Association between Very Low-Density Lipoprotein Cholesterol (VLDL-C) and Carotid Intima-Media Thickness in Postmenopausal Women Without Overt Cardiovascular Disease and on LDL-C Target Levels
Abstract
1. Introduction
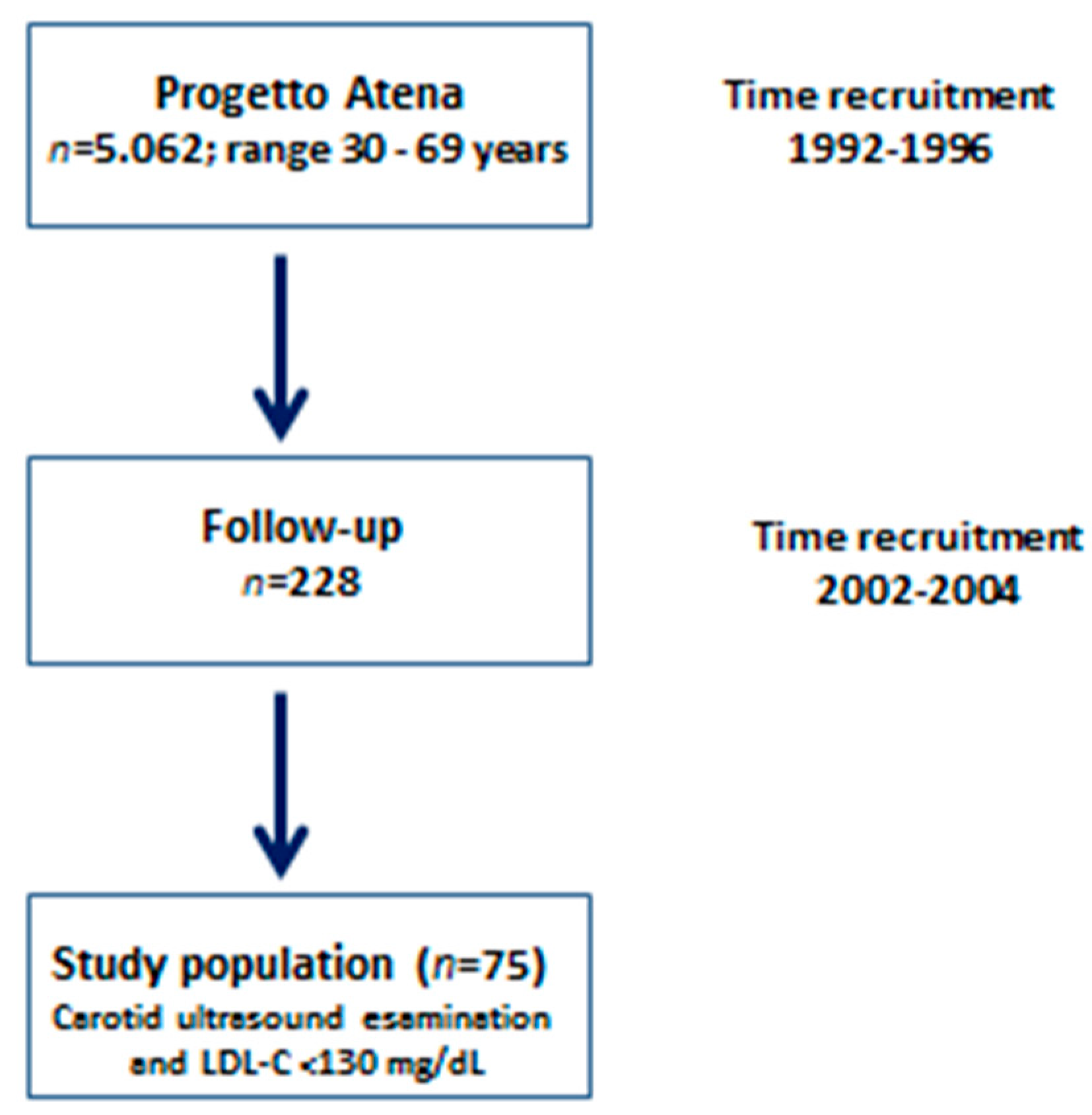
2. Methods
2.1. Clinical and Biochemical Assessment
2.2. Lipoprotein Determination
2.3. High-Resolution Carotid Ultrasound
3. Statistical Analysis
4. Results
5. Discussion
Author Contributions
Conflicts of Interest
References
- Ambrosetti, M.; Abreu, A.; Corrà, U.; Davos, C.H.; Hansen, D.; Frederix, I.; Iliou, M.C.; Pedretti, R.F.; Schmid, J.P.; Vigorito, C.; et al. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the secondary prevention and rehabilitation section of the European association of preventive cardiology. Eur. J. Prev. Cardiol. 2020. [Google Scholar] [CrossRef]
- Buono, F.; Spinelli, L.; Giallauria, F.; Assante di Panzillo, E.; Di Marino, S.; Ferrara, F.; Vigorito, C.; Trimarco, B.; Morisco, C. Usefulness of satisfactory control of low-density lipoprotein cholesterol to predict left ventricular remodeling after a first ST-elevation myocardial infarction successfully reperfused. Am. J. Cardiol. 2011, 107, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.J.; Ginsberg, H.N.; Amarenco, P.; Andreotti, F.; Borén, J.; Catapano, A.L.; Descamps, O.S.; Fisher, E.; Kovanen, P.T.; Kuivenhoven, J.A.; et al. European atherosclerosis society consensus panel. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease. Eur. Heart J. 2011, 32, 1345–1361. [Google Scholar] [CrossRef] [PubMed]
- Alaupovic, P. On the atherogenicity of triglyceride-rich lipoproteins and novel markers for the assessment of their atherogenic potentials. Oléagineux Corps Gras Lipides 2002, 9, 220–226. [Google Scholar] [CrossRef][Green Version]
- Gentile, M.; Iannuzzo, G.; Mattiello, A.; Marotta, G.; Iannuzzi, A.; Panico, S.; Rubba, P. Association between Lp (a) and atherosclerosis in menopausal women without metabolic syndrome. Biomark. Med. 2016, 10, 397–402. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Chapman, M.J.; Ray, K.; Boren, J.; Andreotti, F.; Watts, G.F.; Ginsberg, H.; Amarenco, P.; Catapano, A.; Descamps, O.S.; et al. European atherosclerosis society consensus panel. Lipoprotein (a) as a cardiovascular risk factor: Current status. Eur. Heart J. 2010, 31, 2844–2853. [Google Scholar] [CrossRef]
- Iannuzzi, A.; Gentile, M.; Iannuzzo, G.; Covetti, G.; Panico, C.; Mattiello, A.; Fata, E.; D’Elia, L.; De Michele, M.; Rubba, P.; et al. Atherogenic lipoprotein subfractions and carotid atherosclerosis in menopausal women. Angiology 2018, 69, 666–671. [Google Scholar] [CrossRef]
- Gentile, M.; Panico, S.; Mattiello, A.; Ubaldi, S.; Iannuzzo, G.; De Michele, M.; Iannuzzi, A.; Rubba, P. Association between small dense LDL and early atherosclerosis in a sample of menopausal women. Clin. Chim. Acta 2013, 426, 1–5. [Google Scholar] [CrossRef]
- Rubba, P.; Mercuri, M.; Faccenda, F.; Iannuzzi, A.; Irace, C.; Strisciuglio, P.; Gnasso, A.; Tang, R.; Andria, G.; Bond, M.G.; et al. Premature carotid atherosclerosis: Does it occur in both familial hypercholesterolemia and homocystinuria? Ultrasound assessment of arterial intima-media thickness and blood flow velocity. Stroke 1994, 25, 943–950. [Google Scholar] [CrossRef]
- Panico, S.; Dello Iacovo, R.; Celentano, E.; Galasso, R.; Muti, P.; Salvatore, F.; Mancini, M. Progetto ATENA, a study on the etiology of major chronic diseases in women: Design, rationale and objectives. Eur. J. Epidemiol. 1992, 8, 601–608. [Google Scholar] [CrossRef]
- Gentile, M.; Simeon, V.; Iannuzzo, G.; Mattiello, A.; di Taranto, M.D.; Panico, S.; Rubba, P. Lipoprotein (a) is an independent predictor of cardiovascular events in Mediterranean women (Progetto Atena). Eur. J. Prev. Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. American heart association; national heart, lung, and blood institute diagnosis and management of the metabolic syndrome: An American heart association/national heart, lung, and blood institute scientific statement: Executive summary. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Ridker, P.M. LDL cholesterol: Controversies and future therapeutic directions. Lancet 2014, 384, 607–617. [Google Scholar] [CrossRef]
- Varbo, A.; Nordestgaard, B.G. Remnant lipoproteins. Curr. Opin. Lipidol. 2017, 28, 300–307. [Google Scholar] [CrossRef]
- Generoso, G.; Janovsky, C.C.P.S.; Bittencourt, M.S. Triglycerides and triglyceride-rich lipoproteins in the development and progression of atherosclerosis. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 109–116. [Google Scholar] [CrossRef]
- Hegele, R.A.; Tsimikas, S. Lipid-lowering agents. Circ. Res. 2019, 124, 386–404. [Google Scholar] [CrossRef]
- Lawler, P.R.; Akinkuolie, A.O.; Harada, P.; Glynn, R.J.; Chasman, D.I.; Ridker, P.M.; Mora, S. Residual risk of atherosclerotic cardiovascular events in relation to reductions in very-low-density lipoproteins. J. Am. Heart Assoc. 2017, 6, e007402. [Google Scholar] [CrossRef]
- Boden, W.E.; Bhatt, D.L.; Toth, P.P.; Ray, K.K.; Chapman, M.J.; Lüscher, T.F. Profound reductions in first and total cardiovascular events with icosapent ethyl in the REDUCE-IT trial: Why these results usher in a new era in dyslipidaemia therapeutics. Eur. Heart J. 2019. [Google Scholar] [CrossRef]
- Abi-Ayad, M.; Abbou, A.; Abi-Ayad, F.Z.; Behadada, O.; Benyoucef, M. HDL-C, ApoA1 and VLDL-TG as biomarkers for the carotid plaque presence in patients with metabolic syndrome. Diabetes Metab. Syndr. 2018, 12, 175–179. [Google Scholar] [CrossRef]
- Elshazly, M.B.; Mani, P.; Nissen, S.; Brennan, D.M.; Clark, D.; Martin, S.; Jones, S.R.; Quispe, R.; Donnellan, E.; Nicholls, S.J.; et al. Remnant cholesterol, coronary atheroma progression and clinical events in statin-treated patients with coronary artery disease. Eur. J. Prev. Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Boesen, M.E.; Singh, D.; Menon, B.K.; Frayne, R. A systematic literature review of the effect of carotid atherosclerosis on local vessel stiffness and elasticity. Atherosclerosis 2015, 243, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Motau, T.H.; Norton, G.R.; Sadiq, E.; Manyatsi, N.; Kolkenbeck-Ruh, A.; Robinson, C.; Tade, G.; Mabena, P.; Monareng, T.; Naran, R.; et al. Marked arterial functional changes in patients with arterial vascular events across the early adult lifespan. Arter. Thromb. Vasc. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Giallauria, F.; Ling, S.M.; Schreiber, C.; Maggio, M.; Shetty, V.; Muller, D.; Vigorito, C.; Ferrucci, L.; Najjar, S.S. Arterial stiffness and bone demineralization: The Baltimore longitudinal study of aging. Am. J. Hypertens 2011, 24, 970–975. [Google Scholar] [CrossRef]
- Giallauria, F.; Milaneschi, Y.; Tanaka, T.; Maggio, M.; Canepa, M.; Elango, P.; Vigorito, C.; Lakatta, E.G.; Ferrucci, L.; Strait, J.; et al. Arterial stiffness and vitamin D levels: The Baltimore longitudinal study of aging. J. Clin. Endocrinol. Metab. 2012, 97, 3717–3723. [Google Scholar] [CrossRef]
- Canepa, M.; Ameri, P.; AlGhatrif, M.; Pestelli, G.; Milaneschi, Y.; Strait, J.B.; Giallauria, F.; Ghigliotti, G.; Brunelli, C.; Lakatta, E.G.; et al. Role of bone mineral density in the inverse relationship between body size and aortic calcification: Results from the Baltimore longitudinal study of aging. Atherosclerosis 2014, 235, 169–175. [Google Scholar] [CrossRef]
- Ameri, P.; Canepa, M.; Milaneschi, Y.; Spallarossa, P.; Leoncini, G.; Giallauria, F.; Strait, J.B.; Lakatta, E.G.; Brunelli, C.; Murialdo, G.; et al. Relationship between vitamin D status and left ventricular geometry in a healthy population: Results from the Baltimore longitudinal study of aging. J. Intern. Med. 2013, 273, 253–262. [Google Scholar] [CrossRef]
- Takagi, K.; Ishihara, S.; Kenji, N.; Iha, H.; Kobayashi, N.; Ito, Y.; Nohara, T.; Ohkuma, S.; Mitsuishi, T.; Ishizuka, A.; et al. Clinical significance of arterial stiffness as a factor for hospitalization of heart failure with preserved left ventricular ejection fraction: A retrospective matched case-control study. J. Cardiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Samargandy, S.; Matthews, K.A.; Brooks, M.M.; Barinas-Mitchell, E.; Magnani, J.W.; Janssen, I.; Hollenberg, S.M.; El Khoudary, S.R. Arterial stiffness accelerates within 1 year of the final menstrual period: The SWAN heart study. Arter. Thromb. Vasc. Biol. 2020, 40, 1001–1008. [Google Scholar] [CrossRef]
- Wang, D.; Jackson, E.A.; Karvonen-Gutierrez, C.A.; Elliott, M.R.; Harlow, S.D.; Hood, M.M.; Derby, C.A.; Sternfeld, B.; Janssen, I.; Crawford, S.L.; et al. Healthy lifestyle during the midlife is prospectively associated with less subclinical carotid atherosclerosis: The study of women’s health across the nation. J. Am. Heart Assoc. 2018, 7, e010405. [Google Scholar] [CrossRef]
- Allen, N.B.; Krefman, A.E.; Labarthe, D.; Greenland, P.; Juonala, M.; Kähönen, M.; Lehtimäki, T.; Day, R.S.; Bazzano, L.A.; Van Horn, L.V.; et al. Cardiovascular health trajectories from childhood through middle age and their association with subclinical atherosclerosis. JAMA Cardiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rensma, S.P.; Stehouwer, C.D.A.; Van Boxtel, M.P.J.; Houben, A.J.H.M.; Berendschot, T.T.J.M.; Jansen, J.F.A.; Schalkwijk, C.G.; Verhey, F.R.J.; Kroon, A.A.; Henry, R.M.A.; et al. Associations of arterial stiffness with cognitive performance, and the role of microvascular dysfunction: The maastricht study. Hypertension 2020. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V.; Huang, H.; Zhao, L.; Verble, D.D.; Nutaitis, A.; Tharwani, S.D.; Brown, A.L.; Zetterberg, H.; Hu, W.; Shin, R.; et al. Baseline results: The association between cardiovascular risk and preclinical Alzheimer’s disease pathology (ascend) study. J. Alzheimer’s Dis. 2020, 75, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Wildman, R.P.; Schott, L.L.; Brockwell, S.; Kuller, L.H.; Sutton Tyrrell, K. A dietary and exercise intervention slows menopause associated progression of subclinical atherosclerosis as measured by intima media thickness of the carotid arteries. J. Am. Coll. Cardiol. 2004, 44, 579–585. [Google Scholar] [CrossRef]


| n = 75 | |
|---|---|
| Age (years) | 61.2 ± 9.3 |
| Total cholesterol (mg/dL) | 187.4 ± 20.9 |
| Triglycerides (mg/dL) | 96.9 ± 41.1 |
| High-density lipoprotein cholesterol (mg/dL) | 59.4 ± 14.8 |
| Low-density lipoprotein cholesterol (mg/dL) | 108.6 ± 15.3 |
| Fasting glucose (mg/dL) | 102.8 ± 20.0 |
| Apolipoprotein B (g/L) | 0.9 ± 0.1 |
| Lipoprotein a (mg/dL) | 18.6 ± 23.6 |
| Low-density lipoprotein (LDL) score (mg/dL) | 2.4 ± 4.4 |
| Mean LDL diameter (Å) | 271.2 ± 3.0 |
| High sensitive C-reactive protein (CRP) (mg/L) | 3.4 ± 6.0 |
| Creatinine (mg/dL) | 0.8 ± 0.1 |
| Body mass index (kg/m2) | 27.9 ± 5.4 |
| Waist circumference (cm) | 89.1± 11.7 |
| Homeostatic assessment model index (HOMA) | 1.8 ± 1.3 |
| Systolic blood pressure (mm Hg) | 141.4 ± 24.3 |
| Diastolic blood pressure (mm Hg) | 80.4 ± 9.8 |
| Intima-media thickness (mm) | 1.0 ± 0.2 |
| Active smokers (yes/not) | 29.5% |
| OR | 95%CI | p Value | OR | 95%CI | p Value | |
|---|---|---|---|---|---|---|
| tertile II vs. tertile I (reference) | tertile III vs. tertile I (reference) | |||||
| HDL-C | 0.54 | 0.14–2.16 | 0.38 | 0.52 | 0.13–2.04 | 0.35 |
| LDL-C | 1.27 | 0.25–6.40 | 0.77 | 2.81 | 0.65–12.18 | 0.17 |
| IDL-C | 2.33 | 0.51–10.69 | 0.27 | 2.00 | 0.44–9.07 | 0.37 |
| VLDL-C | 2.56 | 0.58–11.29 | 0.21 | 5.64 | 1.30–24.31 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentile, M.; Iannuzzi, A.; Giallauria, F.; D’Andrea, A.; Venturini, E.; Pacileo, M.; Covetti, G.; Panico, C.; Mattiello, A.; Vitale, G.; et al. Association between Very Low-Density Lipoprotein Cholesterol (VLDL-C) and Carotid Intima-Media Thickness in Postmenopausal Women Without Overt Cardiovascular Disease and on LDL-C Target Levels. J. Clin. Med. 2020, 9, 1422. https://doi.org/10.3390/jcm9051422
Gentile M, Iannuzzi A, Giallauria F, D’Andrea A, Venturini E, Pacileo M, Covetti G, Panico C, Mattiello A, Vitale G, et al. Association between Very Low-Density Lipoprotein Cholesterol (VLDL-C) and Carotid Intima-Media Thickness in Postmenopausal Women Without Overt Cardiovascular Disease and on LDL-C Target Levels. Journal of Clinical Medicine. 2020; 9(5):1422. https://doi.org/10.3390/jcm9051422
Chicago/Turabian StyleGentile, Marco, Arcangelo Iannuzzi, Francesco Giallauria, Antonello D’Andrea, Elio Venturini, Mario Pacileo, Giuseppe Covetti, Camilla Panico, Amalia Mattiello, Giuseppe Vitale, and et al. 2020. "Association between Very Low-Density Lipoprotein Cholesterol (VLDL-C) and Carotid Intima-Media Thickness in Postmenopausal Women Without Overt Cardiovascular Disease and on LDL-C Target Levels" Journal of Clinical Medicine 9, no. 5: 1422. https://doi.org/10.3390/jcm9051422
APA StyleGentile, M., Iannuzzi, A., Giallauria, F., D’Andrea, A., Venturini, E., Pacileo, M., Covetti, G., Panico, C., Mattiello, A., Vitale, G., Sarullo, F. M., Rubba, P., Vigorito, C., Panico, S., & Iannuzzo, G. (2020). Association between Very Low-Density Lipoprotein Cholesterol (VLDL-C) and Carotid Intima-Media Thickness in Postmenopausal Women Without Overt Cardiovascular Disease and on LDL-C Target Levels. Journal of Clinical Medicine, 9(5), 1422. https://doi.org/10.3390/jcm9051422








