Trauma Can Induce Telangiectases in Hereditary Hemorrhagic Telangiectasia
Abstract
1. Introduction
2. Experimental Section
Study Approval
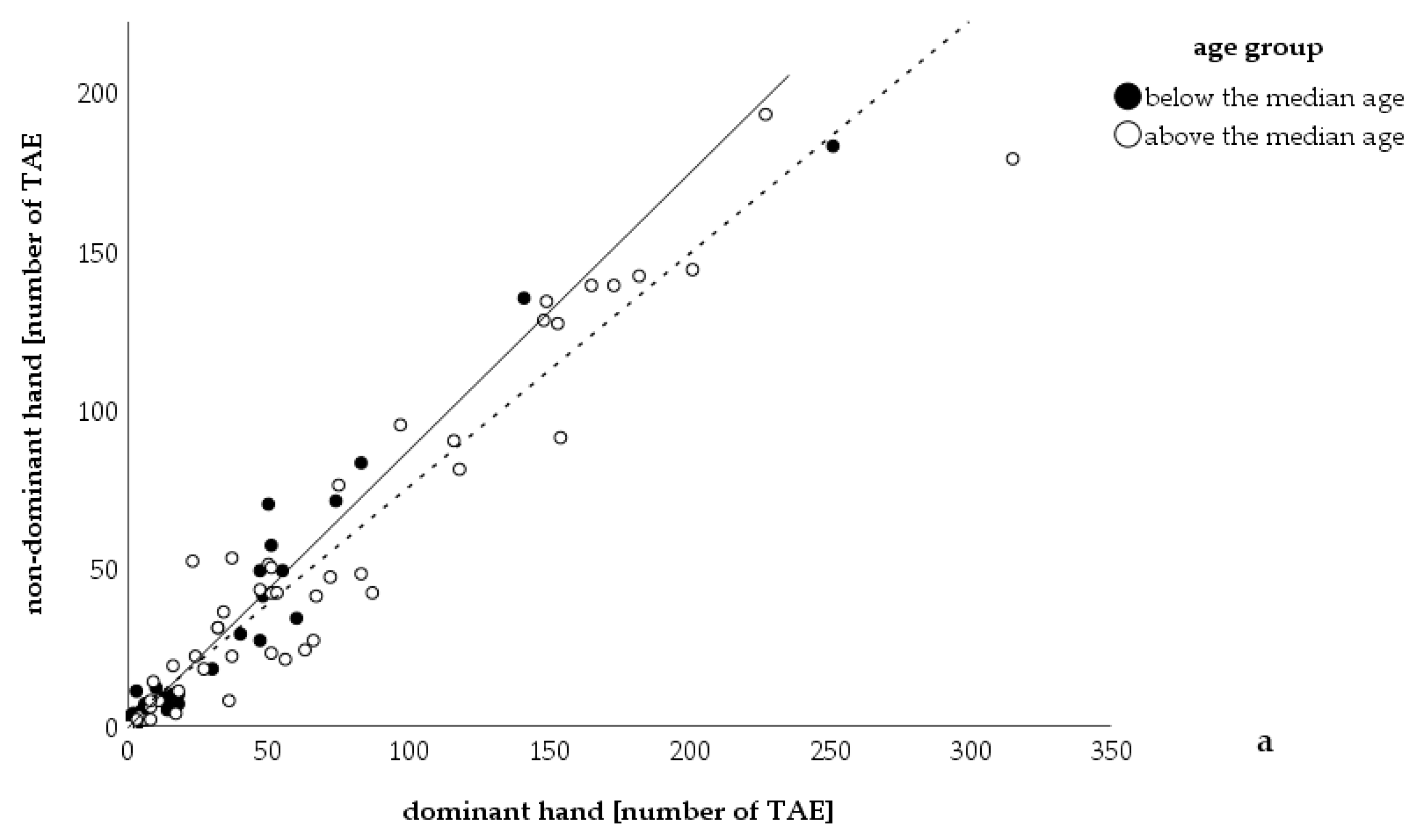
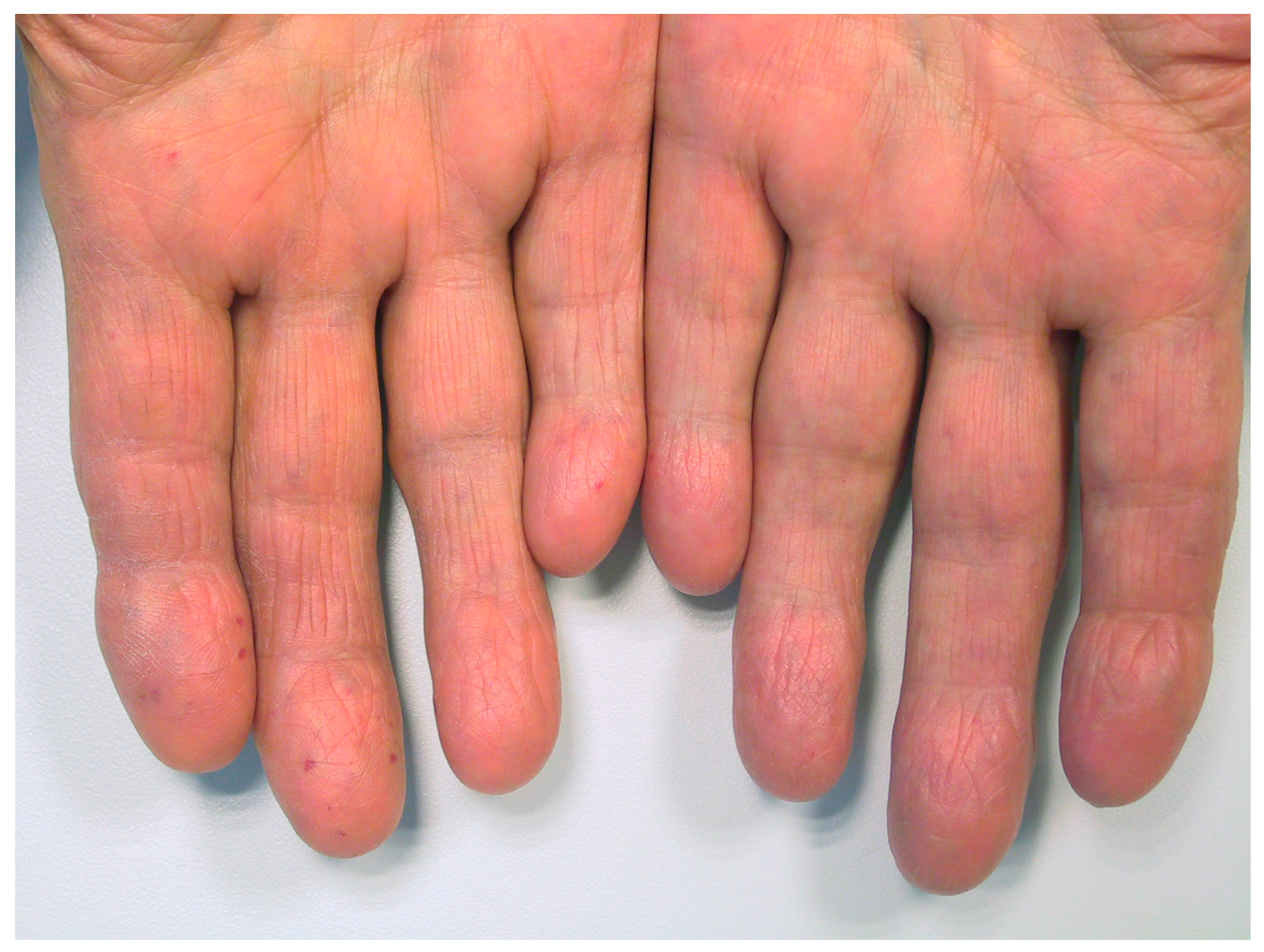
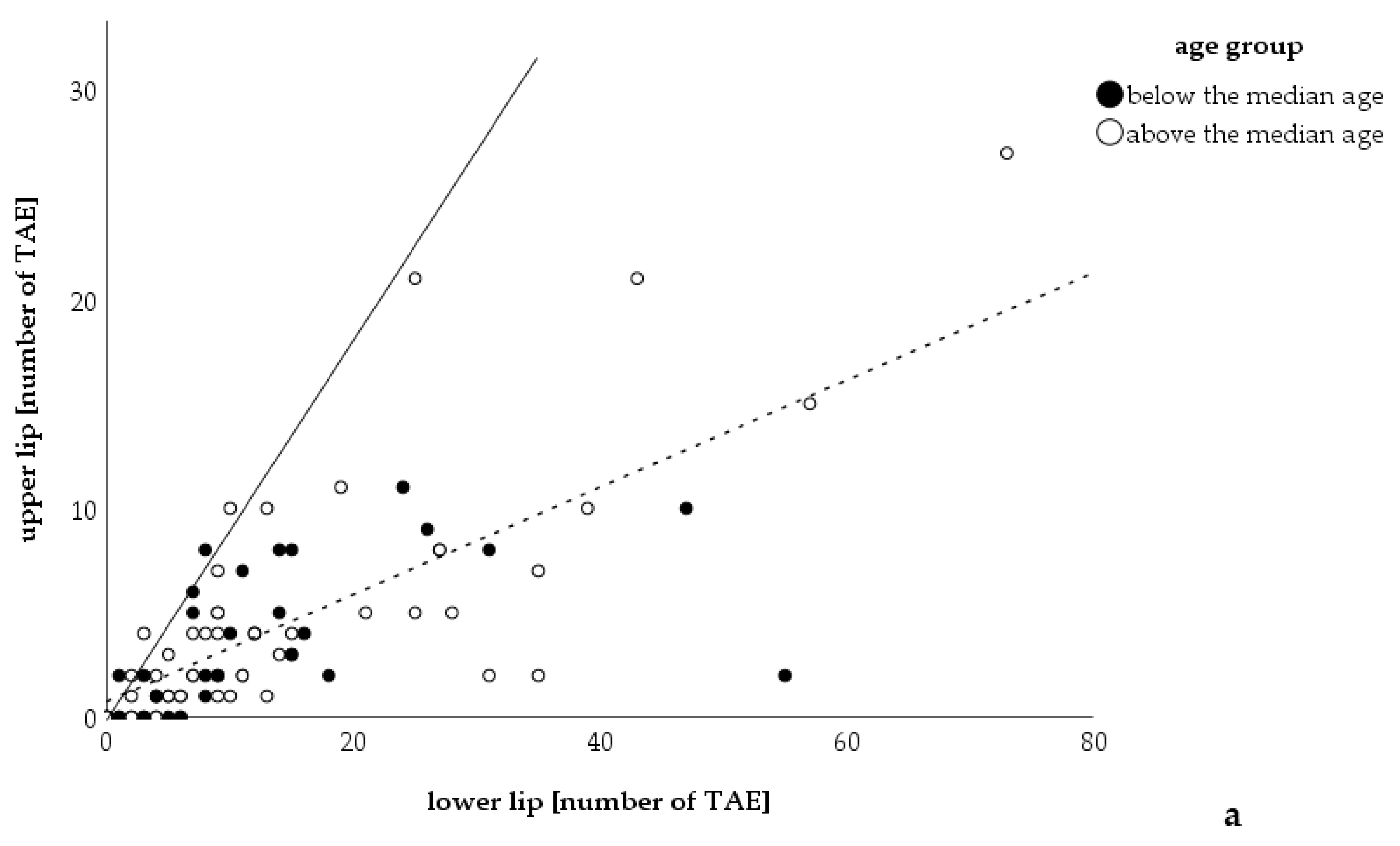
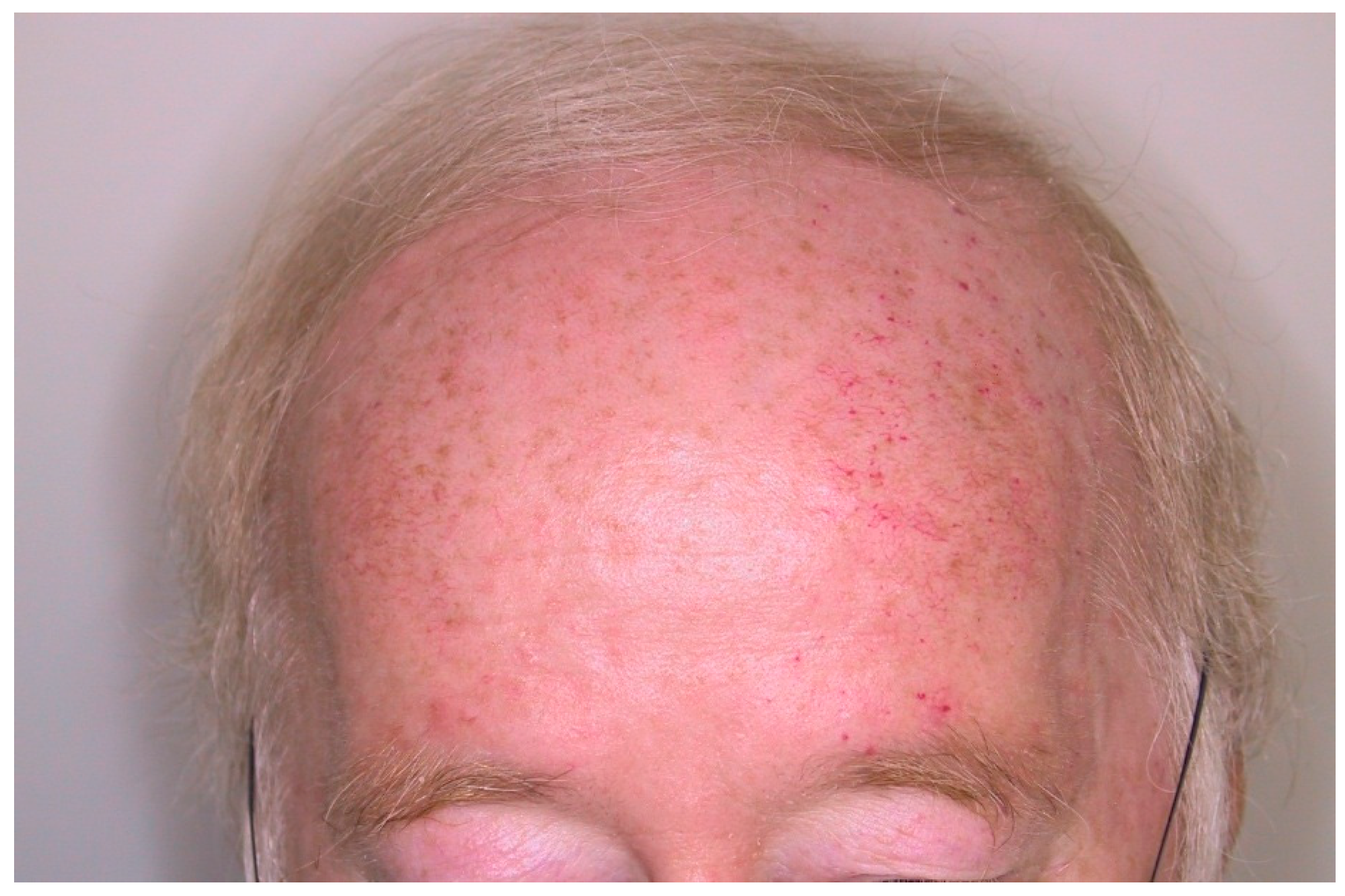
3. Results
4. Discussion
Study Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shovlin, C.L.; Guttmacher, A.E.; Buscarini, E.; Faughnan, M.E.; Hyland, R.H.; Westermann, C.J.; Kjeldsen, A.D.; Plauchu, H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am. J. Med. Genet. 2000, 91, 66–67. [Google Scholar] [CrossRef]
- Osler, W. On a family form of recurring epistaxis associated with multiple telangiectases of the skin and mucous membranes. Johns Hopkins Hosp. Bull. 1901, 12, 333–337. [Google Scholar]
- Sadick, H.; Sadick, M.; Gotte, K.; Naim, R.; Riedel, F.; Bran, G.; Hormann, K. Hereditary hemorrhagic telangiectasia: An update on clinical manifestations and diagnostic measures. Wien. Klin. Wochenschr. 2006, 118, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Geirdal, A.O.; Dheyauldeen, S.; Bachmann-Harildstad, G.; Heimdal, K. Quality of life in patients with hereditary hemorrhagic telangiectasia in Norway: A population based study. Am. J. Med. Genet. A 2012, 158A, 1269–1278. [Google Scholar] [CrossRef]
- Geisthoff, U.W.; Heckmann, K.; D’Amelio, R.; Grunewald, S.; Knobber, D.; Falkai, P.; Konig, J. Health-related quality of life in hereditary hemorrhagic telangiectasia. Otolaryngol. Head Neck Surg. 2007, 136, 726–733; discussion 734–735. [Google Scholar] [CrossRef]
- Lennox, P.A.; Hitchings, A.E.; Lund, V.J.; Howard, D.J. The SF-36 health status questionnaire in assessing patients with epistaxis secondary to hereditary hemorrhagic telangiectasia. Am. J. Rhinol. 2005, 19, 71–74. [Google Scholar] [CrossRef]
- Lenato, G.M.; Guanti, G. Hereditary Haemorrhagic Telangiectasia (HHT): Genetic and molecular aspects. Curr. Pharm. Des. 2006, 12, 1173–1193. [Google Scholar] [CrossRef]
- McDonald, J.E.; Miller, F.J.; Hallam, S.E.; Nelson, L.; Marchuk, D.A.; Ward, K.J. Clinical manifestations in a large hereditary hemorrhagic telangiectasia (HHT) type 2 kindred. Am. J. Med. Genet. 2000, 93, 320–327. [Google Scholar] [CrossRef]
- Lesca, G.; Olivieri, C.; Burnichon, N.; Pagella, F.; Carette, M.F.; Gilbert-Dussardier, B.; Goizet, C.; Roume, J.; Rabilloud, M.; Saurin, J.C.; et al. Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: Data from the French-Italian HHT network. Genet. Med. 2007, 9, 14–22. [Google Scholar] [CrossRef]
- Park, S.O.; Wankhede, M.; Lee, Y.J.; Choi, E.J.; Fliess, N.; Choe, S.W.; Oh, S.H.; Walter, G.; Raizada, M.K.; Sorg, B.S.; et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J. Clin. Investig. 2009, 119, 3487–3496. [Google Scholar] [CrossRef]
- Baeyens, N.; Larrivee, B.; Ola, R.; Hayward-Piatkowskyi, B.; Dubrac, A.; Huang, B.; Ross, T.D.; Coon, B.G.; Min, E.; Tsarfati, M.; et al. Defective fluid shear stress mechanotransduction mediates hereditary hemorrhagic telangiectasia. J. Cell Biol. 2016, 214, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Plauchu, H.; de Chadarevian, J.P.; Bideau, A.; Robert, J.M. Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am. J. Med. Genet. 1989, 32, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.D.; Cipriano, S.D.; Topham, C.A.; Stevenson, D.A.; Whitehead, K.J.; Vanderhooft, S.; Presson, A.P.; McDonald, J. Localization and age distribution of telangiectases in children and adolescents with hereditary hemorrhagic telangiectasia: A retrospective cohort study. J. Am. Acad. Dermatol. 2019, 81, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, C.H.; Burchell, H.B.; Good, C.A.; Clagett, O.T. Hereditary hemorrhagic telangiectasia and pulmonary arteriovenous fistula: Survey of a large family. N. Engl. J. Med. 1959, 261, 625–636. [Google Scholar] [CrossRef]
- McAllister, K.A.; Grogg, K.M.; Johnson, D.W.; Gallione, C.J.; Baldwin, M.A.; Jackson, C.E.; Helmbold, E.A.; Markel, D.S.; McKinnon, W.C.; Murrell, J.; et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet. 1994, 8, 345–351. [Google Scholar] [CrossRef]
- Johnson, D.W.; Berg, J.N.; Baldwin, M.A.; Gallione, C.J.; Marondel, I.; Yoon, S.J.; Stenzel, T.T.; Speer, M.; Pericak-Vance, M.A.; Diamond, A.; et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat. Genet. 1996, 13, 189–195. [Google Scholar] [CrossRef]
- El-Harith el, H.A.; Kuhnau, W.; Schmidtke, J.; Gadzicki, D.; Ahmed, M.; Krawczak, M.; Stuhrmann, M. Hereditary hemorrhagic telangiectasia is caused by the Q490X mutation of the ACVRL1 gene in a large Arab family: Support of homozygous lethality. Eur. J. Med. Genet. 2006, 49, 323–330. [Google Scholar] [CrossRef]
- Bourdeau, A.; Dumont, D.J.; Letarte, M. A murine model of hereditary hemorrhagic telangiectasia. J. Clin. Investig. 1999, 104, 1343–1351. [Google Scholar] [CrossRef]
- Urness, L.D.; Sorensen, L.K.; Li, D.Y. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat. Genet. 2000, 26, 328–331. [Google Scholar] [CrossRef]
- Pagenstecher, A.; Stahl, S.; Sure, U.; Felbor, U. A two-hit mechanism causes cerebral cavernous malformations: Complete inactivation of CCM1, CCM2 or CCM3 in affected endothelial cells. Hum. Mol. Genet. 2009, 18, 911–918. [Google Scholar] [CrossRef]
- Amyere, M.; Aerts, V.; Brouillard, P.; McIntyre, B.A.; Duhoux, F.P.; Wassef, M.; Enjolras, O.; Mulliken, J.B.; Devuyst, O.; Antoine-Poirel, H.; et al. Somatic uniparental isodisomy explains multifocality of glomuvenous malformations. Am. J. Hum. Genet. 2013, 92, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Akers, A.L.; Johnson, E.; Steinberg, G.K.; Zabramski, J.M.; Marchuk, D.A. Biallelic somatic and germline mutations in cerebral cavernous malformations (CCMs): Evidence for a two-hit mechanism of CCM pathogenesis. Hum. Mol. Genet. 2009, 18, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Snellings, D.A.; Gallione, C.J.; Clark, D.S.; Vozoris, N.T.; Faughnan, M.E.; Marchuk, D.A. Somatic mutations in vascular malformations of hereditary hemorrhagic telangiectasia result in Bi-allelic loss of ENG or ACVRL1. Am. J. Hum. Genet. 2019, 105, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Walker, E.J.; Degos, V.; Jun, K.; Kuo, R.; Pile-Spellman, J.; Su, H.; Young, W.L. Endoglin deficiency in bone marrow is sufficient to cause cerebrovascular dysplasia in the adult mouse after vascular endothelial growth factor stimulation. Stroke 2013, 44, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Walker, E.J.; Shen, F.; Oh, S.P.; Arthur, H.M.; Young, W.L.; Su, H. Minimal homozygous endothelial deletion of Eng with VEGF stimulation is sufficient to cause cerebrovascular dysplasia in the adult mouse. Cerebrovasc. Dis. 2012, 33, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Guo, Y.; Walker, E.J.; Shen, F.; Jun, K.; Oh, S.P.; Degos, V.; Lawton, M.T.; Tihan, T.; Davalos, D.; et al. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 305–310. [Google Scholar] [CrossRef]
- Garrido-Martin, E.M.; Nguyen, H.L.; Cunningham, T.A.; Choe, S.W.; Jiang, Z.; Arthur, H.M.; Lee, Y.J.; Oh, S.P. Common and distinctive pathogenetic features of arteriovenous malformations in hereditary hemorrhagic telangiectasia 1 and hereditary hemorrhagic telangiectasia 2 animal models—Brief report. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2232–2236. [Google Scholar] [CrossRef]
- Han, C.; Choe, S.W.; Kim, Y.H.; Acharya, A.P.; Keselowsky, B.G.; Sorg, B.S.; Lee, Y.J.; Oh, S.P. VEGF neutralization can prevent and normalize arteriovenous malformations in an animal model for hereditary hemorrhagic telangiectasia 2. Angiogenesis 2014, 17, 823–830. [Google Scholar] [CrossRef]
- Hiepen, C.; Jatzlau, J.; Knaus, P. Biomechanical stress provides a second hit in the establishment of BMP/TGFbeta-related vascular disorders. Cell Stress 2020, 4, 44–47. [Google Scholar] [CrossRef]
- Crist, A.M.; Lee, A.R.; Patel, N.R.; Westhoff, D.E.; Meadows, S.M. Vascular deficiency of Smad4 causes arteriovenous malformations: A mouse model of Hereditary Hemorrhagic Telangiectasia. Angiogenesis 2018, 21, 363–380. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choe, S.W.; Chae, M.Y.; Hong, S.; Oh, S.P. SMAD4 Deficiency leads to development of arteriovenous malformations in neonatal and adult mice. J. Am. Heart Assoc. 2018, 7, e009514. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Llorente, L.; Gallardo-Vara, E.; Rossi, E.; Smadja, D.M.; Botella, L.M.; Bernabeu, C. Endoglin and alk1 as therapeutic targets for hereditary hemorrhagic telangiectasia. Expert Opin. Ther. Targets 2017, 21, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Shovlin, C.L. Hereditary haemorrhagic telangiectasia: Pathophysiology, diagnosis and treatment. Blood Rev. 2010, 24, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Wang, W.C.; Chen, Y.K.; Lin, L.M. A retrospective study of trauma-associated oral and maxillofacial lesions in a population from southern Taiwan. J. Appl. Oral Sci. 2010, 18, 5–9. [Google Scholar] [CrossRef][Green Version]
- Meltem Koray, T.T. Oral mucosal trauma and injuries. In Trauma in Dentistry; Gözler, S., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Maruccia, M.; Onesti, M.G.; Parisi, P.; Cigna, E.; Troccola, A.; Scuderi, N. Lip cancer: A 10-year retrospective epidemiological study. Anticancer Res. 2012, 32, 1543–1546. [Google Scholar]
- Sawyer, A.R.; See, M.; Nduka, C. 3D stereophotogrammetry quantitative lip analysis. Aesthetic Plast Surg. 2009, 33, 497–504. [Google Scholar] [CrossRef]
- Papadopoulos, O.; Konofaos, P.; Tsantoulas, Z.; Chrisostomidis, C.; Frangoulis, M.; Karakitsos, P. Lip defects due to tumor excision: Apropos of 899 cases. Oral Oncol. 2007, 43, 204–212. [Google Scholar] [CrossRef]
- Montes, D.M.; Ghali, G.E. Lip cancer. In Peterson’s Principles of Oral and Maxillofacial Surgery, 3rd ed.; Miloro, M., Ghali, G.E., Larsen, P.E., Waite, P., Eds.; People’s Medical Publishing House: Shelton, CO, USA, 2011; p. 727. [Google Scholar]
- Halachmi, S.; Israeli, H.; Ben-Amitai, D.; Lapidoth, M. Treatment of the skin manifestations of hereditary hemorrhagic telangiectasia with pulsed dye laser. Lasers Med. Sci. 2014, 29, 321–324. [Google Scholar] [CrossRef]
- Geisthoff, U.W.; Fiorella, M.L.; Fiorella, R. Treatment of recurrent epistaxis in HHT. Curr. Pharm. Des. 2006, 12, 1237–1242. [Google Scholar] [CrossRef]
- Faughnan, M.E.; Palda, V.A.; Garcia-Tsao, G.; Geisthoff, U.W.; McDonald, J.; Proctor, D.D.; Spears, J.; Brown, D.H.; Buscarini, E.; Chesnutt, M.S.; et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J. Med. Genet. 2011, 48, 73–87. [Google Scholar] [CrossRef]
- Jorgensen, G.; Lange, B.; Wanscher, J.H.; Kjeldsen, A.D. Efficiency of laser treatment in patients with hereditary hemorrhagic telangiectasia. Eur. Arch. Otorhinolaryngol. 2011, 268, 1765–1770. [Google Scholar] [CrossRef] [PubMed]
- Abiri, A.; Goshtasbi, K.; Maducdoc, M.; Sahyouni, R.; Wang, M.B.; Kuan, E.C. Laser-assisted control of epistaxis in hereditary hemorrhagic telangiectasia: A systematic review. Lasers Surg. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sexton, A.; Gargan, B.; Taylor, J.; Bogwitz, M.; Winship, I. Living with Hereditary Haemorrhagic Telangiectasia: Stigma, coping with unpredictable symptoms, and self-advocacy. Psychol. Health 2019, 34, 1141–1160. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.A.; Lippert, B.M.; Geisthoff, U.W.; Rudert, H. Nd:YAG laser therapy of recurrent epistaxis in hereditary hemorrhagic telangiectasia. Laryngorhinootologie 1997, 76, 495–501. [Google Scholar] [CrossRef]
- Barolet, D.; Christiaens, F.; Hamblin, M.R. Infrared and skin: Friend or foe. J. Photochem. Photobiol. B 2016, 155, 78–85. [Google Scholar] [CrossRef]
- Werner, J.A.; Geisthoff, U.W.; Lippert, B.M.; Rudert, H. Treatment of recurrent epistaxis in Rendu-Osler-Weber disease. HNO 1997, 45, 673–681. [Google Scholar] [CrossRef]
- Fernandez, L.A.; Garrido-Martin, E.M.; Sanz-Rodriguez, F.; Ramirez, J.R.; Morales-Angulo, C.; Zarrabeitia, R.; Perez-Molino, A.; Bernabeu, C.; Botella, L.M. Therapeutic action of tranexamic acid in hereditary haemorrhagic telangiectasia (HHT): Regulation of ALK-1/endoglin pathway in endothelial cells. Thromb. Haemost. 2007, 97, 254–262. [Google Scholar] [CrossRef]
| Right Handed (n) | Ambidextrous (n) | Left Handed (n) | Total | |
|---|---|---|---|---|
| more TAE on dominant hand (n) | 67 | 0 | 2 | 69 |
| equal number of TAE (n) | 13 | 2 | 1 | 16 |
| more TAE on non-dominant hand (n) | 14 | 2 | 1 | 17 |
| Total | 94 | 4 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geisthoff, U.; Nguyen, H.-L.; Lefering, R.; Maune, S.; Thangavelu, K.; Droege, F. Trauma Can Induce Telangiectases in Hereditary Hemorrhagic Telangiectasia. J. Clin. Med. 2020, 9, 1507. https://doi.org/10.3390/jcm9051507
Geisthoff U, Nguyen H-L, Lefering R, Maune S, Thangavelu K, Droege F. Trauma Can Induce Telangiectases in Hereditary Hemorrhagic Telangiectasia. Journal of Clinical Medicine. 2020; 9(5):1507. https://doi.org/10.3390/jcm9051507
Chicago/Turabian StyleGeisthoff, Urban, Ha-Long Nguyen, Rolf Lefering, Steffen Maune, Kruthika Thangavelu, and Freya Droege. 2020. "Trauma Can Induce Telangiectases in Hereditary Hemorrhagic Telangiectasia" Journal of Clinical Medicine 9, no. 5: 1507. https://doi.org/10.3390/jcm9051507
APA StyleGeisthoff, U., Nguyen, H.-L., Lefering, R., Maune, S., Thangavelu, K., & Droege, F. (2020). Trauma Can Induce Telangiectases in Hereditary Hemorrhagic Telangiectasia. Journal of Clinical Medicine, 9(5), 1507. https://doi.org/10.3390/jcm9051507





