Metabolic Tumour Volume from PSMA PET/CT Scans of Prostate Cancer Patients during Chemotherapy—Do Different Software Solutions Deliver Comparable Results?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. PSMA PET/CT Imaging Protocol
2.3. Image Analysis
2.4. Clinical Response Criteria
2.5. Statistical Analysis
3. Results
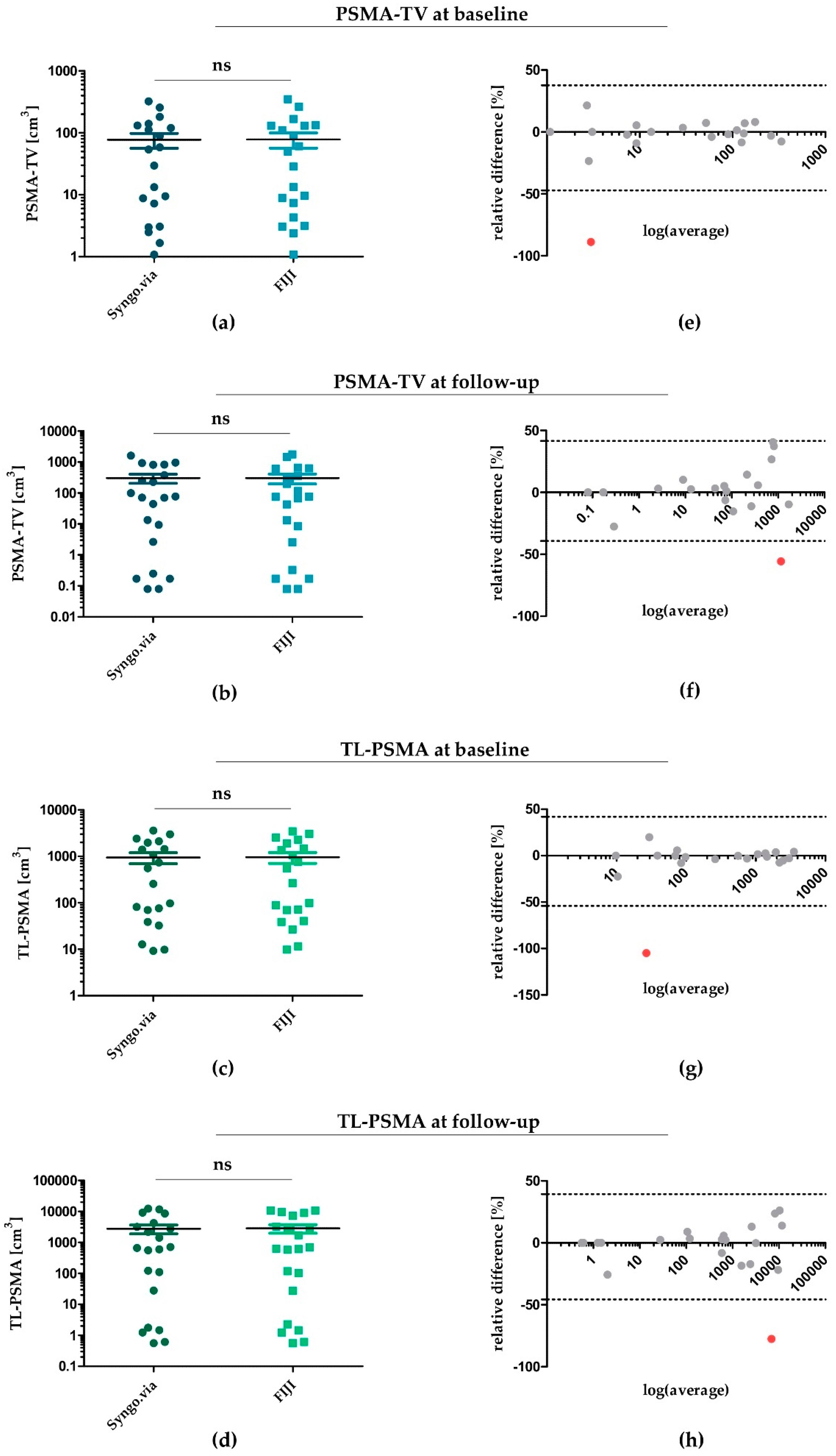
3.1. PSMA-TV and TL-PSMA during Chemotherapy—Determined by Syngo.via and FIJI
3.2. PSMA-TV and TL-PSMA as Imaging-Derived Biomarkers in CRPC
4. Discussion
4.1. Statistical Agreement of PSMA-TV and TL-PSMA Determined with Syngo.via and FIJI
4.2. Clinical Agreement of PSMA-TV and TL-PSMA Determined with Syngo.via and FIJI
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for research and treatment of cancer, national cancer institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50, 122S. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.H.; Kim, J.W.; Han, K.H.; Eo, J.S.; Kang, K.W.; Park, N.-H.; Song, Y.-S.; Chung, J.-K.; Kang, S.-B. Prognostic value of metabolic tumor volume measured by FDG-PET/CT in patients with cervical cancer. Gynecol. Oncol. 2011, 120, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Dibble, E.H.; Alvarez, A.C.L.; Truong, M.-T.; Mercier, G.; Cook, E.F.; Subramaniam, R.M. 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: Adding value to clinical staging. J. Nucl. Med. 2012, 53, 709–715. [Google Scholar] [CrossRef]
- Lim, R.; Eaton, A.; Lee, N.Y.; Setton, J.; Ohri, N.; Rao, S.; Wong, R.; Fury, M.; Schoder, H. 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J. Nucl. Med. 2012, 53, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kang, C.M.; Choi, H.J.; Lee, W.J.; Song, S.Y.; Lee, J.-H.; Lee, J.D. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J. Nucl. Med. 2014, 55, 898–904. [Google Scholar] [CrossRef]
- Camacho, M.R.; Etchebehere, E.; Tardelli, N.; Delamain, M.T.; Vercosa, A.F.A.; Takahashi, M.E.S.; Brunetto, S.Q.; Metze, I.G.H.L.; Souza, C.A.; Cerci, J.J.; et al. Validation of a multi-foci segmentation method for measuring metabolic tumor volume in hodgkin’s lymphoma. J. Nucl. Med. Technol. 2020, 48, 30–35. [Google Scholar] [CrossRef]
- Perrone, A.M.; Dondi, G.; Lima, G.M.; Castellucci, P.; Tesei, M.; Coluccelli, S.; Gasparre, G.; Porcelli, A.M.; Nanni, C.; Fanti, S.; et al. Potential prognostic role of 18F-FDG PET/CT in invasive epithelial ovarian cancer relapse. A preliminary study. Cancers 2019, 11, 713. [Google Scholar] [CrossRef]
- Bauckneht, M.; Capitanio, S.; Donegani, M.I.; Zanardi, E.; Miceli, A.; Murialdo, R.; Raffa, S.; Tomasello, L.; Vitti, M.; Cavo, A.; et al. Role of baseline and post-therapy 18F-FDG PET in the prognostic stratification of metastatic castration-resistant prostate cancer (mCRPC) patients treated with radium-223. Cancers 2019, 12, 31. [Google Scholar] [CrossRef]
- Schmuck, S.; von Klot, C.A.; Henkenberens, C.; Sohns, J.M.; Christiansen, H.; Wester, H.-J.; Ross, T.L.; Bengel, F.M.; Derlin, T. Initial experience with volumetric 68Ga-PSMA I&T PET/CT for assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with prostate cancer. J. Nucl. Med. 2017, 58, 1962–1968. [Google Scholar] [CrossRef]
- Brito, A.E.T.; Mourato, F.A.; de Oliveira, R.P.M.; Leal, A.L.G.; Filho, P.J.A.; de Filho, J.L.L. Evaluation of whole-body tumor burden with 68Ga-PSMA PET/CT in the biochemical recurrence of prostate cancer. Ann. Nucl. Med. 2019, 33, 344–350. [Google Scholar] [CrossRef]
- Acar, E.; Ozdogan, O.; Aksu, A.; Derebek, E.; Bekis, R.; Capa Kaya, G. The use of molecular volumetric parameters for the evaluation of Lu-177 PSMA I&T therapy response and survival. Ann. Nucl. Med. 2019, 33, 681–688. [Google Scholar] [CrossRef]
- Hammes, J.; Tager, P.; Drzezga, A. EBONI: A tool for automated quantification of bone metastasis load in PSMA PET/CT. J. Nucl. Med. 2018, 59, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Gafita, A.; Bieth, M.; Kronke, M.; Tetteh, G.; Navarro, F.; Wang, H.; Gunther, E.; Menze, B.; Weber, W.A.; Eiber, M. qPSMA: Semiautomatic software for whole-body tumor burden assessment in prostate cancer using 68Ga-PSMA11 PET/CT. J. Nucl. Med. 2019, 60, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): Proposed miTNM classification for the interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Seitz, A.K.; Rauscher, I.; Haller, B.; Kronke, M.; Luther, S.; Heck, M.M.; Horn, T.; Gschwend, J.E.; Schwaiger, M.; Eiber, M.; et al. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 602–612. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Jansen, B.H.E.; Cysouw, M.C.F.; Vis, A.N.; van Moorselaar, R.J.A.; Voortman, J.; Bodar, Y.J.L.; Schober, P.R.; Hendrikse, N.H.; Hoekstra, O.S.; Boellaard, R.; et al. Repeatability of quantitative 18F-DCFPyL PET/CT measurements in metastatic prostate cancer. J. Nucl. Med. 2020. [Google Scholar] [CrossRef]
- Gupta, M.; Choudhury, P.S.; Rawal, S.; Goel, H.C.; Rao, S.A. Evaluation of RECIST, PERCIST, EORTC, and MDA criteria for assessing treatment response with Ga68-PSMA PET-CT in metastatic prostate cancer patient with biochemical progression: A comparative study. Nucl. Med. Mol. Imaging 2018, 52, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Choudhury, P.S.; Rawal, S.; Goel, H.C.; Rao, S.A. Evaluation of response in patients of metastatic castration resistant prostate cancer undergoing systemic radiotherapy with lutetium177-prostate-specific membrane antigen: A comparison between response evaluation criteria in solid tumors, positron-emission tomography response criteria in solid tumors, European organization for research and treatment of cancer, and MDA criteria assessed by gallium 68-prostate-specific membrane antigen positron-emission tomography-computed tomography. Urol. Ann. 2019, 11, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kanoun, S.; Tal, I.; Berriolo-Riedinger, A.; Rossi, C.; Riedinger, J.-M.; Vrigneaud, J.-M.; Legrand, L.; Humbert, O.; Casasnovas, O.; Brunotte, F.; et al. Influence of software tool and methodological aspects of total metabolic tumor volume calculation on baseline 18FFDG PET to predict survival in hodgkin lymphoma. PLoS ONE 2015, 10, e0140830. [Google Scholar] [CrossRef] [PubMed]
- Schmidkonz, C.; Cordes, M.; Schmidt, D.; Bauerle, T.; Goetz, T.I.; Beck, M.; Prante, O.; Cavallaro, A.; Uder, M.; Wullich, B.; et al. 68Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | n = 21 |
|---|---|
| age [years] | |
| median (range) | 72 (55–93) |
| previous therapies [n] | |
| radical prostatectomy | 11 (52%) |
| radiotherapy | 13 (62%) |
| androgen deprivation therapy | 21 (100%) |
| brachytherapy | 1 (5%) |
| Abiraterone | 10 (48%) |
| Alpharadin | 2 (10%) |
| 177Lu-PSMA | 2 (10%) |
| Chemotherapy | |
| docetaxel [n] | 15 (71%) |
| cycles; median (range) | 6 (3–13) |
| <6 cycles [n] | 7 (32%) |
| cabazitaxel [n] | 7 (32%) |
| cycles; median (range) | 4 (2–8) |
| serum PSA baseline [ng/mL] | |
| median (range) | 15.0 (0–800) |
| Gleason score | |
| median (range) | 8 (6–10) |
| metastases localization [n] | |
| bone | 16 (76%) |
| lymph nodes | 18 (86%) |
| liver | 4 (19%) |
| lungs | 2 (10%) |
| local recurrence [n] | 4 (19%) |
| time between baseline PET/CT and PSA [d] | |
| median (range) | 13 (1–59) |
| time between follow-up PET/CT and PSA [d] | |
| median (range) | 12 (0–52) |
| time between end of chemotherapy and PET/CT [d] | |
| median (range) | 37 (11–120) |
| Complete Response CR | Partial Response PR | Stable Disease SD | Progressive Disease PD | |
|---|---|---|---|---|
| BR | PSA negative | PSA ≤ 50% | 50% < PSA < 125% | PSA ≥ 125% |
| PERCIST | no malignant PSMA uptake | SUVmax ≤ 70% | 70% < SUVmax < 130% | SUVmax ≥ 130% or new PSMA-lesion |
| PSMA-TV | no measurable PSMA-TV | PSMA-TV ≤ 70% | 70% < PSMA-TV < 130% | PSMA-TV ≥ 130% |
| TL-PSMA | no measurable TL-PSMA | TL-PSMA ≤ 70% | 70% < TL-PSMA < 130% | TL-PSMA ≥ 130% |
| PSA Baseline | PSA Follow-Up | rel. Δ PSA [%] | ||
|---|---|---|---|---|
| Syngo.via | ||||
| PSMA-TV baseline | 0.63 (p < 0.01) | |||
| PSMA-TV follow-up | 0.84 (p < 0.01) | |||
| ΔPSMA-TV [%] | 0.70 (p < 0.01) | |||
| TL-PSMA baseline | 0.55 (p = 0.019) | |||
| TL-PSMA follow-up | 0.80 (p < 0.01) | |||
| ΔTL-PSMA [%] | 0.67 (p < 0.01) | |||
| FIJI | ||||
| PSMA-TV baseline | 0.64 (p < 0.01) | |||
| PSMA-TV follow-up | 0.86 (p < 0.01) | |||
| ΔPSMA-TV [%] | 0.67 (p < 0.01) | |||
| TL-PSMA baseline | 0.56 (p = 0.016) | |||
| TL-PSMA follow-up | 0.84 (p < 0.01) | |||
| ΔTL-PSMA [%] | 0.69 (p < 0.01) |
| Patient | PERCIST | BR | ΔPSMA-TV | ΔTL-PSMA | ΔPSMA-TV | ΔTL-PSMA |
|---|---|---|---|---|---|---|
| Syngo.via | Syngo.via | FIJI | FIJI | |||
| #1 | SD | SD | PR | PR | PR | PR |
| #2 | PD 1 | PD | PD | PD | PD | |
| #3 | PR | PR | PR | PR | PR | PR |
| #4 | PD 1 | PD | PR | PR | PR | PR |
| #5 | PR | PR | PR | PR | PR | PR |
| #6 | PD 1 | PD | PD | PD | PD | PD |
| #7 | PR | PR | PR | PR | PR | PR |
| #8 | PD 1 | PD | SD | SD | SD | SD |
| #9 | PD | SD | SD | SD | SD | SD |
| #10 | PD | SD | PD | PD | PD | PD |
| #11 | PD | PD | PD | PD | PD | PD |
| #12 | PD 1 | PD | PD | PD | PD | PD |
| #13 | PD 1 | PD | PD | PD | PD | PD |
| #14 | PD 1 | PD | ||||
| #15 | PD | PR | PD | PD | PD | PD |
| #16 | PD 1 | PR | PR | PR | PR | PR |
| #17 | PD 1 | PR | PR | PR | PR | |
| #18 | PR | PD | SD | SD | SD | SD |
| #19 | PD 1 | PD | PD | PD | PD | PD |
| #20 | PD | PD | PD | PD | PD | PD |
| #21 | PD 1 | PD | SD | SD | SD | SD |
| Patient | BR | ΔPSMA-TV | ΔPSA [%] | ΔPSMA-TV [%] | OS [d] | Death |
|---|---|---|---|---|---|---|
| #4 | PD | PR | +238% | −76% | 216 | Yes |
| #8 | PD | SD | +786% | −21% | 235 | Yes |
| #10 | SD | PD | +9% | +42% | 1169 | No |
| #15 | PR | PD | −99% | +213% | 720 | No |
| #18 | PD | SD | +291% | −14% | 328 | Yes |
| #21 | PD | SD | +66% | +26% | 1556 | No |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartrampf, P.E.; Heinrich, M.; Seitz, A.K.; Brumberg, J.; Sokolakis, I.; Kalogirou, C.; Schirbel, A.; Kübler, H.; Buck, A.K.; Lapa, C.; et al. Metabolic Tumour Volume from PSMA PET/CT Scans of Prostate Cancer Patients during Chemotherapy—Do Different Software Solutions Deliver Comparable Results? J. Clin. Med. 2020, 9, 1390. https://doi.org/10.3390/jcm9051390
Hartrampf PE, Heinrich M, Seitz AK, Brumberg J, Sokolakis I, Kalogirou C, Schirbel A, Kübler H, Buck AK, Lapa C, et al. Metabolic Tumour Volume from PSMA PET/CT Scans of Prostate Cancer Patients during Chemotherapy—Do Different Software Solutions Deliver Comparable Results? Journal of Clinical Medicine. 2020; 9(5):1390. https://doi.org/10.3390/jcm9051390
Chicago/Turabian StyleHartrampf, Philipp E., Marieke Heinrich, Anna Katharina Seitz, Joachim Brumberg, Ioannis Sokolakis, Charis Kalogirou, Andreas Schirbel, Hubert Kübler, Andreas K. Buck, Constantin Lapa, and et al. 2020. "Metabolic Tumour Volume from PSMA PET/CT Scans of Prostate Cancer Patients during Chemotherapy—Do Different Software Solutions Deliver Comparable Results?" Journal of Clinical Medicine 9, no. 5: 1390. https://doi.org/10.3390/jcm9051390
APA StyleHartrampf, P. E., Heinrich, M., Seitz, A. K., Brumberg, J., Sokolakis, I., Kalogirou, C., Schirbel, A., Kübler, H., Buck, A. K., Lapa, C., & Krebs, M. (2020). Metabolic Tumour Volume from PSMA PET/CT Scans of Prostate Cancer Patients during Chemotherapy—Do Different Software Solutions Deliver Comparable Results? Journal of Clinical Medicine, 9(5), 1390. https://doi.org/10.3390/jcm9051390





