Long-Term Survival Following Surgical Ablation for Atrial Fibrillation Concomitant to Isolated and Combined Coronary Artery Bypass Surgery—Analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK)
Abstract
1. Introduction
2. Experimental Section
2.1. Registry Design
2.2. Data Collection
2.3. Study Population and Clinical Variables
2.4. Statistical Analysis
3. Results
3.1. Operative and Long-Term Data
3.2. Propensity Score Analysis
3.3. Sensitivity and Subgroup Analyses
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Badhwar, V.; Rankin, J.S.; Damiano, R.J., Jr.; Gillinov, A.M.; Bakaeen, F.G.; Edgerton, J.R.; Philpott, J.M.; McCarthy, P.M.; Bolling, S.F.; Roberts, H.G.; et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann. Thorac. Surg. 2017, 103, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, V.; Rankin, J.S.; Ad, N.; Grau-Sepulveda, M.; Damiano, R.J.; Gillinov, A.M.; McCarthy, P.M.; Thourani, V.H.; Suri, R.M.; Jacobs, J.P.; et al. Surgical Ablation of Atrial Fibrillation in the United States: Trends and Propensity Matched Outcomes. Ann. Thorac. Surg. 2017, 104, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Gammie, J.S.; Haddad, M.; Milford-Beland, S.; Welke, K.F.; Ferguson, T.B., Jr.; O’Brien, S.M.; Griffith, B.P.; Peterson, E.D. Atrial fibrillation correction surgery: Lessons from the Society of Thoracic Surgeons National Cardiac Database. Ann. Thorac. Surg. 2008, 85, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Kuck, K.H.; Cappato, R.; Brugada, J.; Camm, A.J.; Chen, S.A.; Crijns, H.J.; Damiano, R.J.; Davies, D.W.; DiMarco, J. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace: European pacing, arrhythmias, and cardiac electrophysiology. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2012, 14, 528–606. [Google Scholar] [CrossRef]
- Quader, M.A.; McCarthy, P.M.; Gillinov, A.M.; Alster, J.M.; Cosgrove, D.M., 3rd; Lytle, B.W.; Blackstone, E.H. Does preoperative atrial fibrillation reduce survival after coronary artery bypass grafting? Ann. Thorac. Surg. 2004, 77, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Doukas, G.; Samani, N.J.; Alexiou, C.; Oc, M.; Chin, D.T.; Stafford, P.G.; Ng, L.L.; Spyt, T.J. Left atrial radiofrequency ablation during mitral valve surgery for continuous atrial fibrillation: A randomized controlled trial. Jama 2005, 294, 2323–2329. [Google Scholar] [CrossRef] [PubMed]
- Suwalski, P.; Kowalewski, M.; Jasinski, M.; Staromlynski, J.; Zembala, M.; Widenka, K.; Brykczynski, M.; Skiba, J.; Zembala, M.O.; Bartus, K.; et al. Survival after surgical ablation for atrial fibrillation in mitral valve surgery: Analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK). J. Thorac. Cardiovasc. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardio Thorac. Surg. Off. J. Eur. Assoc. Cardio Thorac. Surg. 2012, 41, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating; Springer: New York, NY, USA, 2009. [Google Scholar]
- Ad, N.; Damiano, R.J., Jr.; Badhwar, V.; Calkins, H.; La Meir, M.; Nitta, T.; Doll, N.; Holmes, S.D.; Weinstein, A.A.; Gillinov, M. Expert consensus guidelines: Examining surgical ablation for atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2017, 153, 1330–1354. [Google Scholar] [CrossRef] [PubMed]
- Blomstrom-Lundqvist, C.; Johansson, B.; Berglin, E.; Nilsson, L.; Jensen, S.M.; Thelin, S.; Holmgren, A.; Edvardsson, N.; Kallner, G.; Blomstrom, P. A randomized double-blind study of epicardial left atrial cryoablation for permanent atrial fibrillation in patients undergoing mitral valve surgery: The Swedish Multicentre Atrial Fibrillation study (SWEDMAF). Eur. Heart J. 2007, 28, 2902–2908. [Google Scholar] [CrossRef] [PubMed]
- Lawrance, C.P.; Henn, M.C.; Damiano, R.J., Jr. Concomitant Cox-Maze IV techniques during mitral valve surgery. Ann. Cardiothorac. Surg. 2015, 4, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Budera, P.; Straka, Z.; Osmancik, P.; Vanek, T.; Jelinek, S.; Hlavicka, J.; Fojt, R.; Cervinka, P.; Hulman, M.; Smid, M.; et al. Comparison of cardiac surgery with left atrial surgical ablation vs. cardiac surgery without atrial ablation in patients with coronary and/or valvular heart disease plus atrial fibrillation: Final results of the PRAGUE-12 randomized multicentre study. Eur. Heart J. 2012, 33, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Pokushalov, E.; Romanov, A.; Corbucci, G.; Cherniavsky, A.; Karaskov, A. Benefit of ablation of first diagnosed paroxysmal atrial fibrillation during coronary artery bypass grafting: A pilot study. European journal of cardio-thoracic surgery. Off. J. Eur. Assoc. Cardio Thorac. Surg. 2012, 41, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Cherniavsky, A.; Kareva, Y.; Pak, I.; Rakhmonov, S.; Pokushalov, E.; Romanov, A.; Karaskov, A. Assessment of results of surgical treatment for persistent atrial fibrillation during coronary artery bypass grafting using implantable loop recorders. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Rankin, J.S.; Lerner, D.J.; Braid-Forbes, M.J.; Ferguson, M.A.; Badhwar, V. One-year mortality and costs associated with surgical ablation for atrial fibrillation concomitant to coronary artery bypass grafting. European journal of cardio-thoracic surgery. Off. J. Eur. Assoc. Cardio Thorac. Surg. 2017, 52, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Suwalski, P.; Kowalewski, M.; Jasinski, M.; Staromlynski, J.; Zembala, M.; Widenka, K.; Brykczynski, M.; Skiba, J.; Zembala, M.O.; Bartus, K.; et al. Surgical ablation for atrial fibrillation during isolated coronary artery bypass surgery. Eur. J. Cardio Thorac. Surg. Off. J. Eur. Assoc. Cardio Thorac. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Iribarne, A.; Goodney, P.P.; Flores, A.M.; DeSimone, J.; DiScipio, A.W.; Austin, A.; McCullough, J.N. National Trends and Geographic Variation in Bilateral Internal Mammary Artery Use in the United States. Ann. Thorac. Surg. 2017, 104, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, U.; Gaudino, M.; Di Franco, A.; Caputo, M.; Ohmes, L.B.; Grau, J.; Glineur, D.; Girardi, L.N.; Angelini, G.D. Incomplete revascularization and long-term survival after coronary artery bypass surgery. Int. J. Cardiol. 2018, 254, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Madershahian, N.; Scherner, M.; Weber, C.; Kuhn, E.; Choi, Y.H.; Slottosch, I.; Wahlers, T. Temporary biventricular pacing improves bypass graft flows in coronary artery bypass graft patients with permanent atrial fibrillation. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, M.; Jasiński, M.; Staromłyński, J.; Zembala, M.; Widenka, K.; Brykczyński, M.; Skiba, J.; Zembala, M.O.; Bartuś, K.; Hirnle, T.; et al. On-Pump vs Off-Pump Coronary Artery Bypass Surgery in Atrial Fibrillation. Analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK). PLoS ONE 2020, 15, e0231950. [Google Scholar] [CrossRef] [PubMed]
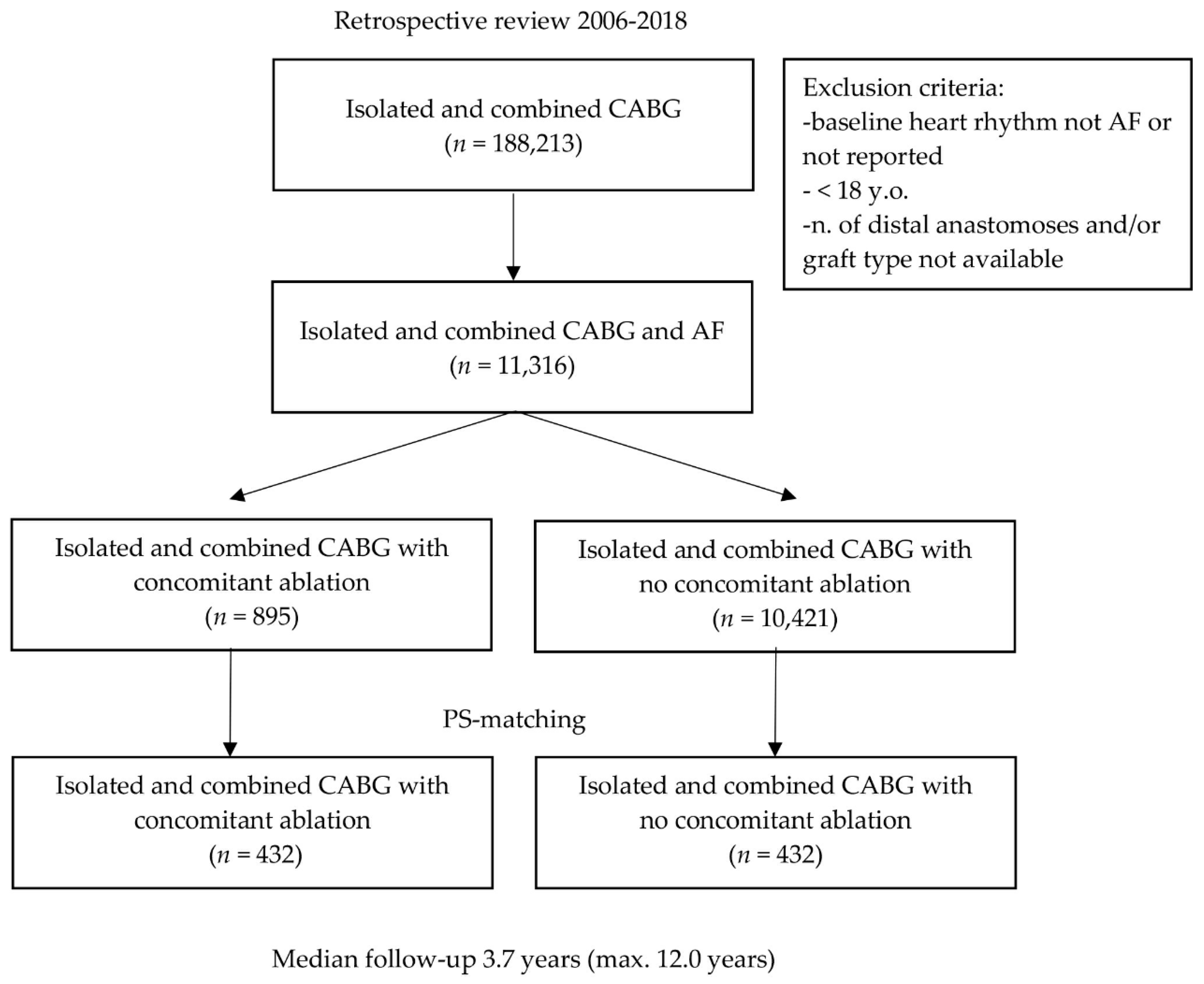
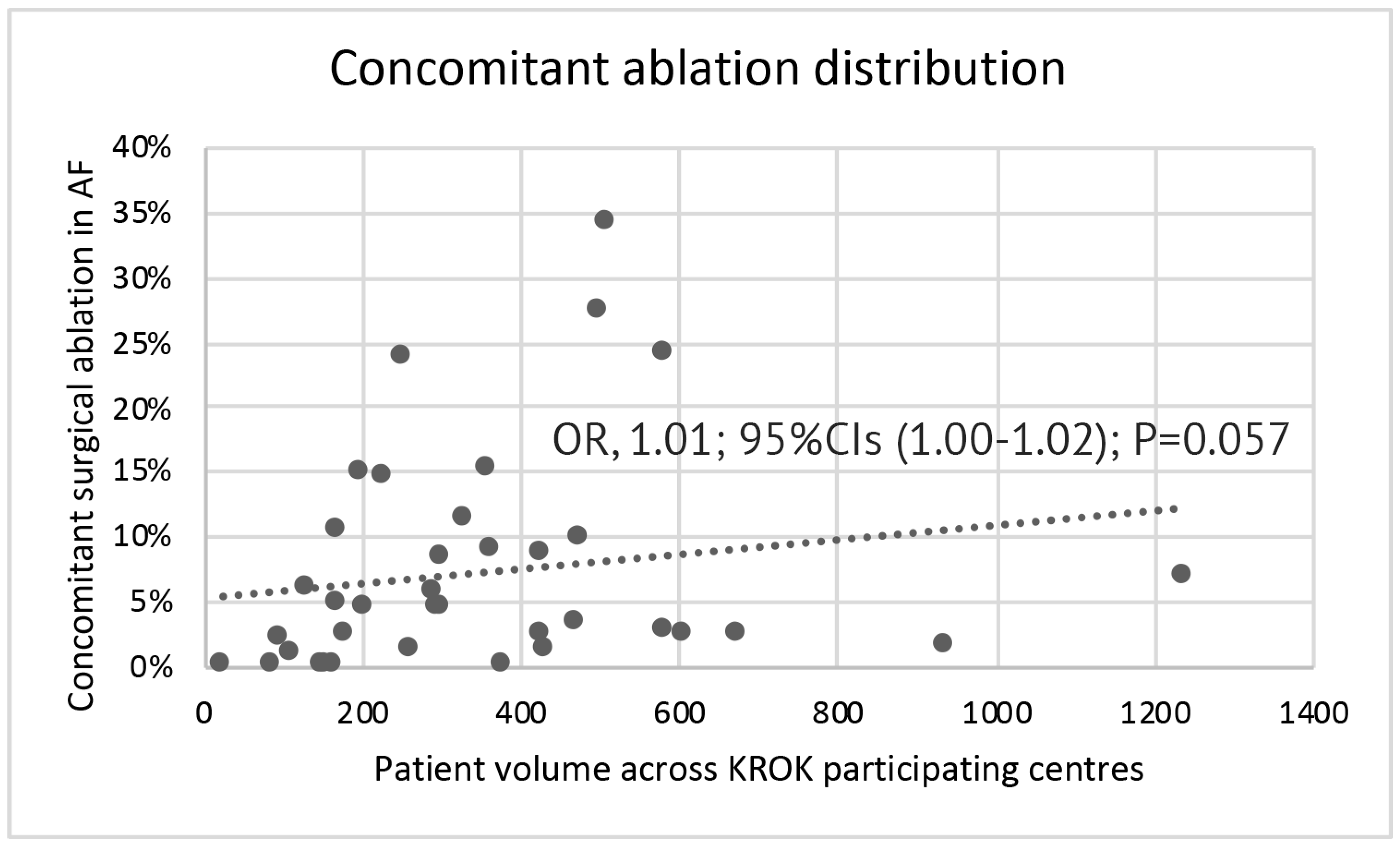
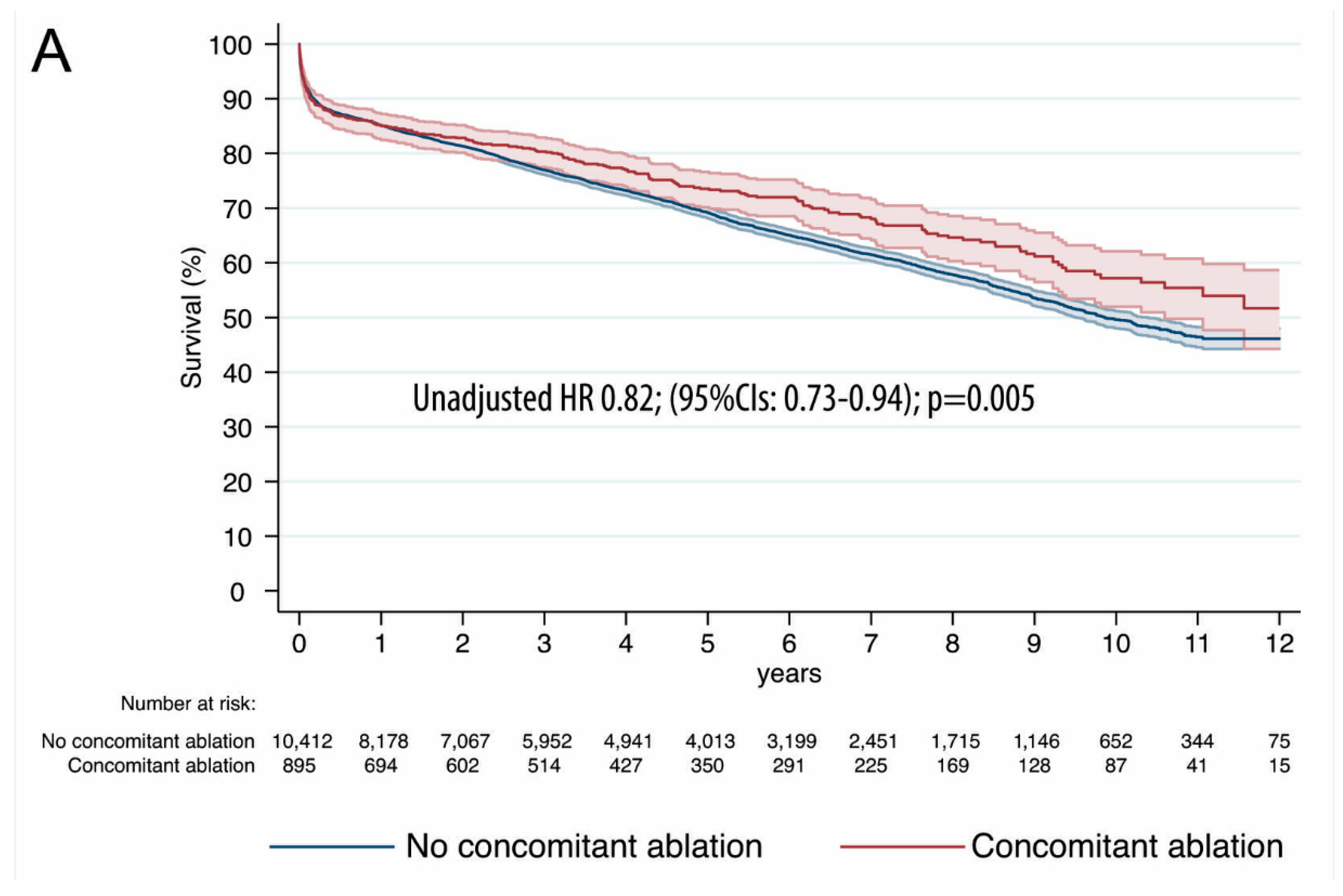
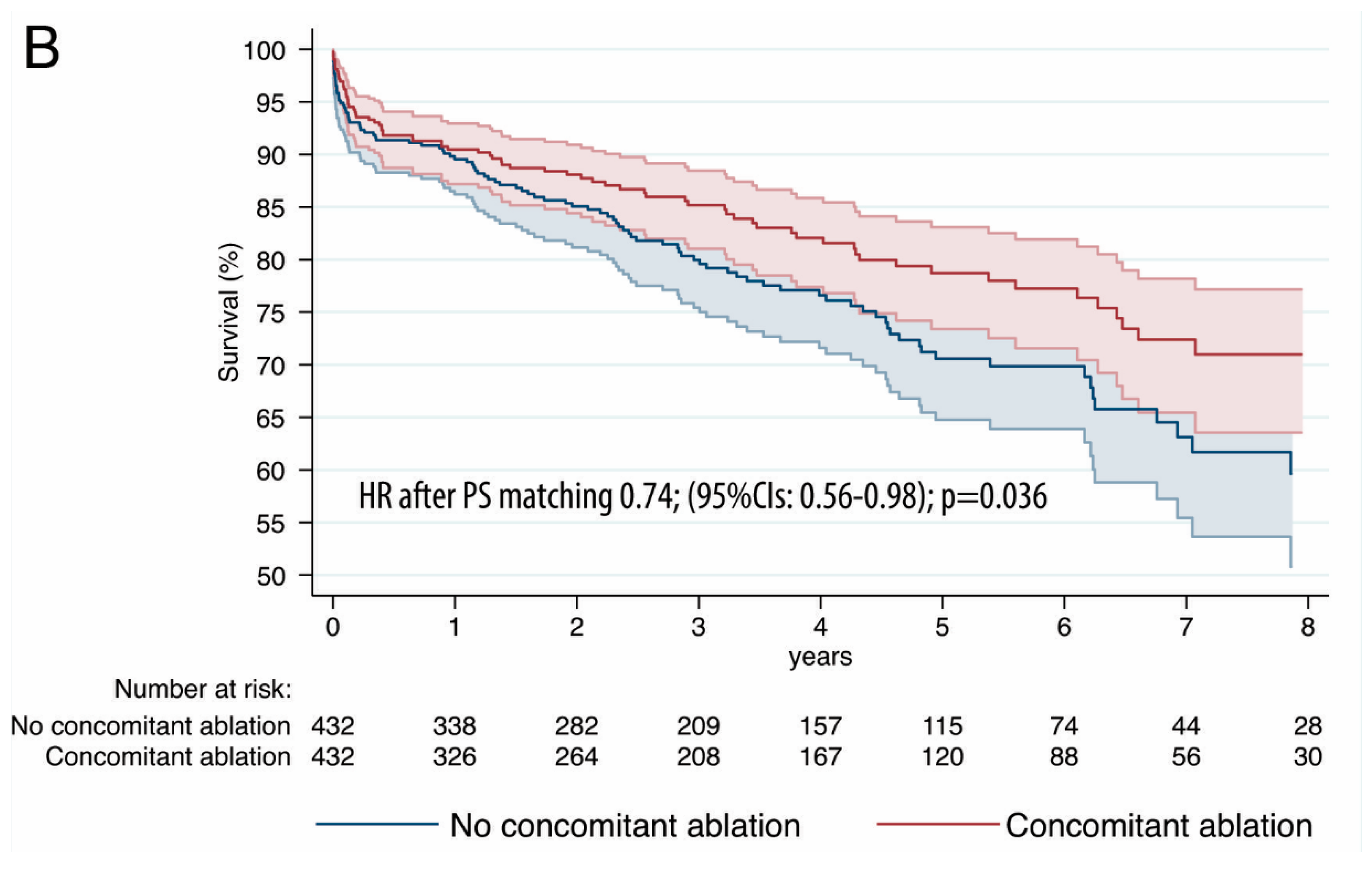
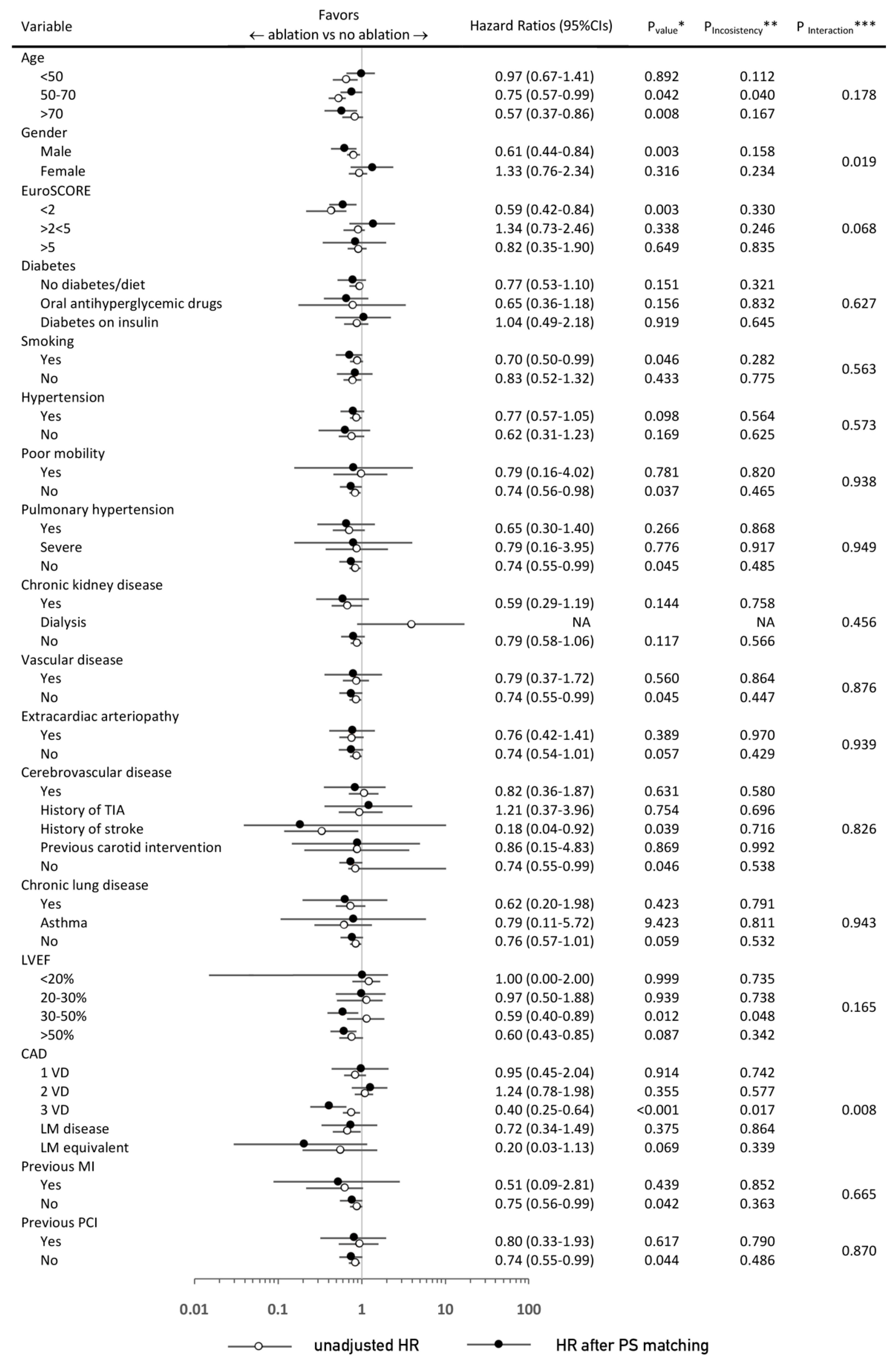
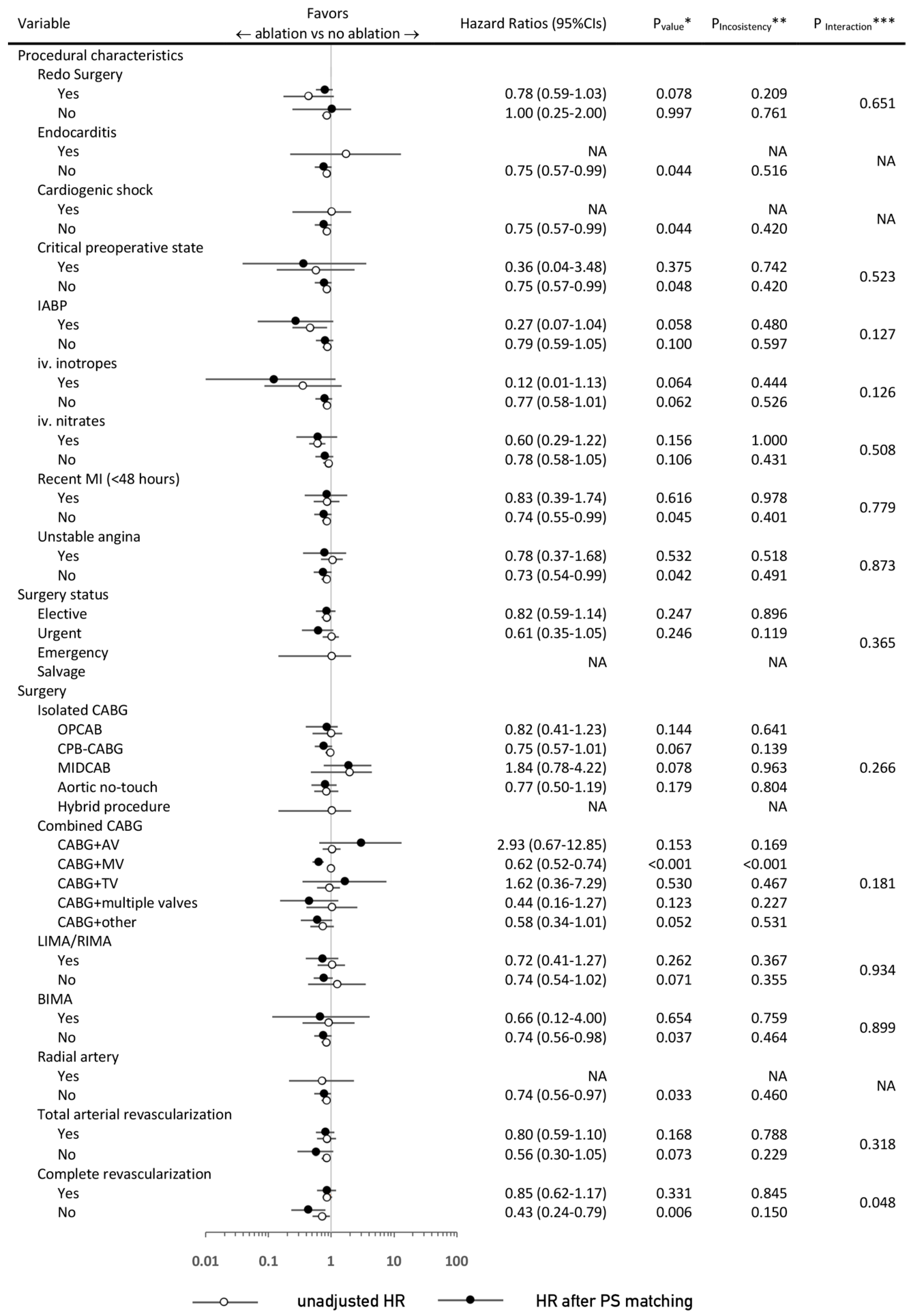
| Variable | PS-Matched Patients | |||
|---|---|---|---|---|
| Total (864) | Concomitant Ablation (432) | No Concomitant Ablation (432) | p-Value | |
| Age years (median (IQR)) | 68.6 (63.19–73.36) | 68.6 (63.2–73.6) | 68.5 (63.1–73.11) | 0.714 |
| <50 | 10 (1.2%) | 5 (1.2%) | 5 (1.2%) | 1.000 |
| 50–70 | 487 (56.4%) | 246 (56.9%) | 241 (55.8%) | 0.732 |
| >70 | 367 (42.5%) | 181 (41.9%) | 186 (43.1%) | 0.731 |
| Gender | ||||
| Male | 657 (76.0%) | 328 (75.9%) | 329 (76.2%) | 0.937 |
| Female | 207 (24.0%) | 104 (24.1%) | 103 (23.8%) | |
| EuroSCORE (median (IQR)) | 1.32 (0.89–2.72) | 1.38 (0.90–2.97) | 1.28 (0.88–2.48) | 0.092 |
| <2 | 568 (65.7%) | 272 (63.0%) | 296 (68.5%) | 0.086 |
| 2–5 | 204 (23.6%) | 110 (25.5%) | 94 (21.8%) | 0.201 |
| >5 | 92 (10.6%) | 50 (11.6%) | 42 (9.7%) | 0.378 |
| Diabetes | 330 (38.2%) | 154 (35.6%) | 176 (40.7%) | 0.124 |
| Insulin dependent | 105 (12.2%) | 46 (10.6%) | 59 (13.7%) | 0.177 |
| Smoking | 538 (62.3%) | 261 (60.4%) | 277 (64.1%) | 0.261 |
| Hypertension | 752 (87.0%) | 377 (87.3%) | 375 (86.8%) | 0.839 |
| Hyperlipidemia | 535 (61.9%) | 259 (60.0%) | 276 (63.9%) | 0.234 |
| Poor mobility | 46 (5.3%) | 20 (4.6%) | 26 (6.0%) | 0.365 |
| BMI (median (IQR)) | 28.25 (25.46–31.23) | 28.08 (23.32–30.92) | 28.38 (25.57–31.57) | 0.919 |
| Pulmonary hypertension | 113 (13.1%) | 50 (11.6%) | 63 (14.6%) | 0.190 |
| Severe (PA systolic > 55 mmHg) | 19 (2.2%) | 10 (2.3%) | 9 (2.1%) | 0.817 |
| Renal impairment | 309 (35.8%) | 152 (35.2%) | 157 (36.3%) | 0.723 |
| moderate (CC > 50 & <85) | 229 (26.5%) | 117 (27.1%) | 112 (25.9%) | 0.700 |
| severe (CC < 50) | 80 (9.3%) | 35 (8.1%) | 45 (10.4%) | 0.241 |
| dialysis (regardless of CC) | 4 (0.5%) | 2 (0.5%) | 2 (0.5%) | 1.000 |
| Peripheral artery disease | 119 (13.8%) | 57 (13.2%) | 62 (14.4%) | 0.622 |
| Cerebrovascular disease | 81 (9.4%) | 35 (8.1%) | 46 (10.6%) | 0.201 |
| Stroke | 30 (3.5%) | 16 (3.7%) | 14 (3.2%) | 0.710 |
| TIA | 207 (24.0%) | 104 (24.1%) | 103 (23.8%) | 0.936 |
| Carotid intervention | 9 (1.0%) | 3 (0.7%) | 6 (1.4%) | 0.325 |
| Chronic lung disease | 63 (7.3%) | 32 (7.4%) | 31 (7.2%) | 0.896 |
| Asthma | 26 (3.0%) | 12 (2.8%) | 14 (3.2%) | 0.691 |
| LVEF (%) (median (IQR)) * | 50.0 (40.0–55.0) | 50.0 (40.0–57.0) | 49.0 (40.0–55.0) | 0.642 |
| <20% | 31 (3.6%) | 13 (3.1%) | 18 (4.2%) | 0.129 |
| 21–30% | 107 (12.4%) | 60 (13.9%) | 47 (10.9%) | 0.180 |
| 31–50% | 421 (48.7%) | 198 (45.8%) | 223 (51.6%) | 0.088 |
| >50% | 305 (35.3%) | 161 (37.2%) | 144 (33.3%) | 0.226 |
| CAD | ||||
| 1 VD | 200 (23.1%) | 97 (22.5%) | 103 (23.8%) | 0.062 |
| 2 VD | 357 (41.3%) | 181 (41.9%) | 176 (40.7%) | 0.713 |
| 3 VD | 307 (35.5%) | 154 (35.6%) | 153 (35.4%) | 0.943 |
| LM disease | 115 (13.3%) | 58 (13.4%) | 57 (13.2%) | 0.902 |
| Previous MI | 30 (3.5%) | 16 (3.7%) | 14 (3.2%) | 0.710 |
| Previous PCI | 100 (11.6%) | 54 (12.5%) | 46 (10.6%) | 0.396 |
| Variable | PS-Matched Patients | |||
|---|---|---|---|---|
| Total (864) | Concomitant Ablation (432) | No Concomitant Ablation (432) | p-Value | |
| Procedural Characteristics | ||||
| Redo surgery | 18 (2.1%) | 9 (2.1%) | 9 (2.1%) | 1.000 |
| Endocarditis | 2 (0.2%) | 1 (0.2%) | 1 (0.2%) | 1.000 |
| Cardiogenic chock | 2 (0.2%) | 1 (0.2%) | 1 (0.2%) | 1.000 |
| Critical preoperative state | 10 (1.2%) | 4 (0.9%) | 6 (1.4%) | 0.528 |
| IABP | 5 (0.6%) | 2 (0.5%) | 3 (0.7%) | 0.656 |
| iv. inotropes | 10 (1.2%) | 5 (1.2%) | 5 (1.2%) | 1.000 |
| iv. nitrates | 116 (13.4%) | 57 (13.2%) | 59 (13.7%) | 0.842 |
| Urgency | ||||
| Elective | 633 (73.3%) | 316 (73.1%) | 317 (73.4%) | 0.939 |
| Urgent | 216 (25.0%) | 109 (25.2%) | 107 (24.8%) | 0.875 |
| Emergency | 12 (1.4%) | 7 (1.6%) | 5 (1.2%) | 0.563 |
| Salvage | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Surgery | ||||
| Isolated CABG | 644 (74.5%) | 322 (75.5%) | 322 (74.5%) | 1.000 |
| OPCAB * | 347 (40.1%) | 170 (39.4%) | 177 (41.0%) | 0.627 |
| CPB-CABG * | 297 (34.4%) | 152 (35.2%) | 145 (33.6%) | 0.616 |
| MIDCAB | 26 (3.0%) | 13 (3.0%) | 13 (3.0%) | 1.000 |
| Aortic no-touch | 92 (10.6%) | 44 (10.2%) | 48 (11.1%) | 0.659 |
| Hybrid procedure | 1 (0.1%) | 1 (0.2%) | 0 (0.0%) | 0.501 |
| Combined CABG | 220 (25.5%) | 110 (25.5%) | 110 (25.5%) | 1.000 |
| CABG + MV | 74 (8.6%) | 35 (8.1%) | 39 (9.0%) | 0.496 |
| CABG + AV | 55 (6.4%) | 29 (6.7%) | 26 (6.0%) | 0.676 |
| CABG + TV | 38 (4.4%) | 17 (3.9%) | 21 (4.9%) | 0.508 |
| CABG + multiple valves | 23 (2.7%) | 10 (2.3%) | 13 (3.0%) | 0.527 |
| CABG + other | 30 (3.5%) | 19 (4.4%) | 11 (2.5%) | 0.141 |
| Grafts and Anastomoses | ||||
| LIMA | 638 (73.8%) | 317 (73.4%) | 321 (74.3%) | 0.757 |
| RIMA | 22 (2.5%) | 9 (2.1%) | 13 (3.0%) | 0.391 |
| BIMA | 19 (2.2%) | 8 (1.9%) | 11 (2.5%) | 0.488 |
| Pedicled IMA * | 438 (50.7%) | 218 (50.5%) | 220 (50.9%) | 0.890 |
| Skeletonized IMA * | 221 (25.6%) | 112 (25.9%) | 109 (25.2%) | 0.815 |
| Radial artery | 22 (2.5%) | 8 (1.9%) | 14 (3.2%) | 0.201 |
| Arterial anastomoses | 282 (40.0%) | 145 (40.8%) | 137 (39.1%) | 0.645 |
| Venous anastomoses | 423 (60.0%) | 210 (59.2%) | 213 (60.9%) | 0.645 |
| Sequential anastomoses | 69 (9.8%) | 38 (10.7%) | 31 (8.9%) | 0.409 |
| Composite anastomoses | 61 (7.1%) | 27 (6.3%) | 34 (7.9%) | 0.353 |
| Total arterial revascularization | 181 (20.9%) | 80 (18.5%) | 101 (23.4%) | 0.080 |
| Completeness of revascularization | 708 (81.9%) | 352 (81.5%) | 356 (82.4%) | 0.724 |
| PS-Matched Patients | ||||
|---|---|---|---|---|
| Concomitant Ablation (432) | No Concomitant Ablation (432) | Risk Ratio (95% CIs) | p-Value | |
| Early postoperative mortality | 4 (0.9%) | 0 (0.0%) | 9.00 (0.49–166.65) | 0.140 |
| 30-day mortality | 21 (4.9%) | 31 (7.2%) | 0.67 (0.40–1.16) | 0.156 |
| Cardiac tamponade and/or rethoracotomy | 35 (7.5%) | 30 (8.1%) | 1.17 (0.73–1.87) | 0.519 |
| Periprocedural MI | 5 (1.2%) | 3 (0.7%) | 1.67 (0.40–6.93) | 0.482 |
| Respiratory failure | 35 (8.1%) | 20 (4.6%) | 1.75 (1.03–2.98) | 0.040 |
| Prolonged ICU stay | 5 (1.2%) | 8 (1.9%) | 0.63 (0.21–1.90) | 0.406 |
| Neurologic complications | 12 (2.8%) | 9 (2.1%) | 1.33 (0.57–3.13) | 0.509 |
| Pulmonary embolism | 3 (0.7%) | 0 (0.0%) | 7.00 (0.36–135.11) | 0.198 |
| Multiogran failure | 10 (2.3%) | 7 (1.6%) | 1.43 (0.55–3.72) | 0.465 |
| Gastrointestinal complications | 5 (1.2%) | 6 (1.4%) | 0.83 (0.26–2.71) | 0.762 |
| Acute kidney failure | 1 (0.2%) | 0 (0.0%) | 3.00 (0.12–73.44) | 0.501 |
| Sternal wound infection | 13 (3.0%) | 15 (3.5%) | 0.87 (0.42–1.80) | 0.701 |
| ECMO | 1 (0.2%) | 0 (0.0%) | 3.00 (0.12–73.44) | 0.501 |
| VAD | 1 (0.2%) | 0 (0.0%) | 3.00 (0.12–73.44) | 0.501 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalewski, M.; Jasiński, M.; Staromłyński, J.; Zembala, M.; Widenka, K.; Zembala, M.O.; Bartuś, K.; Hirnle, T.; Dziembowska, I.; Knapik, P.; et al. Long-Term Survival Following Surgical Ablation for Atrial Fibrillation Concomitant to Isolated and Combined Coronary Artery Bypass Surgery—Analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK). J. Clin. Med. 2020, 9, 1345. https://doi.org/10.3390/jcm9051345
Kowalewski M, Jasiński M, Staromłyński J, Zembala M, Widenka K, Zembala MO, Bartuś K, Hirnle T, Dziembowska I, Knapik P, et al. Long-Term Survival Following Surgical Ablation for Atrial Fibrillation Concomitant to Isolated and Combined Coronary Artery Bypass Surgery—Analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK). Journal of Clinical Medicine. 2020; 9(5):1345. https://doi.org/10.3390/jcm9051345
Chicago/Turabian StyleKowalewski, Mariusz, Marek Jasiński, Jakub Staromłyński, Marian Zembala, Kazimierz Widenka, Michał Oskar Zembala, Krzysztof Bartuś, Tomasz Hirnle, Inga Dziembowska, Piotr Knapik, and et al. 2020. "Long-Term Survival Following Surgical Ablation for Atrial Fibrillation Concomitant to Isolated and Combined Coronary Artery Bypass Surgery—Analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK)" Journal of Clinical Medicine 9, no. 5: 1345. https://doi.org/10.3390/jcm9051345
APA StyleKowalewski, M., Jasiński, M., Staromłyński, J., Zembala, M., Widenka, K., Zembala, M. O., Bartuś, K., Hirnle, T., Dziembowska, I., Knapik, P., Deja, M., Wierzba, W., Tobota, Z., Maruszewski, B. J., & Suwalski, P. (2020). Long-Term Survival Following Surgical Ablation for Atrial Fibrillation Concomitant to Isolated and Combined Coronary Artery Bypass Surgery—Analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK). Journal of Clinical Medicine, 9(5), 1345. https://doi.org/10.3390/jcm9051345






