Macular Ganglion Cell Layer Thickness after Macula-Off Rhegmatogenous Retinal Detachment Repair: Scleral Buckling versus Pars Plana Vitrectomy
Abstract
1. Introduction
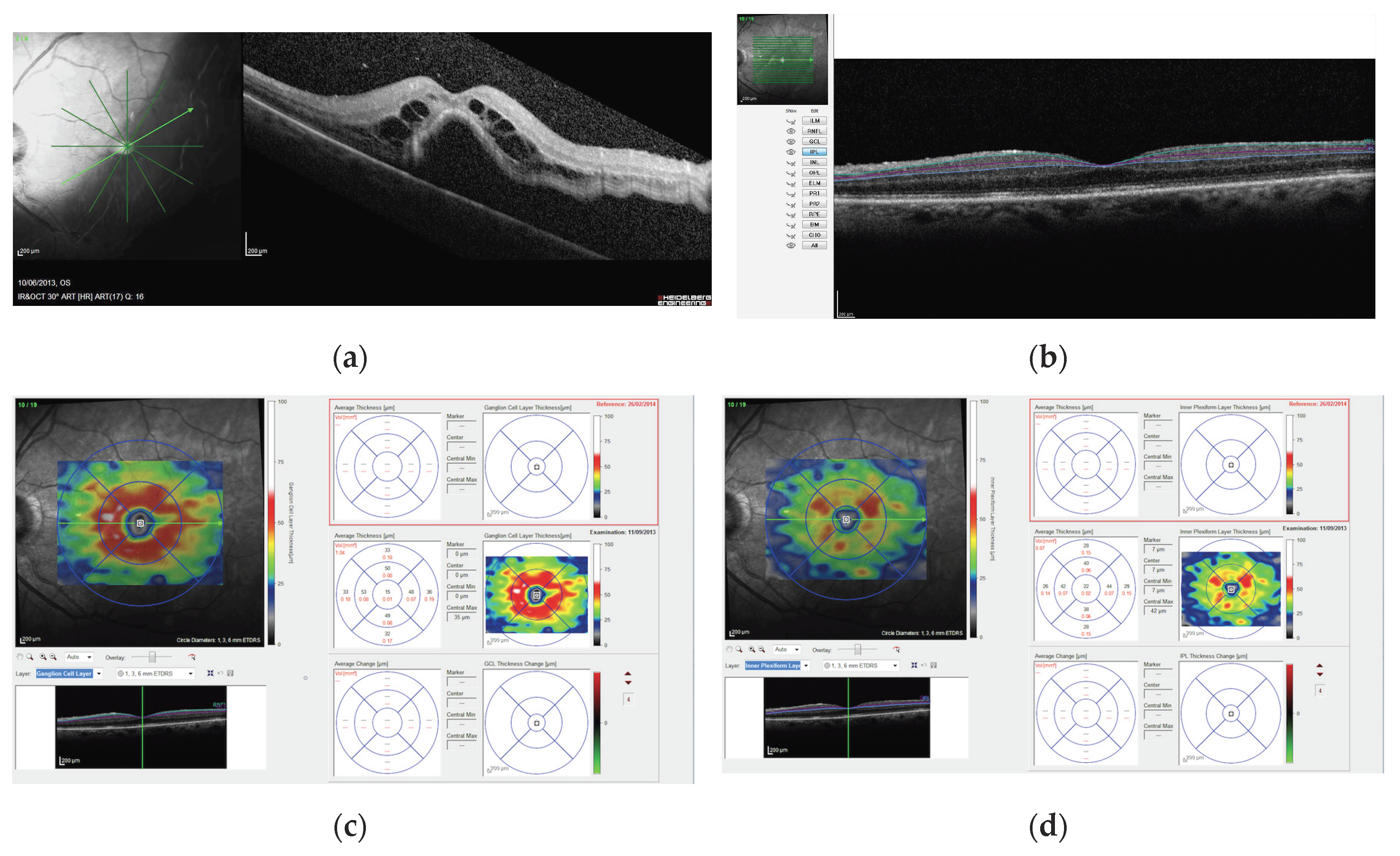
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
References
- Mitry, D.; Charteris, D.G.; Fleck, B.W.; Campbell, H.; Singh, J. The epidemiology of rhegmatogenous retinal detachment: Geographical variation and clinical associations. Br. J. Ophthalmol. 2010, 94, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Yeo, I.Y.; Loh, B.K.; Wong, E.Y.; Wong, D.W.; Ong, S.G.; Ang, C.L.; Lee, S.Y. Scleral buckling versus vitrectomy in the management of macula-off primary rhegmatogenous retinal detachment: A comparison of visual outcomes. Retina 2015, 35, 2552–2557. [Google Scholar] [CrossRef] [PubMed]
- Heimann, H.; Bartz-Schmidt, K.U.; Bornfeld, N.; Weiss, C.; Hilgers, R.D.; Foerster, M.H. Scleral Buckling Versus Primary Vitrectomy in Rhegmatogenous Retinal Detachment: A Prospective Randomized Multicenter Clinical Study. Ophthalmology 2007, 114, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Znaor, L.; Medic, A.; Binder, S.; Vucinovic, A.; Marin Lovric, J.; Puljak, L. Pars plana vitrectomy versus scleral buckling for repairing simple rhegmatogenous retinal detachments. Cochrane Database Syst. Rev. 2019, 3, CD009562. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Oshima, Y.; Fujimoto, H.; Murakami, Y.; Sakaguchi, H.; Kusaka, S.; Tano, Y. Foveal microstructure and visual acuity after retinal detachment repair: Imaging analysis by Fourier-domain optical coherence tomography. Ophthalmology 2009, 116, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Gharbiya, M.; Grandinetti, F.; Scavella, V.; Cecere, M.; Esposito, M.; Segnalini, A.; Gabrieli, C.B. Correlation between spectral-domain optical coherence tomography findings and visual outcome after primary rhegmatogenous retinal detachment repair. Retina 2012, 32, 43–53. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, D.Y.; Ha, H.S.; Kang, S.W. Topographic changes of retinal layers after resolution of acute retinal detachment. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7316–7321. [Google Scholar] [CrossRef][Green Version]
- Lee, S.H.; Han, J.W.; Byeon, S.H.; Kim, S.S.; Koh, H.J.; Lee, S.C.; Kim, M. Retinal layer segmentation after silicone oil or gas tamponade for macula-on retinal detachment using optical coherence tomography. Retina 2018, 38, 310–319. [Google Scholar] [CrossRef]
- Hong, E.H.; Cho, H.; Kim, D.R.; Kang, M.H.; Shin, Y.U.; Seong, M. Changes in Retinal Vessel and Retinal Layer Thickness after Vitrectomy in Retinal Detachment via Swept-Source OCT Angiography. Investig. Ophthalmol. Vis. Sci. 2020, 61, 35. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, J.E.; Shin, Y.I.; Lee, K.M.; Jo, Y.J.; Kim, J.Y. Longitudinal changes in retinal nerve fiber layer thickness after vitrectomy for rhegmatogenous retinal detachment. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5471–5474. [Google Scholar] [CrossRef]
- Menke, M.N.; Kowal, J.H.; Dufour, P.; Wolf-Schnurrbusch, U.E.; Ceklic, L.; Framme, C.; Wolf, S. Retinal layer measurements after successful macula-off retinal detachment repair using optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6575–6579. [Google Scholar] [CrossRef] [PubMed]
- Chylack, L.T.; Wolfe, J.K.; Singer, D.M.; Leske, M.C.; Bullimore, M.A.; Bailey, I.L.; Friend, J.; McCarthy, D.; Wu, S.Y. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch. Ophthalmol. 1993, 111, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Staurenghi, G.; Sadda, S.; Chakravarthy, U.; Spaide, R.F. International Nomenclature for Optical Coherence Tomography (IN•OCT) Panel. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: The IN•OCT consensus. Ophthalmology 2014, 121, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Guérin, C.J.; Lewis, G.P.; Fisher, S.K.; Anderson, D.H. Recovery of photoreceptor outer segment length and analysis of membrane assembly rates in regenerating primate photoreceptor outer segments. Investig. Ophthalmol. Vis. Sci. 1993, 34, 175–183. [Google Scholar]
- Faude, F.; Francke, M.; Makarov, F.; Schuck, J.; Gärtner, U.; Reichelt, W.; Wiedemann, P.; Wolburg, H.; Reichenbach, A. Experimental retinal detachment causes widespread and multilayered degeneration in rabbit retina. J. Neurocytol. 2001, 30, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.P.; Charteris, D.G.; Sethi, C.S.; Leitner, W.P.; Linberg, K.A.; Fisher, S.K. The ability of rapid retinal reattachment to stop or reverse the cellular and molecular events initiated by detachment. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2412–2420. [Google Scholar]
- Sakai, T.; Calderone, J.B.; Lewis, G.P.; Linberg, K.A.; Fisher, S.K.; Jacobs, G.H. Cone photoreceptor recovery after experimental detachment and reattachment: An immunocytochemical, morphological, and electrophysiological study. Investig. Ophthalmol. Vis. Sci. 2003, 44, 416–425. [Google Scholar] [CrossRef]
- Coblentz, F.E.; Radeke, M.J.; Lewis, G.P.; Fisher, S.K. Evidence that ganglion cells react to retinal detachment. Exp. Eye Res. 2003, 76, 333–342. [Google Scholar] [CrossRef]
- Cebulla, C.M.; Ruggeri, M.; Murray, T.G.; Feuer, W.J.; Hernandez, E. Spectral domain optical coherence tomography in a murine retinal detachment model. Exp. Eye Res. 2010, 90, 521–527. [Google Scholar] [CrossRef]
- Koutsandrea, C.; Kanakis, M.; Papaconstantinou, D.; Brouzas, D.; Ladas, I.; Petrou, P.; Georgalas, I. Scleral Buckling versus Vitrectomy for Retinal Detachment Repair: Comparison of Visual Fields and Nerve Fiber Layer Thickness. Ophthalmologica 2016, 235, 10–17. [Google Scholar] [CrossRef]
- Gass, C.A.; Haritoglou, C.; Messmer, E.M.; Schaumberger, M.; Kampik, A. Peripheral visual field defects after macular hole surgery: A complication with decreasing incidence. Br. J. Ophthalmol. 2001, 85, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Ning, M.; Semba, R.; Uji, Y.; Refojo, M.F. Histopathologic abnormalities in rabbit retina after intravitreous injection of expansive gases and air. Retina 2000, 20, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Kokame, G.T. Visual field defects after vitrectomy with fluid-air exchange. Am. J. Ophthalmol. 2000, 130, 653–654. [Google Scholar] [CrossRef]
- Welch, J.C. Dehydration injury as a possible cause of visual field defect after pars plana vitrectomy for macular hole. Am. J. Ophthalmol. 1997, 124, 698–699. [Google Scholar] [CrossRef]
- Skeie, J.M.; Roybal, C.N.; Mahajan, V.B. Proteomic insight into the molecular function of the vitreous. PLoS ONE 2015, 10, e0127567. [Google Scholar] [CrossRef]
- Murthy, K.R.; Goel, R.; Subbannayya, Y.; Jacob, H.K.C.; Murthy, P.R.; Manda, S.S.; Patil, A.H.; Sharma, R.; Sahasrabuddhe, N.A.; Parashar, A. Proteomic analysis of human vitreous humor. Clin. Proteom. 2014, 11, 29. [Google Scholar] [CrossRef]
- Skeie, J.M.; Mahajan, V.B. Proteomic interactions in the mouse vitreous-retina complex. PLoS ONE 2013, 8, e82140. [Google Scholar] [CrossRef]
- Dunker, S.; Sadun, A.A.; Sebag, J. Neuron specific enolase in retinal detachment. Curr. Eye Res. 2001, 23, 382–385. [Google Scholar] [CrossRef]
- Quintyn, J.C.; Pereira, F.; Hellot, M.F.; Brasseur, G.; Coquerel, A. Concentration of neuron-specific enolase and S100 protein in the subretinal fluid of rhegmatogenous retinal detachment. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 1167–1174. [Google Scholar] [CrossRef]
- Wert, K.J.; Velez, G.; Cross, M.R.; Wagner, B.A.; Teoh-Fitzgerald, M.L.; Buettner, G.R.; McAnany, J.J.; Olivier, A.; Tsang, S.H.; Harper, M.M.; et al. Extracellular superoxide dismutase (SOD3) regulates oxidative stress at the vitreoretinal interface. Free Radic. Biol. Med. 2018, 124, 408–419. [Google Scholar] [CrossRef]
- Kawano, H.; Ito, T.; Yamada, S.; Hashiguchi, T.; Maruyama, I.; Hisatomi, T.; Nakamura, M.; Sakamoto, T. Toxic effects of extracellular histones and their neutralization by vitreous in retinal detachment. Lab. Investig. 2014, 94, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Ankamah, E.; Sebag, J.; Ng, E.; Nolan, J.M. Vitreous Antioxidants, Degeneration, and Vitreo-Retinopathy: Exploring the Links. Antioxidants 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.M.; Yang, Y.S. A potential role of crystallin in the vitreous bodies of rats after ischemia-reperfusion injury. Korean J. Ophthalmol. 2012, 26, 248–254. [Google Scholar] [CrossRef]
- Sato, T.; Wakabayashi, T.; Shiraki, N.; Sakaguchi, H. Retinal thickness in parafoveal subfields and visual acuity after vitrectomy for macula-off rhegmatogenous retinal detachment repair. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Ctori, I.; Huntjens, B. Repeatability of Foveal Measurements Using Spectralisn Optical Coherence Tomography Segmentation Software. PLoS ONE 2015, 10, e0129005. [Google Scholar] [CrossRef] [PubMed]
| Scleral Buckling | Pars Plana Vitrectomy | p-Value | |||
|---|---|---|---|---|---|
| Treated Eyes | Fellow Eyes | Treated Eyes | Fellow Eyes | ||
| Age, years | 60.5 ± 11.9 | 62.0 ± 11.6 | 0.5 * | ||
| Sex (male/female) | 24/16 | 19/12 | 0.5 § | ||
| Axial length (mm) | 24.6 ± 0.7 | 24.5 ± 1.0 | 24.3 ± 0.8 | 24.1 ± 0.9 | 0.1 ** |
| Spherical equivalent (dioptres) | −1.1 ± 1.3 | −0.9 ± 1.4 | −0.8 ± 1.5 | −0.6 ± 1.2 | 0.4 ** |
| Lens status (phakic/pseudophakic) | 21/19 | 24/16 | 20/11 | 21/10 | 0.3 § |
| Duration of symptoms (days) | 7.5 ± 6.2 | 6.6 ± 4.4 | 0.5 ** | ||
| Preoperative BCVA (logMAR) | 1.28 ± 0.62 | 1.32 ± 0.67 | 0.8 ** | ||
| Retinal detachment extent >2 quadrants | 16/40 | 21/31 | 0.03 § | ||
| Cystic changes in the retinal layers | 32/40 | 26/31 | 0.8 § | ||
| IOP (mmHg) | 12.1 ± 2.3 | 13.0 ± 1.6 | 11.7 ± 2.1 | 13.3 ± 1.5 | 0.5 * |
| Scleral Buckling | p-Value * | Pars Plana Vitrectomy | p-Value * | p-Value | |||
|---|---|---|---|---|---|---|---|
| Treated Eye | Fellow Eye | Treated Eye | Fellow Eye | ||||
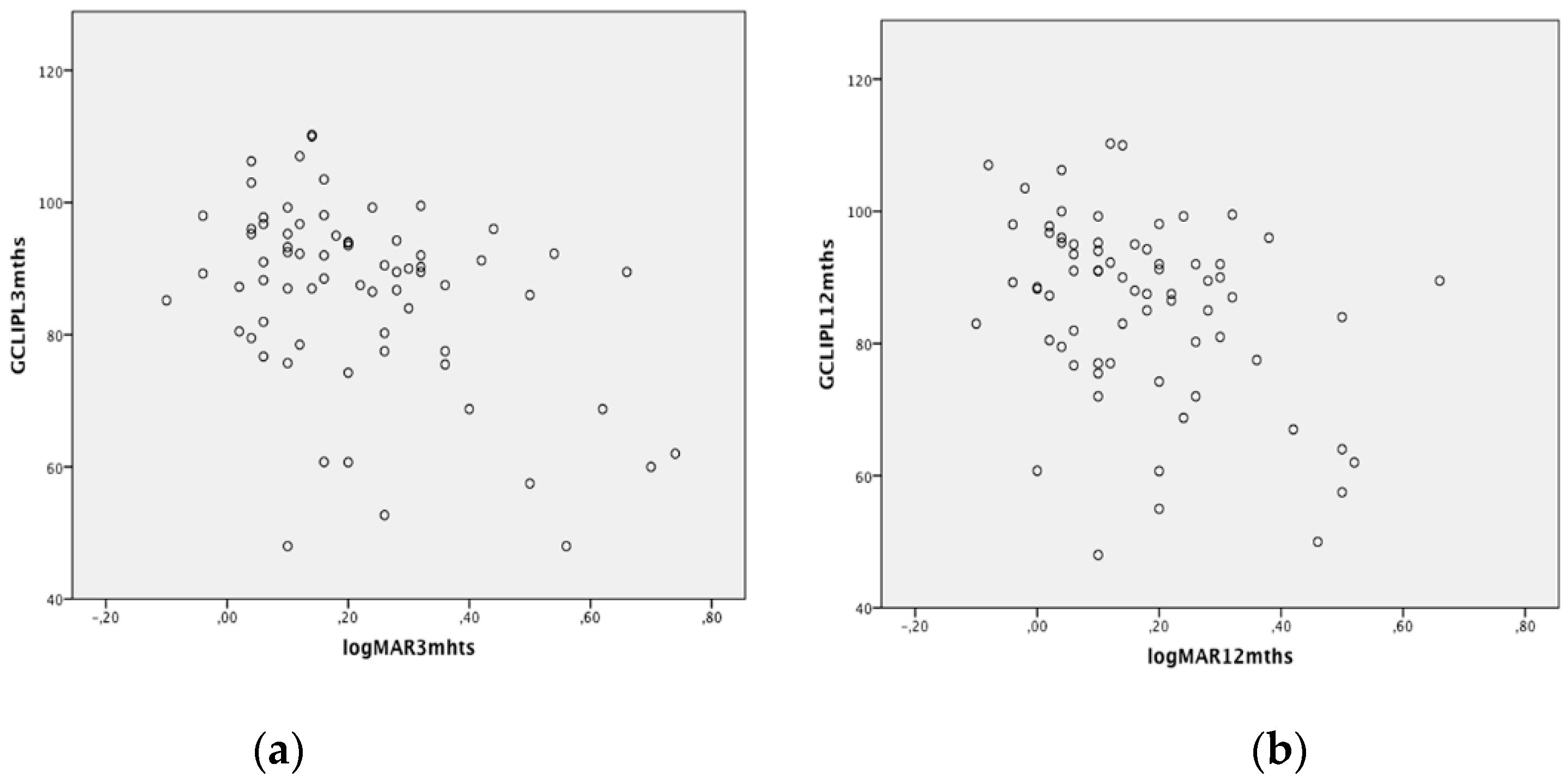
| 1-mm GCL-IPL at 3 months (microns) | 42.1 ± 10.6 | 40.0 ± 8.4 | 0.8 | 41.2 ± 11.4 | 40.7 ± 8.5 | 0.6 | F = 1.17 p = 0.9 ** |
| 3-mm GCL-IPL at 3 months (microns) | 91.8 ± 10.2 | 91.2 ± 8.4 | 0.3 | 79.5 ± 12.6 | 91.9 ± 8.7 | 0.005 | F = 11.45 p = 0.001 ** |
| 1-mm GCL-IPL at 12 months (microns) | 41.4 ± 11.0 | 41.0 ± 9.8 | 0.9 | 40.9 ± 11.7 | 41.2 ± 8.5 | 0.7 | F = 1.65 p = 0.4 ** |
| 3-mm GCL-IPL at 12 months (microns) | 92.2 ± 10.2 | 92.0 ± 8.8 | 0.5 | 77.7 ± 12.9 | 92.1 ± 8.8 | 0.003 | F = 12.37 p = 0.001 ** |
| logMAR BCVA at 3 months | 0.19 ± 0.14 | 0.25 ± 0.17 | p = 0.1 ° | ||||
| logMAR BCVA at 12 months | 0.14 ± 0.14 | 0.21 ± 0.19 | p = 0.08 ° | ||||
| IOP at 3 months (mmHg) | 13.2 ± 1.8 | 13.6 ± 1.5 | 0.3 § | ||||
| IOP at 12 months (mmHg) | 13.3 ± 1.9 | 13.9 ± 1.6 | 0.2 § | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharbiya, M.; Albanese, G.M.; Plateroti, A.M.; Marcelli, M.; Marenco, M.; Lambiase, A. Macular Ganglion Cell Layer Thickness after Macula-Off Rhegmatogenous Retinal Detachment Repair: Scleral Buckling versus Pars Plana Vitrectomy. J. Clin. Med. 2020, 9, 1411. https://doi.org/10.3390/jcm9051411
Gharbiya M, Albanese GM, Plateroti AM, Marcelli M, Marenco M, Lambiase A. Macular Ganglion Cell Layer Thickness after Macula-Off Rhegmatogenous Retinal Detachment Repair: Scleral Buckling versus Pars Plana Vitrectomy. Journal of Clinical Medicine. 2020; 9(5):1411. https://doi.org/10.3390/jcm9051411
Chicago/Turabian StyleGharbiya, Magda, Giuseppe Maria Albanese, Andrea Maria Plateroti, Michela Marcelli, Marco Marenco, and Alessandro Lambiase. 2020. "Macular Ganglion Cell Layer Thickness after Macula-Off Rhegmatogenous Retinal Detachment Repair: Scleral Buckling versus Pars Plana Vitrectomy" Journal of Clinical Medicine 9, no. 5: 1411. https://doi.org/10.3390/jcm9051411
APA StyleGharbiya, M., Albanese, G. M., Plateroti, A. M., Marcelli, M., Marenco, M., & Lambiase, A. (2020). Macular Ganglion Cell Layer Thickness after Macula-Off Rhegmatogenous Retinal Detachment Repair: Scleral Buckling versus Pars Plana Vitrectomy. Journal of Clinical Medicine, 9(5), 1411. https://doi.org/10.3390/jcm9051411