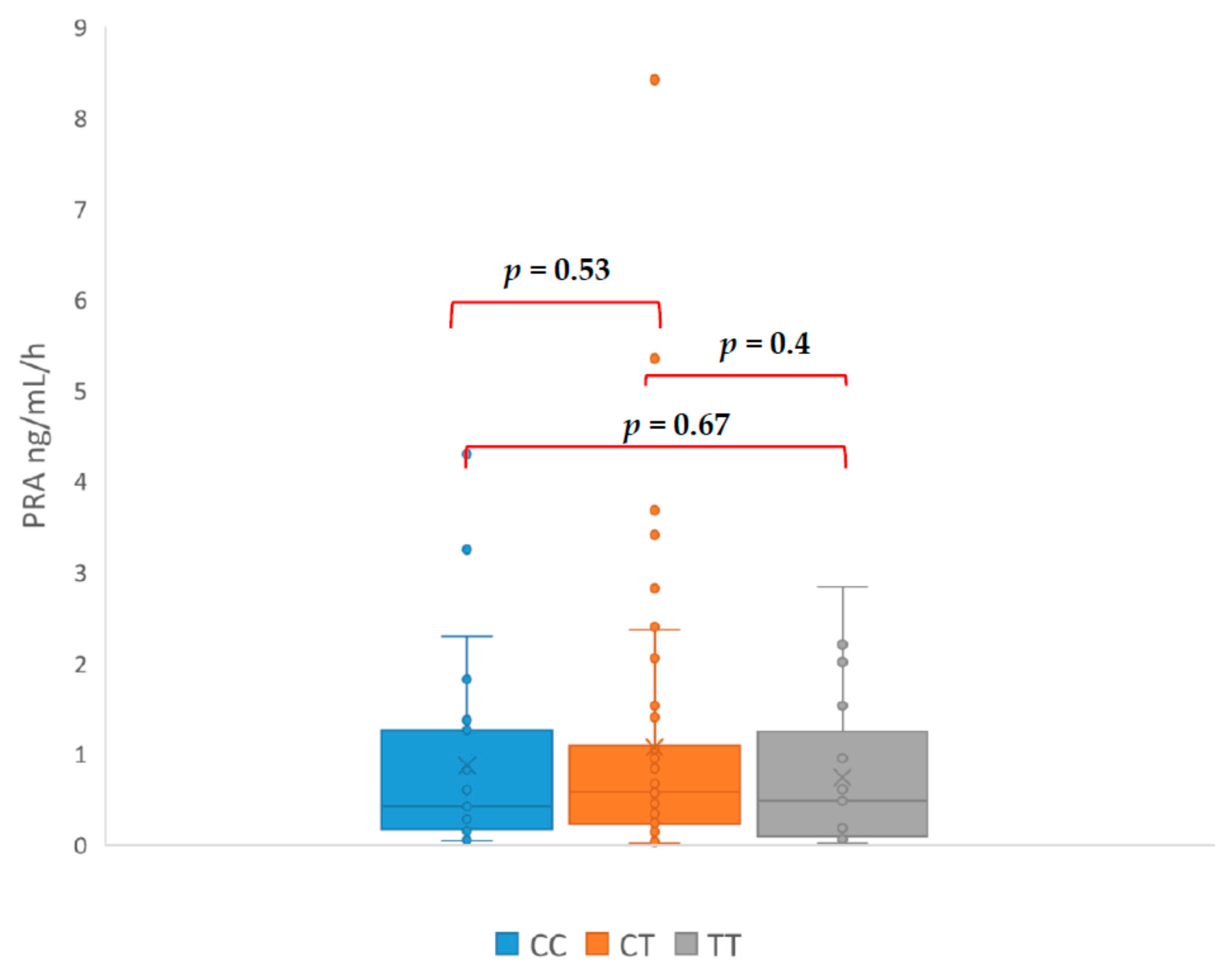
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
References
- Gordon, R.D.; Ziesak, M.D.; Tunny, T.J.; Stowasser, M.; Klemm, S.A. Evidence that primary aldosteronism may not be uncommon: 12% incidence among antihypertensive drug trial volunteers. Clin. Exp. Pharmacol. Physiol. 1993, 20, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.D.; Stowasser, M.; Tunny, T.J.; Klemm, S.A.; Rutherford, J.C. High incidence of primary aldosteronism in 199 patients referred with hypertension. Clin. Exp. Pharmacol. Physiol. 1994, 21, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W. Mineralocorticoid receptor antagonists: Emerging roles in cardiovascular medicine. Integr. Blood Press. Control 2013, 6, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P. The Challenges of Arterial Hypertension. Front. Cardiovasc. Med. 2015, 2, 2. [Google Scholar] [CrossRef]
- Mosso, L.; Carvajal, C.; González, A.; Barraza, A.; Avila, F.; Montero, J.; Huete, A.; Fardella, A.G.C.E. Primary aldosteronism and hypertensive disease. Hypertension 2003, 42, 161–165. [Google Scholar] [CrossRef]
- Baudrand, R.; Guarda, F.J.; Torrey, J.; Williams, G.; Vaidya, A. Dietary Sodium Restriction Increases the Risk of Misinterpreting Mild Cases of Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2016, 101, 3989–3996. [Google Scholar] [CrossRef]
- White, P.C.; Slutsker, L. Haplotype analysis of CYP11B2. Endocr. Res. 1995, 21, 437–442. [Google Scholar] [CrossRef]
- Russo, P.; Siani, A.; Venezia, A.; Iacone, R.; Strazzullo, P. Interaction between the C(-344)T polymorphism of CYP11B2 and age in the regulation of blood pressure and plasma aldosterone levels: Cross-sectional and longitudinal findings of the Olivetti Prospective Heart Study. J. Hypertens. 2002, 20, 1785–1792. [Google Scholar] [CrossRef]
- Davies, E.D.E.; Holloway, C.D.; Ingram, M.C.; Inglis, G.C.; Friel, E.C.; Morrison, C.; Anderson, N.H.; Fraser, R.; Connell, J.M.C. Aldosterone excretion rate and blood pressure in essential hypertension are related to polymorphic differences in the aldosterone synthase gene CYP11B2. Hypertension 1999, 33, 703–707. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Yang, P.; Niu, J.Q.; Wu, Y.; Zhao, D.D.; Wu, S.L. Interaction of ACE and CYP11B2 genes on blood pressure response to hydrochlorothiazide in Han Chinese hypertensive patients. Clin. Exp. Hypertens. 2011, 33, 141–146. [Google Scholar] [CrossRef]
- Kupari, M.; Hautanen, A.; Lankinen, L.; Koskinen, P.; Virolainen, J.; Nikkila, H.; White, P.C. Associations between human aldosterone synthase (CYP11B2) gene polymorphisms and left ventricular size, mass, and function. Circulation 1998, 97, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Hautanena, A.; Lankinen, L.; Kupari, M.; Jänne, O.A.; Adlercreutz, H.; Nikkilä, H.; White, P.C. Associations between aldosterone synthase gene polymorphism and the adrenocortical function in males. J. Intern. Med. 1998, 244, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Paillard, F.; Chansel, D.; Brand, E.; Benetos, A.; Thomas, F.; Czekalski, S.; Ardaillou, R.; Soubrier, F. Genotype-phenotype relationships for the renin-angiotensin-aldosterone system in a normal population. Hypertension 1999, 34, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Komiya, I.; Yamada, T.; Takara, M.; Asawa, T.; Shimabukuro, M.; Nishimori, T.; Takasu, N. Lys(173)Arg and -344T/C variants of CYP11B2 in Japanese patients with low-renin hypertension. Hypertension 2000, 35, 699–703. [Google Scholar] [CrossRef]
- Lim, P.O.; Macdonald, T.M.; Holloway, C.; Friel, E.; Anderson, N.H.; Dow, E.; Jung, R.T.; Davies, E.; Fraser, R.; Connell, J.M.C. Variation at the aldosterone synthase (CYP11B2) locus contributes to hypertension in subjects with a raised aldosterone-to-renin ratio. J. Clin. Endocrinol. Metab. 2002, 87, 4398–4402. [Google Scholar] [CrossRef]
- Nejatizadeh, A.; Kumar, R.; Stobdan, T.; Goyal, A.K.; Gupta, M.; Tyagi, S.; Jain, S.K.; Pasha, M.A.Q. CYP11B2 gene haplotypes independently and in concurrence with aldosterone and aldosterone to renin ratio increase the risk of hypertension. Clin. Biochem. 2010, 3, 136–141. [Google Scholar] [CrossRef]
- Tamaki, S.; Iwai, N.; Tsujita, Y.; Kinoshita, M. Genetic polymorphism of CYP11B2 gene and hypertension in Japanese. Hypertension 1999, 33 Pt 2, 266–270. [Google Scholar] [CrossRef]
- Fardella, C.E.; Rodriguez, H.; Montero, J.; Zhang, G.; Vignolo, P.; Rojas, A.; Villarroel, L.; Miller, W.L. Genetic variation in P450c11AS in Chilean patients with low renin hypertension. J. Clin. Endocrinol. Metab. 1996, 81, 4347–4351. [Google Scholar] [CrossRef][Green Version]
- Pojoga, L.; Gautier, S.; Blanc, H.; Guyene, T.T.; Poirier, O.; Cambien, F.; Benetos, A. Genetic determination of plasma aldosterone levels in essential hypertension. Am. J. Hypertens. 1998, 11, 856–860. [Google Scholar] [CrossRef]
- Wang, W.; Hu, W.; Zhang, X.; Wang, B.; Bin, C.; Huang, H. Predictors of successful outcome after adrenalectomy for primary aldosteronism. Int. Surg. 2012, 97, 104–111. [Google Scholar] [CrossRef]
- Kurland, L.; Melhus, H.; Karlsson, J. Aldosterone synthase (CYP11B2) -344 C/T polymorphism is related to antihypertensive response: Result from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) trial. Am. J. Hypertens. 2002, 15, 389–393. [Google Scholar] [CrossRef]
| Cortisol in Saliva at 11 p.m. | Cortisol in Serum at 11 p.m. | Dexamethasone Suppression Test | Urinary Free Cortisol nmol/24 h | Urinary Metanephrine µg/24 h | Urinary Normetanephrine µg/24 h | |
|---|---|---|---|---|---|---|
| Mean | 0.37 | 2.62 | 1.39 | 49.94 | 110.95 | 345.94 |
| SD | 0.40 | 1.60 | 1.08 | 38.05 | 108.17 | 177.52 |
| Ref. range | <1.2 | <1.8 | <1.8 | <124.2 | <350 | <600 |
| Mean Antyhypertensives Drugs | ||
|---|---|---|
| CC | TT | p |
| 2.361 | 3.080 | 0.044 * |
| TC | TT | p |
| 2.703 | 3.080 | 0.22 |
| TC | CC | p |
| 2.703 | 2.361 | 0.198 |
| Initials | HT Drugs Amount | Genotyp | PRA ng/mL/h | PAC (ng/dL) | ARR | SIT (ng/dL) |
|---|---|---|---|---|---|---|
| CA | 4 | TC | 0.03 | 39.8 | 1300 | 17.8 |
| DM | 3 | TC | 0.12 | 100.6 | 838 | 86.8 |
| LR | 2 | TC | 0.04 | 21.99 | 549 | 10.56 |
| KA | 5 | TT | 0.09 | 34.97 | 388 | 13.09 |
| PT | 4 | TT | 0.06 | 17.21 | 287 | 16.24 |
| WE | 3 | TC | 0.1 | 27.5 | 275 | 8.52 |
| KT | 1 | CC | 0.15 | 17.5 | 117 | 10.3 |
| KG | 4 | TC | 0.03 | 26.61 | 95 | 8.7 |
| MJ | 5 | TT | 0.67 | 62.5 | 93.3 | 67.6 |
| ŁR | 3 | TC | 0.45 | 29.06 | 63 | 8.41 |
| AJ | 3 | TT | 0.48 | 29.93 | 62 | 6.33 |
| DH | 3 | TC | 0.7 | 23.7 | 60 | 7.07 |
| PI | 4 | TT | 0.5 | 28 | 56 | 6.6 |
| KP | 3 | TC | 0.4 | 16.76 | 41.9 | 9.21 |
| IM | 3 | TT | 0.5 | 20.68 | 41 | 16.39 |
| TS | 3 | TC | 0.84 | 30.25 | 36 | 10.91 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żukowski, Ł.; Wawrusiewicz-Kurylonek, N.; Szumowski, P.; Mojsak, M.; Abdelrazek, S.; Myśliwiec, J. The Role of the CYP11B2 Promoter Polymorphism in the Diagnosis of Primary Aldosteronism. J. Clin. Med. 2020, 9, 1519. https://doi.org/10.3390/jcm9051519
Żukowski Ł, Wawrusiewicz-Kurylonek N, Szumowski P, Mojsak M, Abdelrazek S, Myśliwiec J. The Role of the CYP11B2 Promoter Polymorphism in the Diagnosis of Primary Aldosteronism. Journal of Clinical Medicine. 2020; 9(5):1519. https://doi.org/10.3390/jcm9051519
Chicago/Turabian StyleŻukowski, Łukasz, Natalia Wawrusiewicz-Kurylonek, Piotr Szumowski, Małgorzata Mojsak, Saeid Abdelrazek, and Janusz Myśliwiec. 2020. "The Role of the CYP11B2 Promoter Polymorphism in the Diagnosis of Primary Aldosteronism" Journal of Clinical Medicine 9, no. 5: 1519. https://doi.org/10.3390/jcm9051519
APA StyleŻukowski, Ł., Wawrusiewicz-Kurylonek, N., Szumowski, P., Mojsak, M., Abdelrazek, S., & Myśliwiec, J. (2020). The Role of the CYP11B2 Promoter Polymorphism in the Diagnosis of Primary Aldosteronism. Journal of Clinical Medicine, 9(5), 1519. https://doi.org/10.3390/jcm9051519





