Elevated Levels of Monocyte Chemotactic Protein-1 in the Follicular Fluid Reveals Different Populations among Women with Severe Endometriosis
Abstract
1. Introduction
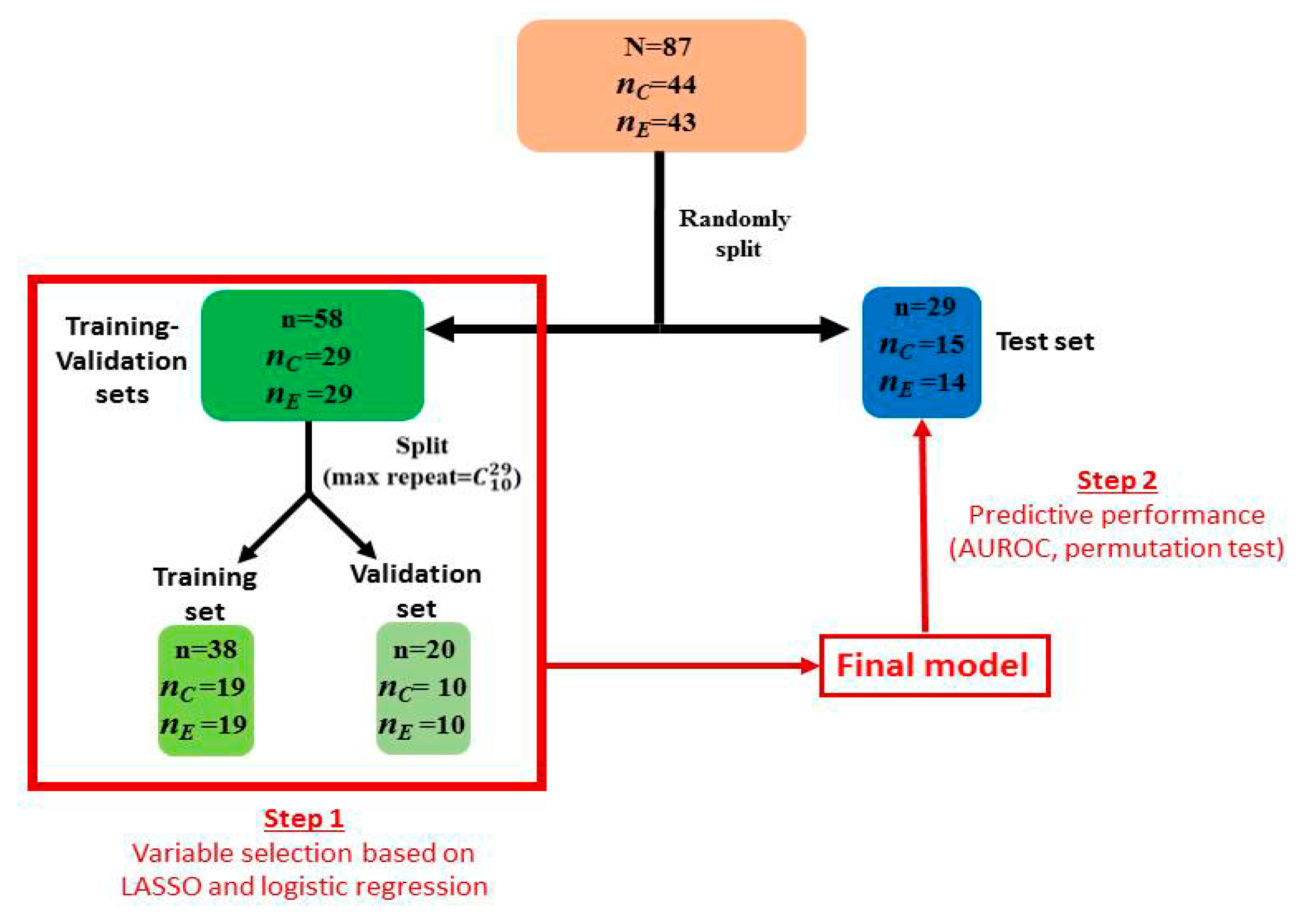
2. Materials and Methods
2.1. Study Participants
2.2. Embryo Culture and FF Samples
2.3. Quantification of Cytokines and other Inflammation-Aassociated Ffactors
2.4. Statistical Analysis
3. Results
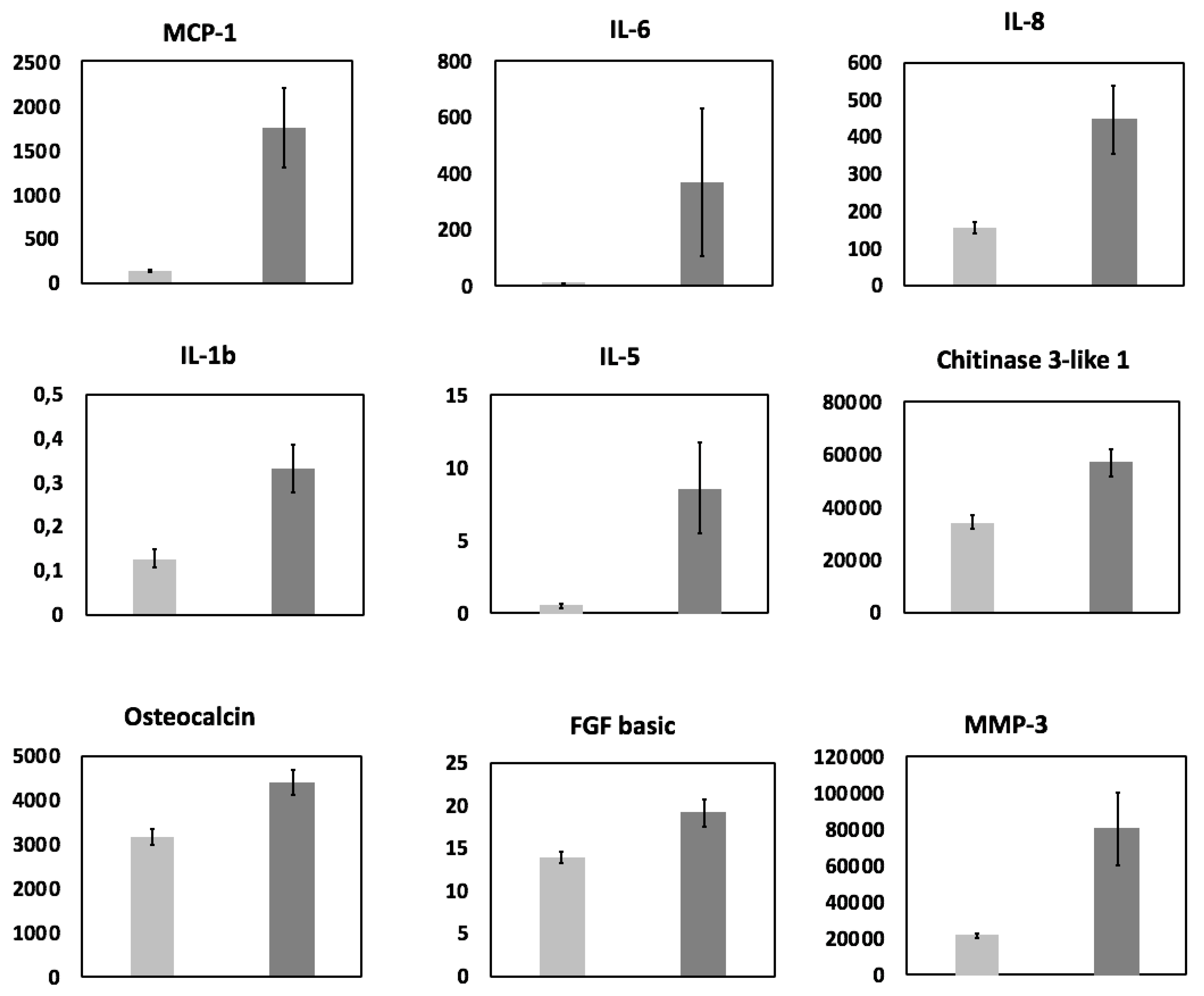
3.1. Cytokine Analysis
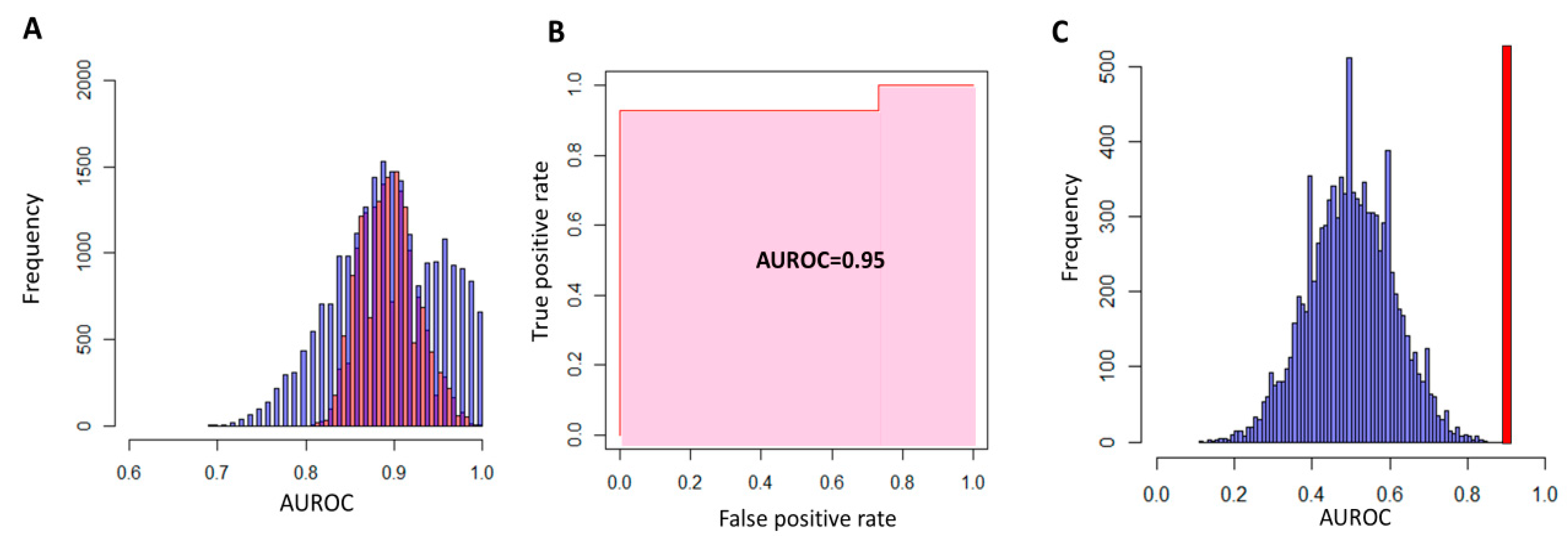
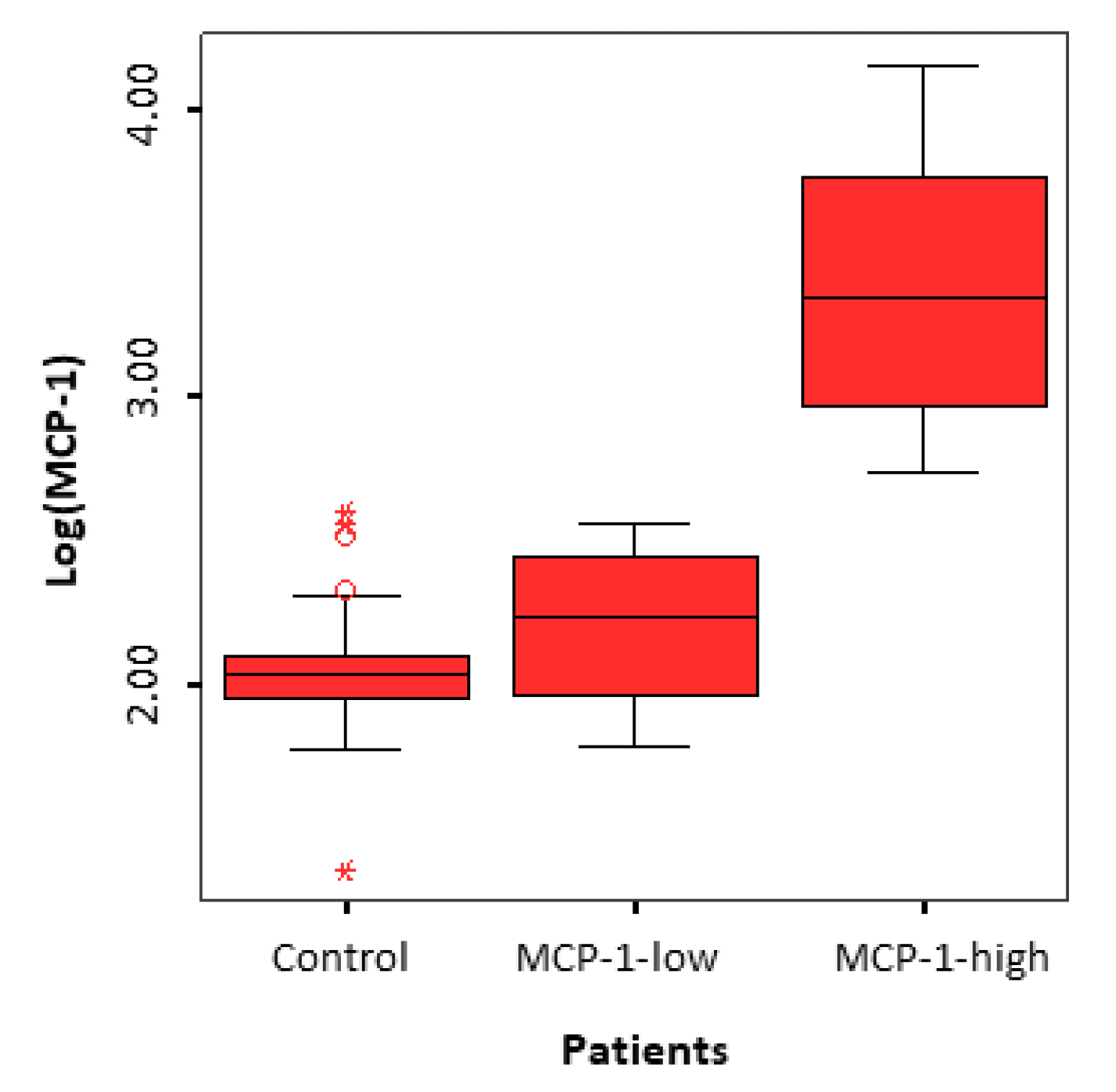
3.2. Identification of two Subgroups of Patients
4. Discussion
4.1. General Considerations
4.2. Summary of Evidence
4.3. Biological Rationale
4.4. Strength and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Schenken, R.S.; Guzick, D.S. Revised endometriosis classification: 1996. Fertil. Steril. 1997, 67, 815-816. [Google Scholar] [CrossRef]
- Wellbery, C. Diagnosis and treatment of endometriosis. Am. Fam. Phys. 1999, 60, 1753–1762. [Google Scholar]
- Macer, M.L.; Taylor, H.S. Endometriosis and infertility: A review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet. Gynecol. Clin. N. Am. 2012, 39, 535–549. [Google Scholar] [CrossRef]
- Hauzmann, E.E.; Garcia-Velasco, J.A.; Pellicer, A. Oocyte donation and endometriosis: What are the lessons? Semin. Reprod. Med. 2013, 31, 173–177. [Google Scholar] [CrossRef]
- Barnhart, K.; Dunsmoor-Su, R.; Coutifaris, C. Effect of endometriosis on in vitro fertilization. Fertil. Steril. 2002, 77, 1148–1155. [Google Scholar] [CrossRef]
- Harb, H.M.; Gallos, I.D.; Chu, J.; Harb, M.; Coomarasamy, A. The effect of endometriosis on in vitro fertilisation outcome: A systematic review and meta-analysis. BJOG 2013, 120, 1308–1320. [Google Scholar] [CrossRef]
- Rossi, A.C.; Prefumo, F. The effects of surgery for endometriosis on pregnancy outcomes following in vitro fertilization and embryo transfer: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2016, 294, 647–655. [Google Scholar] [CrossRef]
- Yang, C.; Geng, Y.; Li, Y.; Chen, C.; Gao, Y. Impact of ovarian endometrioma on ovarian responsiveness and IVF: A systematic review and meta-analysis. Reprod. Biomed. Online 2015, 31, 9–19. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Vanni, V.S.; Bartiromo, L.; Papaleo, E.; Zilberberg, E.; Candiani, M.; Orvieto, R.; Vigano, P. Is the oocyte quality affected by endometriosis? A review of the literature. J. Ovarian Res. 2017, 10, 43. [Google Scholar] [CrossRef]
- Coutinho, L.M.; Ferreira, M.C.; Rocha, A.L.; Carneiro, M.M.; Reis, F.M. New biomarkers in endometriosis. Adv. Clin. Chem. 2019, 89, 59–77. [Google Scholar]
- Zhou, W.J.; Yang, H.L.; Shao, J.; Mei, J.; Chang, K.K.; Zhu, R.; Li, M.Q. Anti-inflammatory cytokines in endometriosis. Cell. Mol. Life Sci. 2019, 76, 2111–2132. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Giorgi, V.S.; Wang, F.; Keefe, D.L.; Albertini, D.; Navarro, P.A. Influence of follicular fluid and cumulus cells on oocyte quality: Clinical implications. J. Assist. Reprod. Genet. 2018, 35, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Revelli, A.; Delle Piane, L.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Lo Turco, E.G.; Cordeiro, F.B.; Lopes, P.H.; Gozzo, F.C.; Pilau, E.J.; Soler, T.B.; da Silva, B.F.; Del Giudice, P.T.; Bertolla, R.P.; Fraietta, R.; et al. Proteomic analysis of follicular fluid from women with and without endometriosis: New therapeutic targets and biomarkers. Mol. Reprod. Dev. 2013, 80, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Bouet, P.E.; Chao de la Barca, J.M.; El Hachem, H.; Descamps, P.; Legendre, G.; Reynier, P.; May-Panloup, P. Metabolomics shows no impairment of the microenvironment of the cumulus-oocyte complex in the follicular fluid of women with isolated endometriosis. Reprod. Biomed. Online 2019, 39, 885–892. [Google Scholar] [CrossRef]
- Cordeiro, F.B.; Cataldi, T.R.; Perkel, K.J.; do Vale Teixeira da Costa, L.; Rochetti, R.C.; Stevanato, J.; Eberlin, M.N.; Zylbersztejn, D.S.; Cedenho, A.P.; Lo Turco, E.G. Lipidomics analysis of follicular fluid by ESI-MS reveals potential biomarkers for ovarian endometriosis. J. Assist. Reprod. Genet. 2015, 32, 1817–1825. [Google Scholar] [CrossRef][Green Version]
- Karaer, A.; Tuncay, G.; Mumcu, A.; Dogan, B. Metabolomics analysis of follicular fluid in women with ovarian endometriosis undergoing in vitro fertilization. Syst. Biol. Reprod. Med. 2019, 65, 39–47. [Google Scholar] [CrossRef]
- Marianna, S.; Alessia, P.; Susan, C.; Francesca, C.; Angela, S.; Francesca, C.; Antonella, N.; Patrizia, I.; Nicola, C.; Emilio, C. Metabolomic profiling and biochemical evaluation of the follicular fluid of endometriosis patients. Mol. Biosyst. 2017, 13, 1213–1222. [Google Scholar] [CrossRef]
- Sun, Z.; Song, J.; Zhang, X.; Wang, A.; Guo, Y.; Yang, Y.; Wang, X.; Xu, K.; Deng, J. Novel SWATHTM technology for follicular fluid metabolomics in patients with endometriosis. Pharmazie 2018, 73, 318–323. [Google Scholar]
- Hammadeh, M.E.; Fischer-Hammadeh, C.; Hoffmeister, H.; Huebner, U.; Georg, T.; Rosenbaum, P.; Schmidt, W. Fibroblast growth factor (FGF) intracellular adhesion molecule (sICAM-1) level in serum and follicular fluid of infertile women with polycystic ovarian syndrome, endometriosis and tubal damage, and their effect on ICSI outcome. Am. J. Reprod. Immunol. 2003, 50, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Karmakar, D.; Tripathi, V.; Luthra, K.; Kumar, S. Correlation of angiogenic cytokines-leptin and IL-8 in stage, type and presentation of endometriosis. Gynecol. Endocrinol. 2012, 28, 224–227. [Google Scholar] [CrossRef]
- Pellicer, A.; Albert, C.; Mercader, A.; Bonilla-Musoles, F.; Remohi, J.; Simon, C. The follicular and endocrine environment in women with endometriosis: Local and systemic cytokine production. Fertil. Steril. 1998, 70, 425–431. [Google Scholar] [CrossRef]
- Singh, A.K.; Dutta, M.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K. Intrafollicular interleukin-8, interleukin-12, and adrenomedullin are the promising prognostic markers of oocyte and embryo quality in women with endometriosis. J. Assit. Reprod. Genet. 2016, 33, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bersinger, N.A.; Mueller, M.D.; von Wolff, M. Intrafollicular inflammatory cytokines but not steroid hormones concentrations are increased in naturally matured follicles of women with proven endometriosis. J. Assist. Reprod. Genet. 2017, 34, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Qiao, J.; Liu, P.; Chen, G. Ovarian suppression treatment prior to in-vitro fertilization and embryo transfer in Chinese women with stage III or IV endometriosis. Int. J. Gynaecol. Obstet. 2008, 100, 167–170. [Google Scholar] [CrossRef]
- Kolanska, K.; Cohen, J.; Bendifallah, S.; Selleret, L.; Antoine, J.M.; Chabbert-Buffet, N.; Darai, E.; d’Argent, E.M. Pregnancy outcomes after controlled ovarian hyperstimulation in women with endometriosis-associated infertility: GnRH-agonist versus GnRH-antagonist. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 681–686. [Google Scholar] [CrossRef]
- ALPHA Scientists in Reproductive Medicine; ESHRE Special Interest Group Embryology. Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Reprod. Biomed. Online 2011, 22, 632–646. [Google Scholar] [CrossRef]
- Jorgensen, H.; Hill, A.S.; Beste, M.T.; Kumar, M.P.; Chiswick, E.; Fedorcsak, P.; Isaacson, K.B.; Lauffenburger, D.A.; Griffith, L.G.; Qviqstad, E. Peritoneal fluid cytokines related to endometriosis in patients evaluated for infertility. Fertil. Steril. 2017, 107, 1191–1199. [Google Scholar] [CrossRef]
- Rakhila, H.; Al-Akoum, M.; Bergeron, M.E.; Leboeuf, M.; Lemyre, M.; Akoum, A.; Pouliot, M. Promotion of angiogenesis and proliferation cytokines patterns in peritoneal fluid from women with endometriosis. J. Reprod. Immunol. 2016, 116, 1–6. [Google Scholar] [CrossRef]
- Ural, U.M.; Tekin, Y.B.; Cüre, M.; Sahin, F.K. Serum YKL-40 levels as a novel marker of inflammation in patients with endometriosis. Clin. Exp. Obstet. Gynecol. 2015, 42, 495–497. [Google Scholar] [PubMed]
- Deshmane, S.L.; Kremley, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interf. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Dahm-Kahler, P.; Ghahremani, M.; Lind, A.K.; Sundfeldt, K.; Brannstrom, M. Monocyte chemotactic protein-1 (MCP-1), its receptor, and macrophages in the perifollicular stroma during the human ovulatory process. Fertil. Steril. 2009, 91, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Dahm-Kahler, P.; Runesson, E.; Lind, A.K.; Brannstrom, M. Monocyte chemotactic protein-1 (MCP-1) in the follicle of the menstrual and IVF cycle. Mol. Hum. Reprod. 2006, 12, 1–6. [Google Scholar] [CrossRef]
- Younis, A.; Hawkins, K.; Mahini, H.; Butler, W.; Garelnabi, M. Serum tumor necrosis factor-α, interleukin-6, monocyte chemotactic protein-1 and paraoxonase-1 profiles in women with endometriosis, PCOS, or unexplained infertility. J. Assist. Reprod. Genet. 2014, 31, 1445–1451. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Kaufmann, A.M.; Abrao, M.S.; Mechsner, S. Addition of MCP-1 and MIP-3beta to the IL-8 appraisal in peritoneal fluid enhances the probability of identifying women with endometriosis. J. Reprod. Immunol. 2015, 109, 66–73. [Google Scholar] [CrossRef]
- Buyuk, E.; Asemota, O.A.; Merhi, Z.; Charron, M.J.; Berger, D.S.; Zapantis, A.; Jindal, S.K. Serum and follicular fluid monocyte chemotactic protein-1 levels are elevated in obese women and are associated with poorer clinical pregnancy rate after in vitro fertilization: A pilot study. Fertil. Steril. 2017, 107, 632–640. [Google Scholar] [CrossRef]
- Zubrzycka, A.; Zubrzycki, M.; Janecka, A.; Zubrzycka, M. New horizons in the etiopathogenesis and non-invasive diagnosis of endometriosis. Curr. Mol. Med. 2015, 15, 697–713. [Google Scholar] [CrossRef]
- Broekmans, F.J. Testing for ovarian reserve in assisted reproduction programs: The current point of view. Facts Views Vis. Obgyn. 2009, 1, 79–87. [Google Scholar]
- Klein, N.A.; Battaglia, D.E.; Fujimoto, V.Y.; Davis, G.S.; Bremner, W.J.; Soules, M.R. Reproductive aging: Accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J. Clin. Endocrinol. Metab. 1996, 81, 1038–1045. [Google Scholar]
- Kitajima, M.; Dolmans, M.M.; Donnez, O.; Masuzaki, H.; Soares, M.; Donnez, J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil. Steril. 2014, 101, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
| Variables | Endometriosis Group (n = 43) | Control Group (n = 44) | p-Value | |
|---|---|---|---|---|
| Age (years) | 32.2 ± 3.9 | 31.4 ± 4.3 | 0.36 | |
| Body Mass Index (kg/m2) | 23.1 ± 3.6 | 23.7 ± 4.0 | 0.46 | |
| Tobacco usage Non-smoker Smoker Former smoker Information missing | - 27 3 10 3 | - 26 6 11 1 | 0.62 | |
| Baseline FSH (IU/L) | 7.40 ± 3.21 | 8.60 ± 2.68 | 0.11 | |
| Serum Estradiol (pg/mL) | 51.5 ± 48.4 | 36.2 ± 16.1 | 0.07 | |
| Baseline Anti-Mullerian Hormone (AMH) (ng/mL) | 2.8 ± 2.08 | 2.9 ± 1.9 | 0.78 | |
| Antral follicle count | 17.6 ± 7.6 | 17.2 ± 6.8 | 0.80 | |
| Total dose of FSH per cycle (IU) | 2810 ± 1072 | 2375 ± 900 | 0.04* | |
| Stimulation protocol | Antagonist Agonist | 23 20 | 43 1 | < 0.001* |
| Stimulation | FSH FSH + Luteinizing Hormone (LH) | 32 11 | 35 9 | 0.57 |
| Treatment type | IVF ICSI | 14 29 | 18 26 | 0.42 |
| Oocytes retrieved | 8.8 ± 7.0 | 13.4 ± 5.9 | 0.002* | |
| Embryos per oocytes retrieved (%) | 53.5 ± 27.1 | 52.1 ± 24.5 | 0.72 | |
| Good quality embryos (%) | 58.2 ± 31.5 | 58.5 ± 28.1 | 0.97 | |
| Variables | Endometriosis Group (n = 43) | Subgroup MCP-1-high (n = 20) | Sub-group MCP-1-low (n = 23) | p* |
|---|---|---|---|---|
| Locations of endometriosis Ovaries Fallopian tubes Torus uterinus/uterosacral ligaments Rectovaginal septum Digestive tract Bladder | 41 12 25 11 9 5 | 20 3 12 4 4 1 | 21 9 13 7 5 4 | 0.56 0.15 0.82 0.67 - 0.44 |
| Presence of adhesions | 14 | 6 | 8 | 0.74 |
| Endometriosis diagnosis made by: Surgery MRI or ultrasound | 31 12 | 15 5 | 16 7 | 0.69 0.69 |
| History of surgery: Ovarian Deep endometriosis | 19 17 | 9 8 | 10 9 | 0.92 0.95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouet, P.-E.; Chao de la Barca, J.-M.; Boucret, L.; Descamps, P.; Legendre, G.; Hachem, H.E.; Blanchard, S.; Jeannin, P.; Reynier, P.; May-Panloup, P. Elevated Levels of Monocyte Chemotactic Protein-1 in the Follicular Fluid Reveals Different Populations among Women with Severe Endometriosis. J. Clin. Med. 2020, 9, 1306. https://doi.org/10.3390/jcm9051306
Bouet P-E, Chao de la Barca J-M, Boucret L, Descamps P, Legendre G, Hachem HE, Blanchard S, Jeannin P, Reynier P, May-Panloup P. Elevated Levels of Monocyte Chemotactic Protein-1 in the Follicular Fluid Reveals Different Populations among Women with Severe Endometriosis. Journal of Clinical Medicine. 2020; 9(5):1306. https://doi.org/10.3390/jcm9051306
Chicago/Turabian StyleBouet, Pierre-Emmanuel, Juan-Manuel Chao de la Barca, Lisa Boucret, Philippe Descamps, Guillaume Legendre, Hady El Hachem, Simon Blanchard, Pascale Jeannin, Pascal Reynier, and Pascale May-Panloup. 2020. "Elevated Levels of Monocyte Chemotactic Protein-1 in the Follicular Fluid Reveals Different Populations among Women with Severe Endometriosis" Journal of Clinical Medicine 9, no. 5: 1306. https://doi.org/10.3390/jcm9051306
APA StyleBouet, P.-E., Chao de la Barca, J.-M., Boucret, L., Descamps, P., Legendre, G., Hachem, H. E., Blanchard, S., Jeannin, P., Reynier, P., & May-Panloup, P. (2020). Elevated Levels of Monocyte Chemotactic Protein-1 in the Follicular Fluid Reveals Different Populations among Women with Severe Endometriosis. Journal of Clinical Medicine, 9(5), 1306. https://doi.org/10.3390/jcm9051306






