The Effect of a Multicomponent Dual-Task Exercise on Cortical Thickness in Older Adults with Cognitive Decline: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
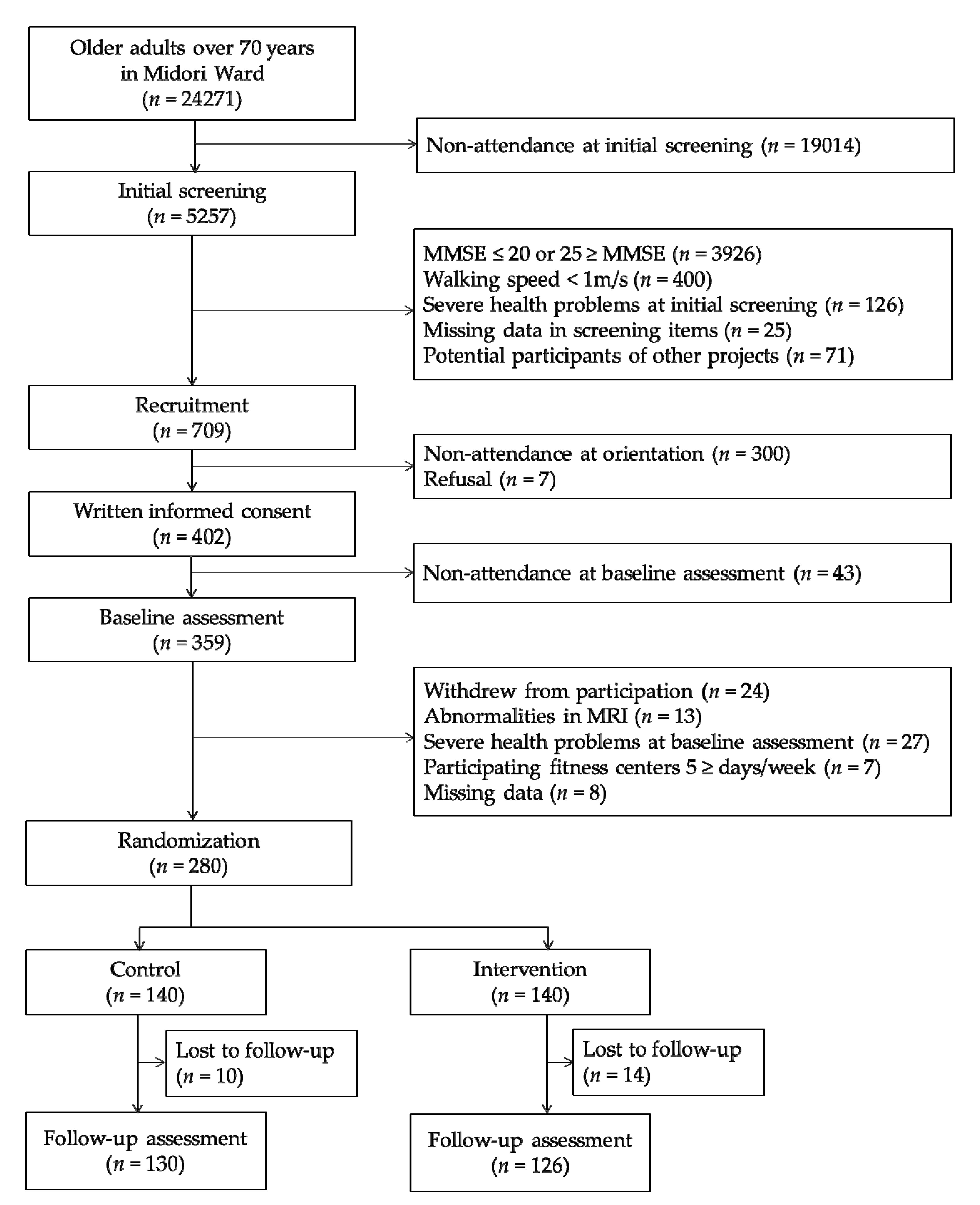
2.1. Participants and Procedures
2.2. Intervention
2.3. Neuropsychological Test Battery
2.4. MRI Acquisition
2.5. Regional Cortical Thickness Measurement
2.6. Aerobic Fitness
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Completion Rates
3.2. The Effects of Training on Cortical Thickness
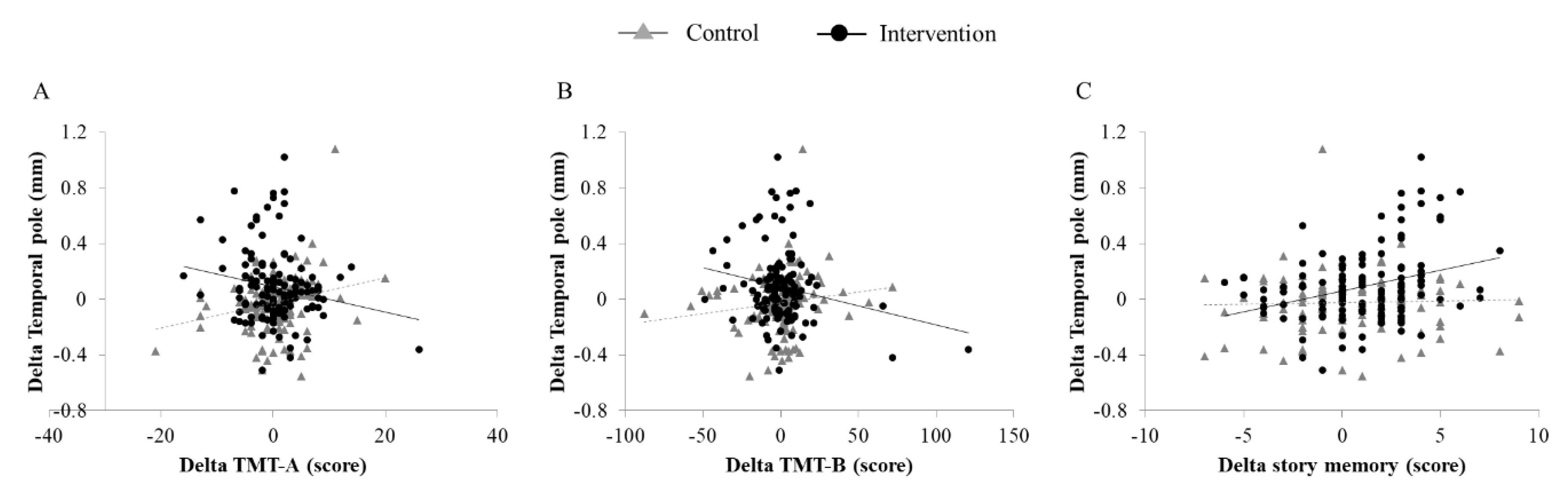
3.3. Association Between Changes in Cortical Thickness and Cognitive Performance
3.4. Association Between Changes in Cortical Thickness and Aerobic Fitness
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Alzheimer Report 2015—The Global Impact of Dementia. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf (accessed on 14 March 2019).
- Gates, N.J.; Sachdev, P. Is cognitive training an effective treatment for preclinical and early Alzheimer’s disease? J. Alzheimers Dis. 2014, 42 (Suppl. 4), 551–559. [Google Scholar] [CrossRef] [PubMed]
- Groot, C.; Hooghiemstra, A.M.; Raijmakers, P.G.; van Berckel, B.N.; Scheltens, P.; Scherder, E.J.; van der Flier, W.M.; Ossenkoppele, R. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res. Rev. 2016, 25, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, J.; An, L.; Hui, F.; Ren, T.; Ma, H.; Zhao, Q. Does music therapy enhance behavioral and cognitive function in elderly dementia patients? A systematic review and meta-analysis. Ageing Res. Rev. 2017, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rodakowski, J.; Saghafi, E.; Butters, M.A.; Skidmore, E.R. Non-pharmacological interventions for adults with mild cognitive impairment and early stage dementia: An updated scoping review. Mol. Asp. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Daviglus, M.L.; Bell, C.C.; Berrettini, W.; Bowen, P.E.; Connolly, E.S.; Cox, N.J.; Dunbar-Jacob, J.M.; Granieri, E.C.; Hunt, G.; McGarry, K.; et al. National Institutes of Health State-of-the-Science Conference statement: Preventing Alzheimer disease and cognitive decline. Ann. Intern. Med. 2010, 153, 176–181. [Google Scholar] [CrossRef]
- Lautenschlager, N.T.; Cox, K.L.; Flicker, L.; Foster, J.K.; van Bockxmeer, F.M.; Xiao, J.G.; Greenop, K.R.; Almeida, O.P. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease—A randomized trial. JAMA J. Am. Med. Assoc. 2008, 300, 1027–1037. [Google Scholar] [CrossRef]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H.; Strauman, T.A.; Welsh-Bohmer, K.; Browndyke, J.N.; Sherwood, A. Aerobic Exercise and Neurocognitive Performance: A Meta-Analytic Review of Randomized Controlled Trials. Psychosom. Med. 2010, 72, 239–252. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. Ser. A 2006, 61, 1166–1170. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- ten Brinke, L.F.; Bolandzadeh, N.; Nagamatsu, L.S.; Hsu, C.L.; Davis, J.C.; Miran-Khan, K.; Liu-Ambrose, T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Sports Med. 2015, 49, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.J.; Matthews, F.E.; Brayne, C.; Ince, P.; Halliday, G.; Kril, J.J.; Dalton, M.A.; Richardson, K.; Forster, G.; Sachdev, P.S.; et al. Multiple biological pathways link cognitive lifestyle to protection from dementia. Biol. Psychiatry 2012, 71, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Lipton, R.B.; Katz, M.J.; Hall, C.B.; Derby, C.A.; Kuslansky, G.; Ambrose, A.F.; Sliwinski, M.; Buschke, H. Leisure activities and the risk of dementia in the elderly. N. Engl. J. Med. 2003, 348, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.J.; Sachdev, P.; Wen, W.; Chen, X.; Brodaty, H. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS ONE 2008, 3, e2598. [Google Scholar] [CrossRef] [PubMed]
- Landau, S.M.; Marks, S.M.; Mormino, E.C.; Rabinovici, G.D.; Oh, H.; O’Neil, J.P.; Wilson, R.S.; Jagust, W.J. Association of Lifetime Cognitive Engagement and Low beta-Amyloid Deposition. Arch. Neurol. 2012, 69, 623–629. [Google Scholar]
- Law, L.L.; Barnett, F.; Yau, M.K.; Gray, M.A. Effects of combined cognitive and exercise interventions on cognition in older adults with and without cognitive impairment: A systematic review. Ageing Res. Rev. 2014, 15, 61–75. [Google Scholar] [CrossRef]
- Tait, J.L.; Duckham, R.L.; Milte, C.M.; Main, L.C.; Daly, R.M. Influence of Sequential vs. Simultaneous Dual-Task Exercise Training on Cognitive Function in Older Adults. Front. Aging Neurosci. 2017, 9, 368. [Google Scholar] [CrossRef]
- Zhu, X.; Yin, S.; Lang, M.; He, R.; Li, J. The more the better? A meta-analysis on effects of combined cognitive and physical intervention on cognition in healthy older adults. Ageing Res. Rev. 2016, 31, 67–79. [Google Scholar] [CrossRef]
- Yang, C.; Moore, A.; Mpofu, E.; Dorstyn, D.; Li, Q.; Yin, C. Effectiveness of Combined Cognitive and Physical Interventions to Enhance Functioning in Older Adults With Mild Cognitive Impairment: A Systematic Review of Randomized Controlled Trials. Gerontologist 2019. [Google Scholar] [CrossRef]
- Nishiguchi, S.; Yamada, M.; Tanigawa, T.; Sekiyama, K.; Kawagoe, T.; Suzuki, M.; Yoshikawa, S.; Abe, N.; Otsuka, Y.; Nakai, R.; et al. A 12-Week Physical and Cognitive Exercise Program Can Improve Cognitive Function and Neural Efficiency in Community-Dwelling Older Adults: A Randomized Controlled Trial. J. Am. Geriatr. Soc. 2015, 63, 1355–1363. [Google Scholar] [CrossRef]
- Anstey, K.J.; Wood, J.; Lord, S.; Walker, J.G. Cognitive, sensory and physical factors enabling driving safety in older adults. Clin. Psychol. Rev. 2005, 25, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Ito, K.; Shimokata, H.; Washimi, Y.; Endo, H.; Kato, T. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS ONE 2013, 8, e61483. [Google Scholar] [CrossRef] [PubMed]
- Fabel, K.; Kempermann, G. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromol. Med. 2008, 10, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Fabel, K.; Wolf, S.A.; Ehninger, D.; Babu, H.; Leal-Galicia, P.; Kempermann, G. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front. Neurosci. 2009, 3, 50. [Google Scholar] [CrossRef] [PubMed]
- Law, L.L.F.; Mok, V.C.T.; Yau, M.M.K. Effects of functional tasks exercise on cognitive functions of older adults with mild cognitive impairment: A randomized controlled pilot trial. Alzheimers Res. Ther. 2019, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef]
- Dotson, V.M.; Szymkowicz, S.M.; Sozda, C.N.; Kirton, J.W.; Green, M.L.; O’Shea, A.; McLaren, M.E.; Anton, S.D.; Manini, T.M.; Woods, A.J. Age Differences in Prefrontal Surface Area and Thickness in Middle Aged to Older Adults. Front. Aging Neurosci. 2015, 7, 250. [Google Scholar] [CrossRef]
- Kabani, N.; Le Goualher, G.; MacDonald, D.; Evans, A.C. Measurement of cortical thickness using an automated 3-D algorithm: A validation study. Neuroimage 2001, 13, 375–380. [Google Scholar] [CrossRef]
- Fjell, A.M.; Westlye, L.T.; Amlien, I.; Espeseth, T.; Reinvang, I.; Raz, N.; Agartz, I.; Salat, D.H.; Greve, D.N.; Fischl, B.; et al. High Consistency of Regional Cortical Thinning in Aging across Multiple Samples. Cereb. Cortex 2009, 19, 2001–2012. [Google Scholar] [CrossRef]
- Jonasson, L.S.; Nyberg, L.; Kramer, A.F.; Lundquist, A.; Riklund, K.; Boraxbekk, C.J. Aerobic Exercise Intervention, Cognitive Performance, and Brain Structure: Results from the Physical Influences on Brain in Aging (PHIBRA) Study. Front. Aging Neurosci. 2016, 8, 336. [Google Scholar] [CrossRef]
- Reiter, K.; Nielson, K.A.; Smith, T.J.; Weiss, L.R.; Alfini, A.J.; Smith, J.C. Improved Cardiorespiratory Fitness Is Associated with Increased Cortical Thickness in Mild Cognitive Impairment. J. Int. Neuropsychol. Soc. 2015, 21, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Cao, X.; Li, T.; Tang, Y.; Li, W.; Wang, J.; Chan, R.C.; Li, C. Cortical Thickness Changes Correlate with Cognition Changes after Cognitive Training: Evidence from a Chinese Community Study. Front. Aging Neurosci. 2016, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Bugg, J.M.; Head, D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol. Aging 2011, 32, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Engvig, A.; Fjell, A.M.; Westlye, L.T.; Moberget, T.; Sundseth, O.; Larsen, V.A.; Walhovd, K.B. Effects of memory training on cortical thickness in the elderly. Neuroimage 2010, 52, 1667–1676. [Google Scholar] [CrossRef]
- Cowell, P.E.; Turetsky, B.I.; Gur, R.C.; Grossman, R.I.; Shtasel, D.L.; Gur, R.E. Sex-Differences in Aging of the Human Frontal and Temporal Lobes. J. Neurosci. 1994, 14, 4748–4755. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, T.L.; Archibald, S.L.; Fennema-Notestine, C.; Gamst, A.C.; Stout, J.C.; Bonner, J.; Hesselink, J.R. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol. Aging 2001, 22, 581–594. [Google Scholar] [CrossRef]
- Bartzokis, G.; Beckson, M.; Lu, P.H.; Nuechterlein, K.H.; Edwards, N.; Mintz, J. Age-related changes in frontal and temporal lobe volumes in men—A magnetic resonance imaging study. Arch. Gen. Psychiatry 2001, 58, 461–465. [Google Scholar] [CrossRef]
- McDonald, C.R.; McEvoy, L.K.; Gharapetian, L.; Fennema-Notestine, C.; Hagler, D.J.; Holland, D.; Koyama, A.; Brewer, J.B.; Dale, A.M. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurobiology 2009, 73, 457–465. [Google Scholar] [CrossRef]
- Harada, K.; Lee, S.; Lee, S.; Bae, S.; Harada, K.; Shimada, H. Changes in objectively measured outdoor time and physical, psychological, and cognitive function among older adults with cognitive impairments. Arch. Gerontol. Geriatr. 2018, 78, 190–195. [Google Scholar] [CrossRef]
- Harada, K.; Lee, S.; Lee, S.; Bae, S.; Harada, K.; Shimada, H. Environmental predictors of objectively measured out-of-home time among older adults with cognitive decline. Arch. Gerontol. Geriatr. 2019, 82, 259–265. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Doi, T.; Tsutsumimoto, K.; Lee, S.; Suzuki, T. Cognitive Impairment and Disability in Older Japanese Adults. PLoS ONE 2016, 11, e0158720. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-mental state. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Suzuki, T.; Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Lee, S.; Park, H. Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: A randomized controlled trial. BMC Neurol. 2012, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Makizako, H.; Shimada, H.; Park, H.; Doi, T.; Yoshida, D.; Uemura, K.; Tsutsumimoto, K.; Suzuki, T. Evaluation of multidimensional neurocognitive function using a tablet personal computer: Test-retest reliability and validity in community-dwelling older adults. Geriatr. Gerontol. Int. 2013, 13, 860–866. [Google Scholar] [CrossRef]
- Lezak, M.D.; Lezak, M.D. Neuropsychological Assessment, 4th ed.; Oxford University Press: Oxford, NY, USA, 2004. [Google Scholar]
- Royer, F.L.; Gilmore, G.C.; Gruhn, J.J. Normative Data for the Symbol Digit Substitution Task. J. Clin. Psychol 1981, 37, 608–614. [Google Scholar] [CrossRef]
- Yesavage, J.A. Geriatric Depression Scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Desikan, R.S.; Segonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Steffen, T.M.; Hacker, T.A.; Mollinger, L. Six-minute walk test: A valuable test, when properly standardized—Author response. Phys. Ther. 2002, 82, 827–828. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Burzynska, A.Z.; Nagel, I.E.; Preuschhof, C.; Gluth, S.; Backman, L.; Li, S.C.; Lindenberger, U.; Heekeren, H.R. Cortical thickness is linked to executive functioning in adulthood and aging. Hum. Brain Mapp. 2012, 33, 1607–1620. [Google Scholar] [CrossRef]
- Righart, R.; Duering, M.; Gonik, M.; Jouvent, E.; Reyes, S.; Herve, D.; Chabriat, H.; Dichgans, M. Impact of regional cortical and subcortical changes on processing speed in cerebral small vessel disease. Neuroimage Clin. 2013, 2, 854–861. [Google Scholar] [CrossRef] [PubMed][Green Version]
- MacPherson, S.E.; Cox, S.R.; Dickie, D.A.; Karama, S.; Starr, J.M.; Evans, A.C.; Bastin, M.E.; Wardlaw, J.M.; Deary, I.J. Processing speed and the relationship between Trail Making Test-B performance, cortical thinning and white matter microstructure in older adults. Cortex 2017, 95, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, E.A.; Geisler, M.W.; Squires, N.K. Construct validity in the Trail Making Test: What makes Part B harder? J. Clin. Exp. Neuropsychol. 1995, 17, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Bakkour, A.; Morris, J.C.; Dickerson, B.C. The cortical signature of prodromal AD Regional thinning predicts mild AD dementia. Neurology 2009, 72, 1048–1055. [Google Scholar] [CrossRef]
- Lerch, J.P.; Pruessner, J.C.; Zijdenbos, A.; Hampel, H.; Teipel, S.J.; Evans, A.C. Focal decline of cortical thickness in Alzheimer’s disease identified by computational neuroanatomy. Cereb. Cortex 2005, 15, 995–1001. [Google Scholar] [CrossRef]
- Jouvent, E.; Mangin, J.F.; Porcher, R.; Viswanathan, A.; O’Sullivan, M.; Guichard, J.P.; Dichgans, M.; Bousser, M.G.; Chabriat, H. Cortical changes in cerebral small vessel diseases: A 3D MRI study of cortical morphology in CADASIL. Brain 2008, 131, 2201–2208. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, L.G.; Green, A.E.; Babakchanian, S.; Hwang, K.S.; Chou, Y.Y.; Toga, A.W.; Thompson, P.M. Hippocampal Atrophy and Ventricular Enlargement in Normal Aging, Mild Cognitive Impairment (MCI), and Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2012, 26, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; Aisen, P.S.; Trojanowski, J.Q.; Shaw, L.M.; Bernstein, M.A.; Petersen, R.C.; Weiner, M.W.; et al. Evidence for ordering of Alzheimer disease biomarkers. Arch. Neurol. 2011, 68, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Cabral, H.J.; Hess, C.P.; Dillon, W.P.; Glastonbury, C.M.; Weiner, M.W.; Schmansky, N.J.; Greve, D.N.; Salat, D.H.; Buckner, R.L.; et al. Automated MRI measures identify individuals with mild cognitive impairment and Alzheimers disease. Brain 2009, 132, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Gomar, J.J.; Bobes-Bascaran, M.T.; Conejero-Goldberg, C.; Davies, P.; Goldberg, T.E.; Neuroimaging, A.s.D. Utility of Combinations of Biomarkers, Cognitive Markers, and Risk Factors to Predict Conversion From Mild Cognitive Impairment to Alzheimer Disease in Patients in the Alzheimer’s Disease Neuroimaging Initiative. Arch. Gen. Psychiatry 2011, 68, 961–969. [Google Scholar] [CrossRef]
- Devanand, D.P.; Pradhaban, G.; Liu, X.; Khandji, A.; De Santi, S.; Segal, S.; Rusinek, H.; Pelton, G.H.; Honig, L.S.; Mayeux, R.; et al. Hippocampal and entorhinal atrophy in mild cognitive impairment—Prediction of Alzheimer disease. Neurology 2007, 68, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, J.L.; Przybelski, S.A.; Weigand, S.D.; Knopman, D.S.; Boeve, B.F.; Petersen, R.C.; Jack, C.R. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain 2007, 130, 1777–1786. [Google Scholar] [CrossRef]
- Williams, V.J.; Hayes, J.P.; Forman, D.E.; Salat, D.H.; Sperling, R.A.; Verfaellie, M.; Hayes, S.M. Cardiorespiratory fitness is differentially associated with cortical thickness in young and older adults. Neuroimage 2017, 146, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Siddarth, P.; Burggren, A.C.; Eyre, H.A.; Small, G.W.; Merrill, D.A. Sedentary behavior associated with reduced medial temporal lobe thickness in middle-aged and older adults. PLoS ONE 2018, 13, e0195549. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Perreau, V.M.; Cotman, C.W. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol. Aging 2005, 26, 511–520. [Google Scholar] [CrossRef]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 20, 2580–2590. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002, 25, 295–301. [Google Scholar] [CrossRef]
- LLorens-Martin, M.; Torres-Aleman, I.; Trejo, J.L. Exercise modulates insulin-like growth factor 1-dependent and -independent effects on adult hippocampal neurogenesis and behaviour. Mol. Cell Neurosci. 2010, 44, 109–117. [Google Scholar] [CrossRef]
- Pervaiz, N.; Hoffman-Goetz, L. Freewheel Training Alters Mouse Hippocampal Cytokines. Int. J. Sports Med. 2011, 32, 889–895. [Google Scholar] [CrossRef]
- Santin, K.; da Rocha, R.F.; Cechetti, F.; Quincozes-Santos, A.; de Souza, D.F.; Nardin, P.; Rodrigues, L.; Leite, M.C.; Moreira, J.C.F.; Salbego, C.G.; et al. Moderate exercise training and chronic caloric restriction modulate redox status in rat hippocampus. Brain Res. 2011, 1421, 1–10. [Google Scholar] [CrossRef]
- Farmer, J.; Zhao, X.; van Praag, H.; Wodtke, K.; Gage, F.H.; Christie, B.R. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 2004, 124, 71–79. [Google Scholar] [CrossRef] [PubMed]
- van Praag, H. Exercise and the brain: Something to chew on. Trends Neurosci. 2009, 32, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Maviel, T.; Durkin, T.P.; Menzaghi, F.; Bontempi, B. Sites of neocortical reorganization critical for remote spatial memory. Science 2004, 305, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Trachtenberg, J.T.; Chen, B.E.; Knott, G.W.; Feng, G.; Sanes, J.R.; Welker, E.; Svoboda, K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 2002, 420, 788–794. [Google Scholar] [CrossRef]
- Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Theill, N.; Schumacher, V.; Adelsberger, R.; Martin, M.; Jancke, L. Effects of simultaneously performed cognitive and physical training in older adults. BMC Neurosci. 2013, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Curlik, D.M.; Shors, T.J. Training your brain: Do mental and physical (MAP) training enhance cognition through the process of neurogenesis in the hippocampus? Neuropharmacology 2013, 64, 506–514. [Google Scholar] [CrossRef]
- Yu, F.; Lin, F.V.; Salisbury, D.L.; Shah, K.N.; Chow, L.; Vock, D.; Nelson, N.W.; Porsteinsson, A.P.; Jack, C., Jr. Efficacy and mechanisms of combined aerobic exercise and cognitive training in mild cognitive impairment: Study protocol of the ACT trial. Trials 2018, 19, 700. [Google Scholar] [CrossRef]
- Fabre, C.; Chamari, K.; Mucci, P.; Masse-Biron, J.; Prefaut, C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int. J. Sports Med. 2002, 23, 415–421. [Google Scholar] [CrossRef]
- Oswald, W.D.; Gunzelmann, T.; Rupprecht, R.; Hagen, B. Differential effects of single versus combined cognitive and physical training with older adults: The SimA study in a 5-year perspective. Eur. J. Ageing 2006, 3, 179. [Google Scholar] [CrossRef]
- Reiman, E.M.; Caselli, R.J.; Yun, L.S.; Chen, K.W.; Bandy, D.; Minoshima, S.; Thibodeau, S.N.; Osborne, D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N. Engl. J. Med. 1996, 334, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Rajji, T.K.; Shulman, K.I. Brief cognitive screening instruments: An update. Int. J. Geriatr. Psychiatr. 2010, 25, 111–120. [Google Scholar] [CrossRef] [PubMed]
- O’Bryant, S.E.; Humphreys, J.D.; Smith, G.E.; Ivnik, R.J.; Graff-Radford, N.R.; Petersen, R.C.; Lucas, J.A. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch. Neurol. 2008, 65, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.J.; Trempe, C.L. The End of Alzheimer’s: The Brain and Beyond, 2nd ed.; Academic Press: London, UK, 2017. [Google Scholar]
- Mungas, D. In-Office Mental Status Testing—A Practical Guide. Geriatrics 1991, 46, 54–58. [Google Scholar]
- Patnode, C.D.; Perdue, L.A.; Rossom, R.C.; Rushkin, M.C.; Redmond, N.; Thomas, R.G.; Lin, J.S. Screening for Cognitive Impairment in Older Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2020, 323, 764–785. [Google Scholar] [CrossRef]
| Control (n = 140) | Intervention (n = 140) | p-Value | |
|---|---|---|---|
| Age (years) | 76.4 ± 4.2 | 76.3 ± 4.1 | 0.761 |
| Gender (female), % | 37.1 | 42.1 | 0.392 |
| Year of education (years) | 12.1 ± 2.5 | 11.7 ± 2.6 | 0.150 |
| GDS (score) | 2.6 ± 2.3 | 2.8 ± 2.2 | 0.441 |
| eTIV (cm3) | 1399.6 | 1385.5 | 0.421 |
| 6MWT (m) | 450 ± 53 | 453 ± 60 | 0.610 |
| Cognitive performance | |||
| TMT-A (sec) | 20.9 ± 4.9 | 20.9 ± 5.2 | 0.943 |
| TMT-B (sec) | 43.2 ± 21.9 | 42.4 ± 17.0 | 0.740 |
| SDST (score) | 51.6 ± 9.7 | 52.6± 10.3 | 0.382 |
| Story memory composite (score) | 11.9 ± 3.4 | 11.8 ± 3.7 | 0.724 |
| Mean Difference (95% CI) Between Baseline and Postintervention Values | Group | Time | Group × Time | Cohen’s d | |||
|---|---|---|---|---|---|---|---|
| Hemi | Intervention (n = 126) | Control (n = 130) | pa | pa | pa | ||
| Frontal lobe, mm | |||||||
| Superior frontal | L | −0.008 (−0.017 to 0.001) | −0.007 (−0.016 to 0.001) | 0.904 | 0.017 | 0.928 | 0.011 |
| R | −0.008 (−0.017 to 0.001) | −0.005 (−0.013 to 0.003) | 0.551 | 0.027 | 0.519 | 0.081 | |
| Rostral middle frontal | L | −0.009 (−0.018 to 0.001) | −0.003 (−0.011 to 0.006) | 0.898 | 0.063 | 0.308 | 0.128 |
| R | 0.004 (−0.005 to 0.014) | 0.004 (−0.005 to 0.013) | 0.965 | 0.195 | 0.971 | 0.004 | |
| Caudal middle frontal | L | −0.007 (−0.019 to 0.005) | −0.007 (−0.019 to 0.005) | 0.267 | 0.102 | 0.991 | 0.004 |
| R | −0.004 (−0.017 to 0.009) | −0.007 (−0.020 to 0.005) | 0.087 | 0.209 | 0.724 | 0.044 | |
| Lateral orbitofrontal | L | −0.006 (−0.019 to 0.007) | 0.003 (−0.009 to 0.016) | 0.672 | 0.795 | 0.312 | 0.127 |
| R | −0.011 (−0.028 to 0.006) | 0.009 (−0.008 to 0.026) | 0.731 | 0.858 | 0.106 | 0.204 | |
| Medial orbitofrontal | L | −0.009 (−0.023 to 0.005) | 0.009 (−0.006 to 0.023) | 0.137 | 0.98 | 0.09 | 0.214 |
| R | 0.000 (−0.015 to 0.016) | 0.012 (−0.003 to 0.027) | 0.693 | 0.254 | 0.292 | 0.132 | |
| Frontal pole | L | 0.006 (−0.023 to 0.036) | 0.004 (−0.025 to 0.033) | 0.488 | 0.609 | 0.915 | 0.013 |
| R | 0.013 (−0.015 to 0.041) | 0.035 (0.007 to 0.063) | 0.826 | 0.017 | 0.269 | 0.139 | |
| Precentral | L | 0.001 (−0.012 to 0.014) | −0.002 (−0.015 to 0.011) | 0.859 | 0.913 | 0.706 | 0.047 |
| R | −0.007 (−0.024 to 0.009) | −0.003 (−0.020 to 0.014) | 0.4 | 0.382 | 0.713 | 0.046 | |
| Temporal lobe, mm | |||||||
| Superior temporal | L | 0.002 (−0.013 to 0.018) | −0.017 (−0.032 to −0.002) | 0.954 | 0.185 | 0.08 | 0.221 |
| R | −0.010 (−0.019 to 0.000) | −0.013 (−0.023 to −0.004) | 0.856 | 0.001 | 0.634 | 0.06 | |
| Middle temporal | L | 0.095 (0.060 to 0.130) | −0.008 (−0.043 to 0.026) | 0.002 | 0.001 | <0.001 | 0.519 |
| R | −0.006 (−0.016 to 0.005) | −0.010 (−0.020 to 0.001) | 0.902 | 0.039 | 0.594 | 0.067 | |
| Inferior temporal | L | −0.007 (−0.029 to 0.005) | −0.009 (−0.021 to 0.003) | 0.134 | 0.071 | 0.839 | 0.025 |
| R | −0.003 (−0.016 to 0.010) | 0.002 (−0.011 to 0.015) | 0.095 | 0.886 | 0.63 | 0.06 | |
| Fusiform | L | −0.003 (−0.018 to 0.011) | −0.002 (−0.016 to 0.012) | 0.679 | 0.594 | 0.902 | 0.015 |
| R | −0.007 (−0.020 to 0.006) | 0.005 (−0.008 to 0.017) | 0.15 | 0.783 | 0.203 | 0.16 | |
| Temporal pole | L | 0.086 (0.045 to 0.127) | −0.032 (−0.073 to 0.008) | 0.104 | 0.069 | <0.001 | 0.508 |
| R | −0.035 (−0.070 to 0.000) | −0.045 (−0.079 to −0.011) | 0.702 | 0.001 | 0.69 | 0.05 | |
| Parahippocampal | L | −0.030 (−0.050 to −0.011) | −0.032 (−0.051 to −0.013) | 0.063 | 0 | 0.901 | 0.016 |
| R | −0.006 (−0.023 to 0.011) | −0.007 (−0.024 to 0.010) | 0.901 | 0.312 | 0.944 | 0.009 | |
| Control Group | Intervention Group | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Δ Left Middle Temporal | Δ Left Temporal Pole | Δ Left Middle Temporal | Δ Left Temporal Pole | |||||||||||||
| r | pa | β | pb | r | pa | β | pb | r | pa | β | pb | r | pa | β | pb | |
| Δ TMT-A | −0.014 | 0.872 | 0.002 | 0.984 | 0.235 | 0.008 | 0.223 | 0.013 | −0.084 | 0.351 | −0.082 | 0.347 | −0.182 | 0.042 | −0.179 | 0.041 |
| Δ TMT-B | 0.005 | 0.959 | −0.007 | 0.937 | 0.143 | 0.11 | 0.123 | 0.178 | −0.060 | 0.503 | −0.123 | 0.164 | −0.194 | 0.03 | −0.266 | 0.003 |
| Δ SDST | 0.09 | 0.314 | 0.101 | 0.272 | 0.06 | 0.506 | 0.033 | 0.721 | 0.164 | 0.068 | 0.152 | 0.086 | 0.182 | 0.042 | 0.142 | 0.116 |
| Δ Story memory | 0.09 | 0.317 | 0.111 | 0.229 | 0.035 | 0.695 | 0.025 | 0.785 | 0.236 | 0.008 | 0.212 | 0.017 | 0.313 | 0.001 | 0.277 | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, S.; Harada, K.; Lee, S.; Harada, K.; Makino, K.; Chiba, I.; Park, H.; Shimada, H. The Effect of a Multicomponent Dual-Task Exercise on Cortical Thickness in Older Adults with Cognitive Decline: A Randomized Controlled Trial. J. Clin. Med. 2020, 9, 1312. https://doi.org/10.3390/jcm9051312
Bae S, Harada K, Lee S, Harada K, Makino K, Chiba I, Park H, Shimada H. The Effect of a Multicomponent Dual-Task Exercise on Cortical Thickness in Older Adults with Cognitive Decline: A Randomized Controlled Trial. Journal of Clinical Medicine. 2020; 9(5):1312. https://doi.org/10.3390/jcm9051312
Chicago/Turabian StyleBae, Seongryu, Kenji Harada, Sangyoon Lee, Kazuhiro Harada, Keitaro Makino, Ippei Chiba, Hyuntae Park, and Hiroyuki Shimada. 2020. "The Effect of a Multicomponent Dual-Task Exercise on Cortical Thickness in Older Adults with Cognitive Decline: A Randomized Controlled Trial" Journal of Clinical Medicine 9, no. 5: 1312. https://doi.org/10.3390/jcm9051312
APA StyleBae, S., Harada, K., Lee, S., Harada, K., Makino, K., Chiba, I., Park, H., & Shimada, H. (2020). The Effect of a Multicomponent Dual-Task Exercise on Cortical Thickness in Older Adults with Cognitive Decline: A Randomized Controlled Trial. Journal of Clinical Medicine, 9(5), 1312. https://doi.org/10.3390/jcm9051312







