Contemporary Management of Acute Lower Limb Ischemia: Determinants of Treatment Choice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgery
2.3. Endovascular Treatment
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Walker, T.G. Acute Limb Ischemia. Tech. Vasc. Interv. Radiol. 2009, 12, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, M.; Käunicke, M.; Hailer, B. Percutaneous mechanical thrombectomy for treatment of acute femoropopliteal bypass occlusion. Vasc. Health Risk Manag. 2012, 8, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukasiewicz, A. Treatment of acute lower limb ischaemia. Vasa 2016, 45, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Hennebry, T.A. Percutaneous isolated pharmaco-mechanical thrombolysis-thrombectomy system for the management of acute arterial limb ischemia: 30-day results from a single-center experience. Catheter. Cardiovasc. Interv. 2012, 80, 636–643. [Google Scholar] [CrossRef]
- Björck, M.; Earnshaw, J.J.; Acosta, S.; Gonçalves, F.B.; Cochennec, F.; Debus, E.S.; Hinchliffe, R.; Jongkind, V.; Koelemay, M.J.; Menyhei, G.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Acute Limb Ischaemia. Eur. J. Vasc. Endovasc. Surg. 2019, 59, 173–218. [Google Scholar] [CrossRef] [Green Version]
- Investigators, S. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. Ann. Surg. 1994, 220, 251–266. [Google Scholar]
- Ouriel, K.; Veith, F.J.; Sasahara, A.A. A Comparison of Recombinant Urokinase with Vascular Surgery as Initial Treatment for Acute Arterial Occlusion of the Legs. N. Engl. J. Med. 1998, 338, 1105–1111. [Google Scholar] [CrossRef]
- Veenstra, E.B.; Van Der Laan, M.J.; Zeebregts, C.J.; De Heide, E.-J.; Kater, M.; Bokkers, R.P. A systematic review and meta-analysis of endovascular and surgical revascularization techniques in acute limb ischemia. J. Vasc. Surg. 2019, 71, 654–668. [Google Scholar] [CrossRef]
- Taha, A.G.; Byrne, R.M.; Avgerinos, E.D.; Marone, L.K.; Makaroun, M.S.; Chaer, R.A. Comparative effectiveness of endovascular versus surgical revascularization for acute lower extremity ischemia. J. Vasc. Surg. 2015, 61, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutherford, R.B.; Baker, J.; Ernst, C.; Johnston, K.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538. [Google Scholar] [CrossRef] [Green Version]
- Lukasiewicz, A.; Lichota, W.; Thews, M. Outcomes of accelerated catheter-directed thrombolysis in patients with acute arterial thrombosis. Vasc. Med. 2016, 21, 453–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.B.; Choi, H.C.; Lee, S.M.; Na, J.B.; Park, M.J.; Shin, H.S.; Won, J.H.; Lee, C.E.; Park, S.E. Combined treatment (image-guided thrombectomy and endovascular therapy with open femoral access) for acute lower limb ischemia: Clinical efficacy and outcomes. PLoS ONE 2019, 14, e0225136. [Google Scholar] [CrossRef] [PubMed]
- Weeda, E.R.; Bohm, N. Association between comorbid cancer and outcomes among admissions for acute ischemic stroke receiving systemic thrombolysis. Int. J. Stroke 2018, 14, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Tehan, P.E.; Sebastian, M.; Barwick, A.; Chuter, V.H. How sensitive and specific is continuous-wave Doppler for detecting peripheral arterial disease in people with and without diabetes? A cross-sectional study. Diabetes Vasc. Dis. Res. 2018, 15, 396–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, P.; Eckstein, H.H.; De Rango, P.; Setacci, C.; Ricco, J.B.; de Donato, G.; Becker, F.; Robert-Ebadi, H.; Diehm, N.; Schmidli, J. Chapter II: Diagnostic methods. Eur. J. Vasc. Endovasc. Surg. 2011, 42, 13–32. [Google Scholar] [CrossRef] [Green Version]
- Olinic, D.M.; Stanek, A.; Tataru, A.-D.; Homorodean, C.; Olinic, M. Acute Limb Ischemia: An Update on Diagnosis and Management. J. Clin. Med. 2019, 8, 1215. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, W.J.; Forstner, R.; Kofler, B.; Binder, K.; Ugurluoglu, A.; Magometschnigg, H. Pedal artery imaging—A comparison of selective digital subtraction angiography, contrast enhanced magnetic resonance angiography and duplex ultrasound. Eur. J. Vasc. Endovasc. Surg. 2002, 24, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Clinical classification of acute limb ischemia. J. Vasc. Surg. 2000, 31, 142–145.
- Lipsitz, E.C.; Veith, F.J. Fluoroscopically assisted thromboembolectomy: Should it be routine? Semin. Vasc. Surg. 2001, 14, 100–106. [Google Scholar] [CrossRef]
- Zaraca, F.; Stringari, C.; Ebner, J.A.; Ebner, H. Routine versus selectiveuse of intraoperative angiography during thromboembolectomy for acute lower limb ischemia: Analysis of outcomes. Ann. Vasc. Surg. 2010, 24, 621–627. [Google Scholar] [CrossRef]
- Byrne, R.M.; Taha, A.G.; Avgerinos, E.D.; Marone, L.K.; Makaroun, M.S.; Chaer, R.A. Contemporary outcomes of endovascular interventions for acute limb ischemia. J. Vasc. Surg. 2014, 59, 988–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varki A Trousseau’s syndrome: Multiple definitions and multiple mechanisms. Blood 2007, 110, 1723–1729. [CrossRef] [PubMed]
| Thrombolysis Degree | Description |
|---|---|
| 0 | No effect of thrombolysis visible on angiography |
| 1 | Partial lysis without axial flow |
| 2 | Partial lysis with axial flow reconstitution |
| 3 | Complete thrombus lysis |
| Overall (%) | Surgery/ | Endovascular | p | |
|---|---|---|---|---|
| Hybrid (%) | ||||
| N (%) | 307 | 191 (62.2) | 94 (30.6) | |
| Age | 68 | 67.8 | 68.4 | NS |
| Sex (male) | 208 (67.8) | 120 (62.8) | 75 (79.8) | 0.004 # |
| BMI | 26.6 | 26.7 | 26.2 | NS |
| Duration | 1 * | 1 * | 4 * | <0.0001 ** |
| Rutherford 1 | 82 (26.7) | 39 (20.4) | 36 (38.3) | 0.0013 # |
| Rutherford 2A | 98 (31.9) | 46 (24.1) | 41 (43.6) | 0.001 # |
| Rutherford 2B | 38 (12.4) | 28 (14.7) | 10 (10.6) | NS |
| Rutherford 3 | 89 (29) | 78 (40.8) | 7 (7.4) | 0.00001 # |
| Etiology | 0.00001 ## | |||
| Embolus | 98 (31.9) | 85 (44.5) | 5 (5.3) | |
| Thrombosis | 209 (68.1) | 106 (55.5) | 89 (94.7) | |
| Location | ||||
| Aorta | 10 (3.3) | 7 (3.7) | 1 (1.1) | NS |
| Iliac | 79 (25.7) | 57 (29.8) | 17 (18.9) | 0.054 |
| Femoral | 194 (63.2) | 125 (65.4) | 59 (61.1) | NS |
| Popliteal | 99 (32.2) | 50 (26.2) | 42 (45.6) | 0.002 # |
| Tibial | 72 (23.5) | 34 (17.8) | 31 (34.4) | 0.004 # |
| Multilevel | 105 (34.2) | 50 (30.4) | 41 (43.6) | 0.003# |
| Smoker | 196 (66.7) | 113 (62.1) | 74 (80.4) | 0.001 # |
| HA | 170 (55.4) | 105 (55) | 54 (57.4) | NS |
| CAD | 102 (33.2) | 70 (36.6) | 22 (23.4) | 0.02 # |
| DM | 75 (24.4) | 49 (25.7) | 22 (23.4) | NS |
| CHF | 41 (13.4) | 34 (17.8) | 4 (4.3) | 0.002 # |
| STROKE | 31 (10.1) | 24 (12.6) | 6 (6.5) | NS |
| CRI | 20 (6.5) | 14 (7.3) | 4 (4.3) | NS |
| MI | 12 (3.9) | 11 (5.8) | 0 (0) | 0.02 ## |
| FA | 71 (23.1) | 59 (30.9) | 5 (5.3) | 0.00001 ## |
| ACTIVE CANCER | 43 (13.2) | 35 (18.3) | 4 (4.3) | 0.0008 ## |
| COLD | 21 (6.8) | 12 (6.3) | 6 (6.4) | NS |
| Overall | Surgery/ | Endovascular | p | |
|---|---|---|---|---|
| (307 patients) | Hybrid | (94 patients) | ||
| (191 patients) | ||||
| Procedures | ||||
| Conservative | 22 (7.2) | |||
| Surgical (overall) | 161 (52.4) | |||
| Embolectomy | 85 (27.7) | |||
| Thrombectomy | 83 (27) | |||
| Endarterectomy | 70 (22.8) | |||
| By-pass | 68 (22.2) | |||
| Angiography | 142 (46.3) | |||
| Endovascular (overall) | 94 (30.6) | |||
| Thrombolysis | 89 (29) | |||
| Mechanical thrombectomy | 4 (1.3) | |||
| Angioplasty/stent | 95 (30.9) | |||
| Stentgraft | 6 (2) | |||
| Hybrid | 30 (9.8) | |||
| Postoperative hospitalization | 4! | 5! | 2! | |
| Primary procedure success | 238 (77.5) | 156 (81.7) | 71 (75.5) | NS # |
| Final treatment success | 248 (80.8) | 156 (81.7) | 82 (86.2) | NS # |
| Reintervention | 36 (11.7) | 17 (8.9) | 19 (20.2) | 0.007 # |
| Major amputation (in hospital) | 23 (7.5) | 17 (8.9) | 4 (4.3) | NS * |
| Major amputation (6 months) | 14 (6.1) | 7 (5) | 7 (8.8) | NS * |
| Hospital mortality | 26 (8.5) | 18 (9.4) | 3 (3.2) | 0.09 * |
| Mortality (6 months) | 32 (10.4) | 24 (12.6) | 3 (3.2) | 0.001 * |
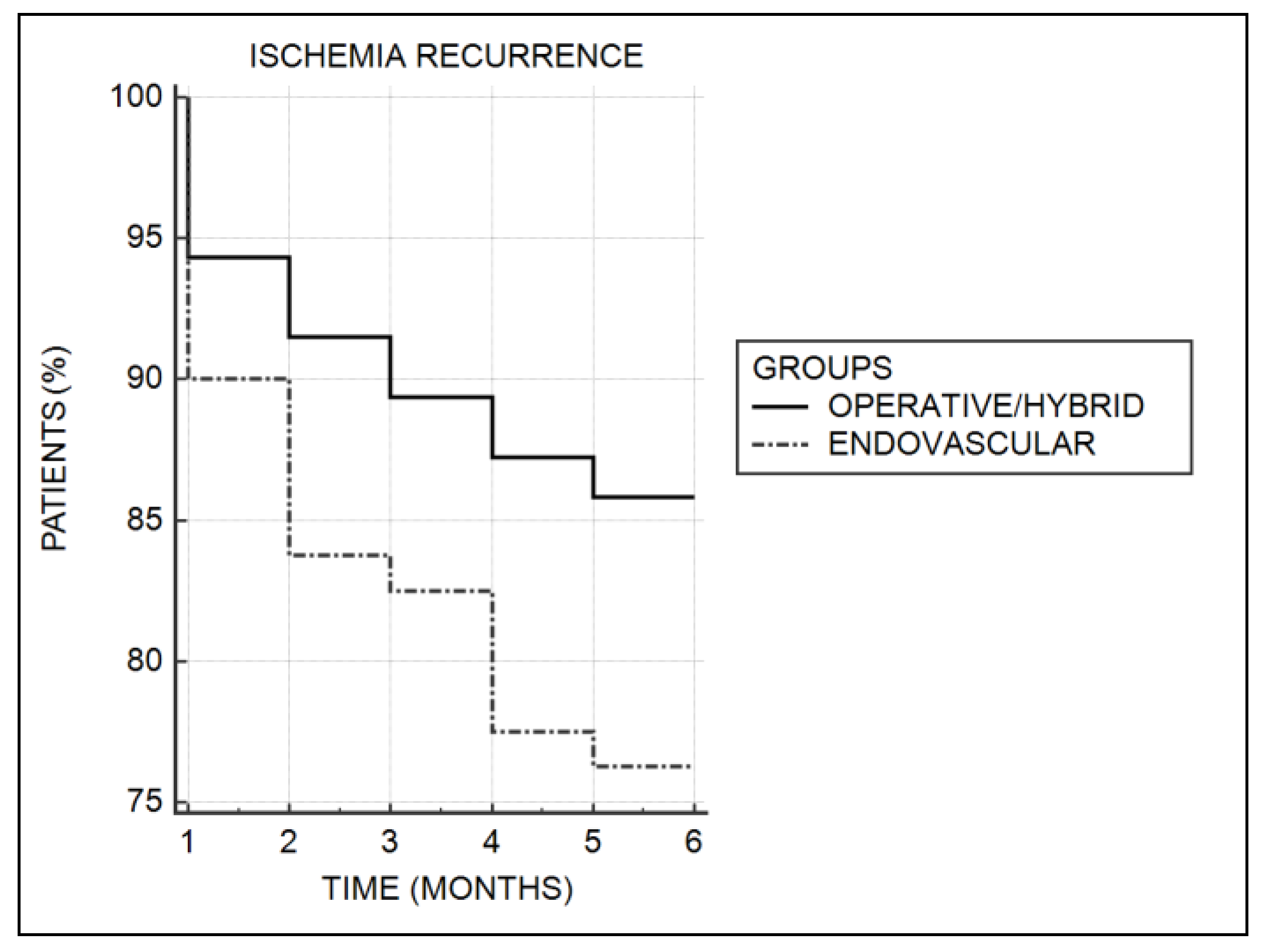
| Recurrent ischemia | 41 (17.7) | 20 (14.3) | 19 (23.8) | 0.07 # |
| Number of Patients (%) | |
|---|---|
| Endoluminal Treatment | 16 (17) |
| Hematoma | 6 (6.4) |
| Bleeding | 4 (4.3) |
| Intracranial bleeding | 1 (1.1) |
| Spinal cord hematoma | 1 (1.1) |
| Vascular bypass thrombosis | 1 (1.1) |
| Myocardial infarction | 1 (1.1) |
| Acute heart failure | 1 (1.1) |
| Symptomatic bradycardia | 1 (1.1) |
| Surgical treatment | 60 (34.1) |
| Recurrent thrombosis | 9 (5.1) |
| Multiorgan failure | 8 (4.5) |
| Surgical site infection | 6 (3.4) |
| Hematoma | 6 (3.4) |
| Lymphorrhea | 5 (2.8) |
| Bypass thrombosis | 5 (2.8) |
| Pulmonary edema | 1 (0.6) |
| Cardiac arrest | 3 (1.7) |
| Superior mesenteric artery embolus | 2 (1.1) |
| Surgical site bleeding | 2 (1.1) |
| Bypass infection | 2 (1.1) |
| Acute renal failure | 2 (1.1) |
| Myocardial infarction | 2 (1.1) |
| Gastrointestinal bleeding | 2 (1.1) |
| Hematuria | 2 (1.1) |
| Fever | 1 (0.6) |
| Pneumonia | 1 (0.6) |
| Stroke | 1 (0.6) |
| Hybrid Treatment | 10 (33.3) |
| Hematoma | 2 (6.7) |
| Urinary tract infection | 1 (3.3) |
| Bypass thrombosis | 1 (3.3) |
| Bleeding | 1 (3.3) |
| Acute cardiac failure | 2 (6.7) |
| Acute renal failure | 1 (3.3) |
| Surgical site infection | 1 (3.3) |
| Lymphorrhea | 1 (3.3) |
| Factor | Treatment | Complications | Amputation (p) | Death (p) | Recurrence |
|---|---|---|---|---|---|
| Success (p) | (p) | (p) | |||
| Rutherford class | <0.0001 ## | 0.009 ## | 0.004 ## | 0.0002 ## | 0.04 ## |
| Lesion type | |||||
| Embolus | NS # | NS # | NS # | 0.04 # | 0.053 # |
| Native thrombosis | NS # | 0.03 # | NS # | NS # | 0.03 # |
| Bypass thrombosis | NS # | NS # | NS # | NS # | 0.0001 # |
| Lesion location: | |||||
| Popliteal | NS # | NS # | 0.01 # | NS # | NS # |
| Tibial | 0.01 # | NS # | 0.0001 # | NS # | NS # |
| Multilevel | NS # | NS # | NS # | NS # | 0.01 # |
| Comorbidities | |||||
| ARI | 0.02 * | 0.0001 # | NS # | 0.002 # | NS # |
| CHF | 0.009 # | 0.05 # | 0.01 # | 0.0001 # | NS # |
| CRI | 0.06 # | NS # | NS # | 0.056 # | NS # |
| FA | 0.02 # | 0.02 # | NS # | 0.02 # | NS # |
| MI | NS # | NS # | NS # | <0.0001 # | NS # |
| NEO | NS # | 0.02 # | NS # | NS # | NS # |
| Treatment | |||||
| Conservative | NS # | NS # | NS # | 0.01 # | NS # |
| Complications | 0.0001 # | - | 0.0001 # | <0.0001 # | NS # |
| Reintervention | NS # | NS # | NS # | NS # | 0.04 # |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukasiewicz, A. Contemporary Management of Acute Lower Limb Ischemia: Determinants of Treatment Choice. J. Clin. Med. 2020, 9, 1501. https://doi.org/10.3390/jcm9051501
Lukasiewicz A. Contemporary Management of Acute Lower Limb Ischemia: Determinants of Treatment Choice. Journal of Clinical Medicine. 2020; 9(5):1501. https://doi.org/10.3390/jcm9051501
Chicago/Turabian StyleLukasiewicz, Aleksander. 2020. "Contemporary Management of Acute Lower Limb Ischemia: Determinants of Treatment Choice" Journal of Clinical Medicine 9, no. 5: 1501. https://doi.org/10.3390/jcm9051501
APA StyleLukasiewicz, A. (2020). Contemporary Management of Acute Lower Limb Ischemia: Determinants of Treatment Choice. Journal of Clinical Medicine, 9(5), 1501. https://doi.org/10.3390/jcm9051501





