Luteinizing Hormone Levels Relate to the Unfavorable Pathology of Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADT | androgen deprivation therapy |
| BCR | biochemical recurrence |
| CI | confidence interval |
| CRPC | castration-resistant prostate cancer |
| FSH | follicle-stimulating hormone |
| HSPC | hormone-sensitive prostate cancer |
| LH | luteinizing hormone |
| LHRH | luteinizing hormone releasing hormone |
| OR | odds ratio |
| PSA | Prostate-specific antigen |
| SHBG | sex hormone-binding globulin |
References
- Huggins, C.; Hodges, C.V. Studies on Prostatic Cancer: I. The Effect of Castration, Of Estrogen and of Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. CA A Cancer J. Clin. 1972, 22, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Tombal, B.; Miller, K.; Boccon-Gibod, L.; Schröder, F.; Shore, N.; Moul, J.W.; Jensen, J.-K.; Olesen, T.K.; Persson, B.-E. A Phase III Extension Trial with a 1-Arm Crossover from Leuprolide to Degarelix: Comparison of Gonadotropin-Releasing Hormone Agonist and Antagonist Effect on Prostate Cancer. J. Urol. 2011, 186, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Kluth, L.A.; Shariat, S.F.; Kratzik, C.; Tagawa, S.; Sonpavde, G.; Rieken, M.; Scherr, U.S.; Pummer, K. The hypothalamic–pituitary–gonadal axis and prostate cancer: Implications for androgen deprivation therapy. World J. Urol. 2013, 32, 669–676. [Google Scholar] [CrossRef]
- Weckermann, D.; Harzmann, R. Hormone Therapy in Prostate Cancer: LHRH Antagonists versus LHRH Analogues. Eur. Urol. 2004, 46, 279–284. [Google Scholar] [CrossRef]
- Van Poppel, H.; Nilsson, S. Testosterone Surge: Rationale for Gonadotropin-Releasing Hormone Blockers? Urol. 2008, 71, 1001–1006. [Google Scholar] [CrossRef]
- Yano, M.; Imamoto, T.; Suzuki, H.; Fukasawa, S.; Kojima, S.; Komiya, A.; Naya, Y.; Ichikawa, T. The Clinical Potential of Pretreatment Serum Testosterone Level to Improve the Efficiency of Prostate Cancer Screening. Eur. Urol. 2007, 51, 375–380. [Google Scholar] [CrossRef]
- Morgentaler, A. Testosterone and Prostate Cancer: An Historical Perspective on a Modern Myth. Eur. Urol. 2006, 50, 935–939. [Google Scholar] [CrossRef]
- Crawford, E.D.; Schally, A.V.; Pinthus, J.H.; Block, N.L.; Rick, F.; Garnick, M.B.; Eckel, R.H.; Keane, T.E.; Shore, N.D.; Dahdal, D.N.; et al. The potential role of follicle-stimulating hormone in the cardiovascular, metabolic, skeletal, and cognitive effects associated with androgen deprivation therapy. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 183–191. [Google Scholar] [CrossRef]
- Ingles, S.A.; Liu, S.; Pinski, J. LHRH and LHR genotypes and prostate cancer incidence and survival. Int. J. Mol. Epidemiol. Genet. 2013, 4, 228–234. [Google Scholar]
- Vermeulen, A.; Verdonck, L.; Kaufman, J.M. A Critical Evaluation of Simple Methods for the Estimation of Free Testosterone in Serum. J. Clin. Endocrinol. Metab. 1999, 84, 3666–3672. [Google Scholar] [CrossRef]
- Garde, S.V.; Sheth, A.R.; Shah, M.G.; Kulkarni, S.A. Prostate—an extrapituitary source of follicle-stimulating hormone (FSH): Occurrence, localization, and de novo biosynthesis and its hormonal modulation in primates and rodents. Prostate 1991, 18, 271–287. [Google Scholar] [CrossRef]
- Mariani, S.; Salvatori, L.; Basciani, S.; Arizzi, M.; Franco, G.; Petrangeli, E.; Spera, G.; Gnessi, L. Expression and Cellular Localization of Follicle-Stimulating Hormone Receptor in Normal Human Prostate, Benign Prostatic Hyperplasia and Prostate Cancer. J. Urol. 2006, 175, 2072–2077. [Google Scholar] [CrossRef]
- Klotz, C.L.; Shore, N.D.; Andreou, C.; Persson, B.-E.; Cantor, P.; Jensen, J.-K.; Olesen, T.K.; Schröder, F.H.; Boccon-Gibod, L. The efficacy and safety of degarelix: A 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008, 102, 1531–1538. [Google Scholar] [CrossRef]
- Bull, P.; Morales, P.; Huyser, C.; Socías, T.; Castellón, E. Expression of GnRH receptor in mouse and rat testicular germ cells. Mol. Hum. Reprod. 2000, 6, 582–586. [Google Scholar] [CrossRef][Green Version]
- Sonntag, B.; Kiesel, L.; Nieschlag, E.; Behre, H. Association of Inhibin B Serum Levels with Parameters of Follicular Response in a Randomized Controlled Trial Comparing GnRH Agonist Versus Antagonist Protocols for Ovarian Hyperstimulation. J. Assist. Reprod. Genet. 2004, 21, 249–255. [Google Scholar] [CrossRef]
- Tao, Y.-X.; Bao, S.; Ackermann, D.M.; Lei, Z.M.; Rao, C. Expression of luteinizing hormone/human chorionic gonadotropin receptor gene in benign prostatic hyperplasia and in prostate carcinoma in humans. Biol. Reprod. 1997, 56, 67–72. [Google Scholar] [CrossRef]
- Pinski, J.K.; Xiong, S.; Wang, Q.; Stanczyk, F.; Hawes, D.; Liu, S. Effect of luteinizing hormone on the steroidogenic pathway in prostate cancer. Prostate 2010, 71, 892–898. [Google Scholar] [CrossRef]
- Montgomery, R.B.; Mostaghel, E.A.; Vessella, R.; Hess, D.L.; Kalhorn, T.F.; Higano, C.S.; True, L.D.; Nelson, P.S. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008, 68, 4447–4454. [Google Scholar] [CrossRef]
- Hilz, H.; Graefen, M.; Noldus, J.; Hammerer, P.; Knabbe, C.; Huland, E.; Huland, H. Advanced prostate cancer is associated with a decrease in serum luteinizing hormone. Eur. Urol. 2000, 38, 243–249. [Google Scholar] [CrossRef]
- Hsing, A.W.; Chu, L.W.; Stanczyk, F.Z. Androgen and Prostate Cancer: Is the Hypothesis Dead? Cancer Epidemiol. Biomark. Prev. 2008, 17, 2525–2530. [Google Scholar] [CrossRef]
- Claps, M.; Petrelli, F.; Caffo, O.; Amoroso, V.; Roca, E.; Mosca, A.; Maines, F.; Barni, S.; Berruti, A. Testosterone Levels and Prostate Cancer Prognosis: Systematic Review and Meta-analysis. Clin. Genitourin. Cancer 2018, 16, 165–175. [Google Scholar] [CrossRef]
- Léon, P.; Seisen, T.; Cussenot, O.; Drouin, S.J.; Cattarino, S.; Compérat, E.; Renard-Penna, R.; Mozer, P.; Bitker, M.-O.; Rouprêt, M. Low circulating free and bioavailable testosterone levels as predictors of high-grade tumors in patients undergoing radical prostatectomy for localized prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 384.e21–384.e27. [Google Scholar] [CrossRef]
- Vigano, A.; Piccioni, M.; Trutschnigg, B.; Hornby, L.; Chaudhury, P.; Kilgour, R. Male hypogonadism associated with advanced cancer: A systematic review. Lancet Oncol. 2010, 11, 679–684. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Heber, D. Hypogonadism in male patients with metastatic cancer prior to chemotherapy. Cancer Res. 1982, 42, 2495–2498. [Google Scholar]
- Lee, S.E.; Chung, J.S.; Han, B.K.; Park, C.S.; Moon, K.H.; Byun, S.-S.; Choea, G.; Hong, S.K. Preoperative Serum Sex Hormone-Binding Globulin as a Predictive Marker for Extraprostatic Extension of Tumor in Patients with Clinically Localized Prostate Cancer. Eur. Urol. 2008, 54, 1324–1332. [Google Scholar] [CrossRef]
- De Nunzio, C.; Lombardo, R.; Albisinni, S.; Gacci, M.; Tubaro, A. Serum levels of sex hormone binding globulin (SHBG) are not predictive of prostate cancer diagnosis and aggressiveness: Results from an Italian biopsy cohort. Int. Braz. Urol. 2014, 39, 793–799. [Google Scholar] [CrossRef]
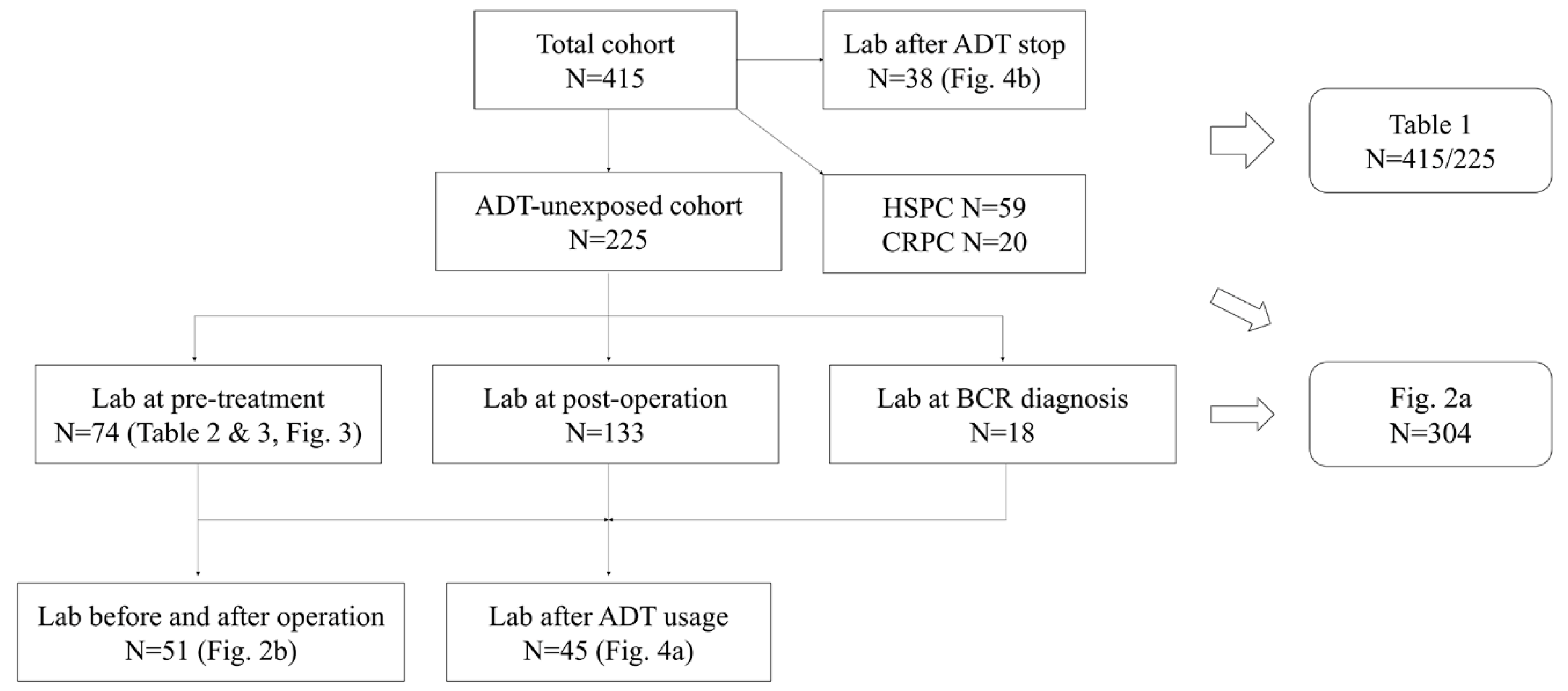
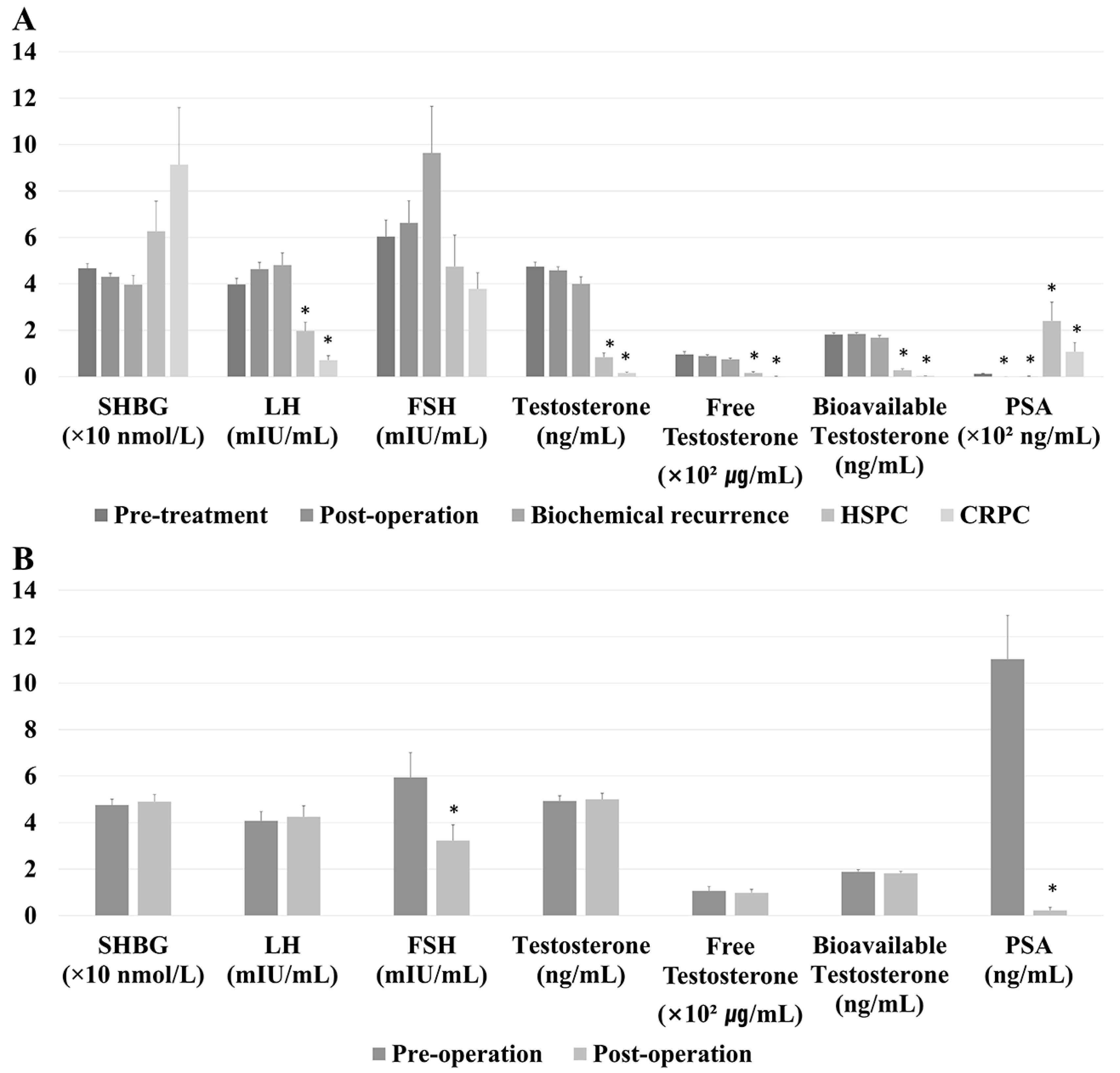
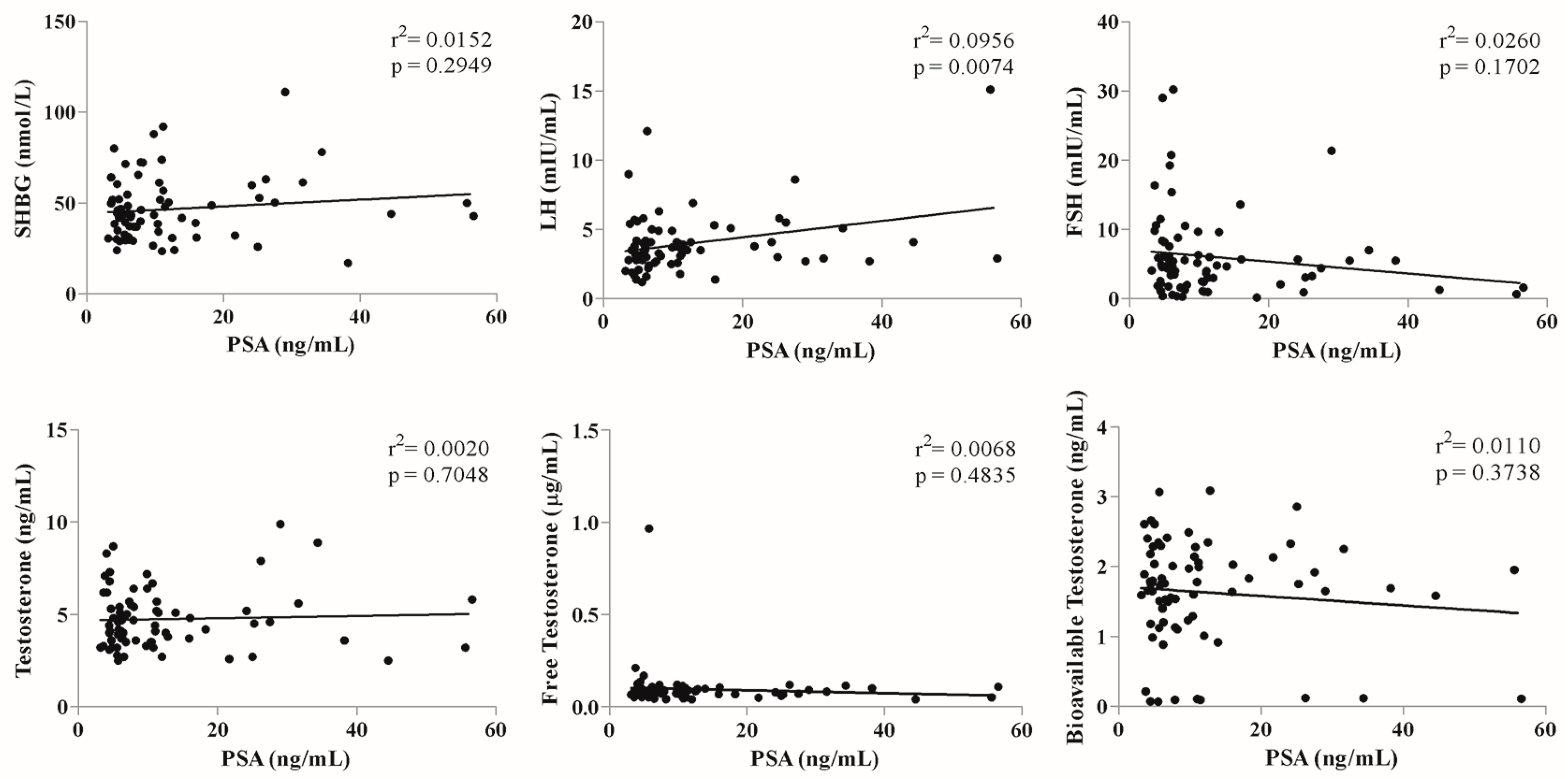

| Total Cohort (n = 415) | ADT-Unexposed Cohort (n = 225) | |
|---|---|---|
| Mean ± SD or Number (%) | Mean ± SD or Number (%) | |
| Age at diagnosis (years) | 67.3 ± 7.6 | 65.7 ± 6.5 |
| Age at the first exam (years) | 68.5 ± 7.4 | 66.8 ± 6.5 |
| 5α-Reductase inhibitor history | 32 (7.7%) | 18 (8.0%) |
| PSA level (ng/mL) | 73.5 ± 287.7 | 11.8 ± 16.7 |
| Grade group at biopsy | ||
| - ≤2 | 157 (37.8%) | 124 (55.1%) |
| - ≥3 | 224 (54.0%) | 99 (44.0%) |
| Percent of positive core (%) | 49.0 ± 34.3 | 34.1 ± 24.1 |
| Maximum percent of positive core (%) | 53.9 ± 32.5 | 41.1 ± 27.0 |
| Condition at the first exam | ||
| - Pre-treatment | 130 (31.3%) | 74 (32.8%) |
| - Post-operation | 171 (41.2%) | 133 (59.1%) |
| - Biochemical recurrence | 35 (8.4%) | 18 (8.0%) |
| - Hormone-sensitive prostate cancer | 59 (14.2%) | 0 (0.0%) |
| - Castration-resistant prostate cancer | 20 (4.8%) | 0 (0.0%) |
| Medication at the first exam | ||
| - None | 266 (64.1%) | 225 (100.0%) |
| - LHRH agonist | 115 (27.7%) | 0 (0.0%) |
| - LHRH antagonist | 10 (2.4%) | 0 (0.0%) |
| Clinical T stage | ||
| - 2 | 227 (54.7%) | 158 (70.9%) |
| - 3a | 100 (24.1%) | 51 (22.9%) |
| - 3b | 51 (12.3%) | 10 (4.5%) |
| - 4 | 26 (6.3%) | 4 (1.8%) |
| Clinical N1 stage | 68 (16.7%) | 1 (0.4%) |
| Clinical M1 stage | 63 (15.5%) | 0 (0.0%) |
| Prostate volume (cc) | 35.5 ± 20.6 | 32.8 ± 17.5 |
| Mean ± SD or Number (%) | |
|---|---|
| Grade group | |
| - ≤2 | 31 (41.9%) |
| - ≥3 | 43 (58.1%) |
| Pathologic T stage | |
| - ≤2 | 41 (55.5%) |
| - 3a | 22 (29.7%) |
| - 3b | 11 (14.9%) |
| Pathologic N1 stage | 1 (1.4%) |
| Tumor percent (%) | 18.3 ± 17.6 |
| Tumor volume (cc) | 5.4 ± 6.4 |
| Perineural invasion | 51 (68.9%) |
| Lymphovascular invasion | 23 (31.1%) |
| Unfavorable pathology | 50 (67.6%) |
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% | CI | p | OR | 95% | CI | p | |
| SHBG level | 1.01 | 0.98 | 1.04 | 0.4439 | ||||
| LH level | 1.53 | 1.02 | 2.29 | 0.0397 | 1.59 | 1.03 | 2.47 | 0.0376 |
| FSH level | 1.01 | 0.93 | 1.09 | 0.8398 | ||||
| Testosterone level | 1.00 | 0.74 | 1.35 | 0.9957 | ||||
| Free testosterone level | 0.01 | 0.00 | 558.61 | 0.3698 | ||||
| Bioavailable testosterone level | 0.70 | 0.29 | 1.69 | 0.4334 | ||||
| Preoperative PSA level | 1.10 | 1.02 | 1.19 | 0.0124 | 1.13 | 1.03 | 1.24 | 0.0133 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.Y.; Chi, B.H.; Lee, W.; Lim, B.; You, D.; Kim, C.-S. Luteinizing Hormone Levels Relate to the Unfavorable Pathology of Prostate Cancer. J. Clin. Med. 2020, 9, 1281. https://doi.org/10.3390/jcm9051281
Choi SY, Chi BH, Lee W, Lim B, You D, Kim C-S. Luteinizing Hormone Levels Relate to the Unfavorable Pathology of Prostate Cancer. Journal of Clinical Medicine. 2020; 9(5):1281. https://doi.org/10.3390/jcm9051281
Chicago/Turabian StyleChoi, Se Young, Byung Hoon Chi, Wonchul Lee, Bumjin Lim, Dalsan You, and Choung-Soo Kim. 2020. "Luteinizing Hormone Levels Relate to the Unfavorable Pathology of Prostate Cancer" Journal of Clinical Medicine 9, no. 5: 1281. https://doi.org/10.3390/jcm9051281
APA StyleChoi, S. Y., Chi, B. H., Lee, W., Lim, B., You, D., & Kim, C.-S. (2020). Luteinizing Hormone Levels Relate to the Unfavorable Pathology of Prostate Cancer. Journal of Clinical Medicine, 9(5), 1281. https://doi.org/10.3390/jcm9051281




